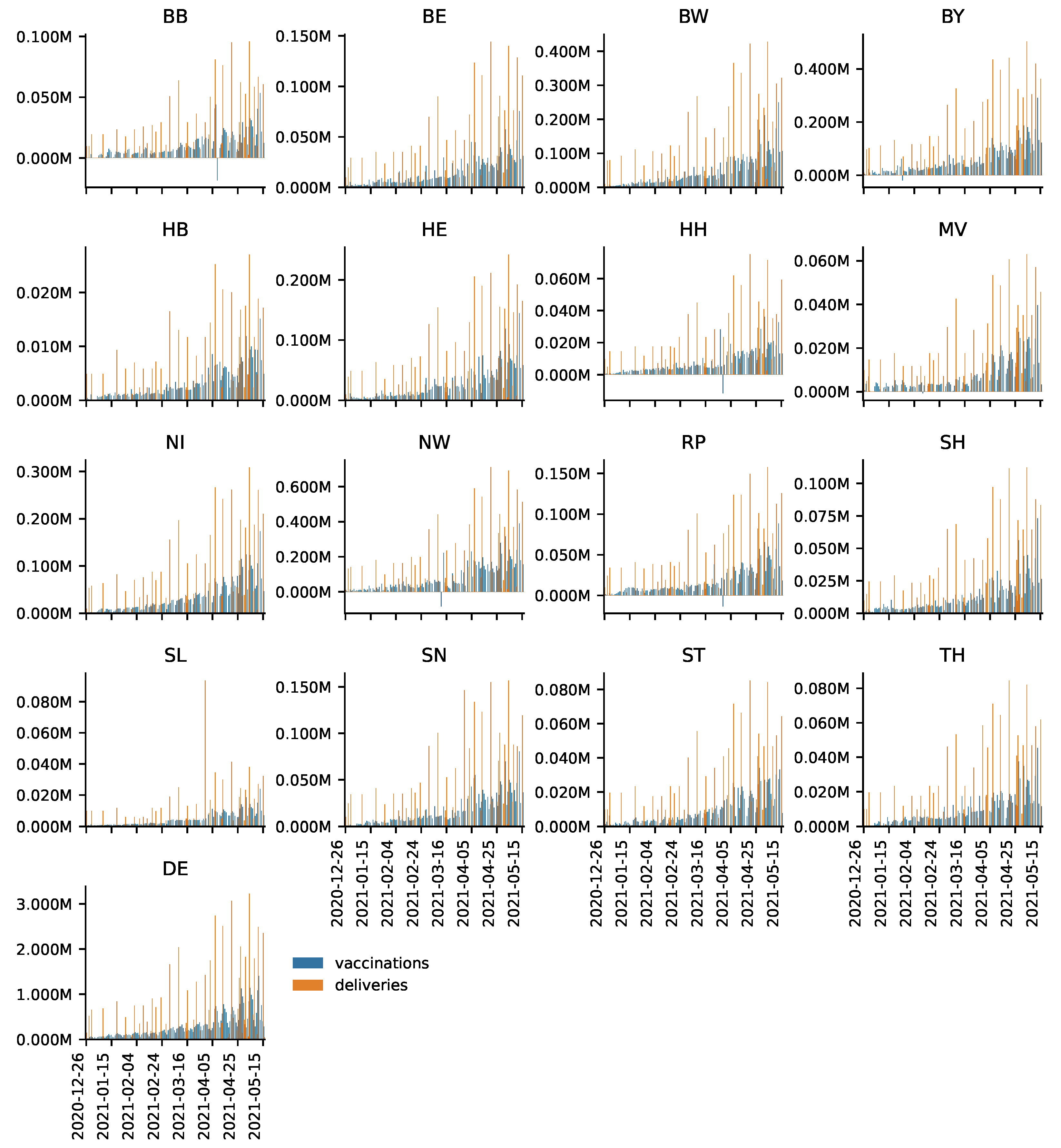
Appendix A. DEA
The influence of diminishing returns to scale was controlled for in the VRS DEA. However, there are further factors potentially affecting the results.
It is documented that there were occasional failures to deliver vaccines to certain areas (
https://bit.ly/3uGEIul; accessed on 28 June 2021). Even though these are out of the control of the decision makers, it is unlikely that these delivery failures to individual federal states systematically affects the DEA scores over a period of over 4 months. The same holds for large-scale delivery failures of the vaccine of Astra-Zeneca (
https://bit.ly/3jok2oe; accessed on 28 June 2021)). Moreover, the delivery failure of Astra-Zeneca’s vaccine affected Germany as a whole so that it should not affect the relative efficiencies of the federal states’ vaccination roll-outs.
One could argue that it is more difficult for the elderly to make and keep their appointments. This means that the broader the range of age cohorts eligible of being vaccinated the more strongly this could affect the DEA scores because, e.g., it requires more effort to vaccinate the elderly. In the first quarter the majority of vaccinations were given to the groups with highest (aged 80+) and second highest (aged 70–79) priority. Note that in the official regulation (“CoronaImpfV”) priority groups within the population are not only defined by age but also by other factors, such as prior diseases, social relevance or exposition to infected people. The official regulation can be found here:
https://bit.ly/2TbIpdR (accessed on 28 June 2021). It does not appear to be the case that demographics largely impact our results. Despite some overlaps, the federal states’ age profiles (see
https://bit.ly/349YNhb; accessed on 28 June 2021) do not seem to affect the results of our DEA. The following example illustrates this. If demographics had a significant impact on the relative success of the federal states’ vaccination roll-outs, federal states with a younger population such as Hamburg (HH) with an average age of 42.1 would have an advantage over those with an older population such as Thuringia (TH) with an average age of 47.4 years. Hamburg and Thuringia have about the same population while Hamburg is significantly smaller in terms of area. However, according to the results presented in
Table 3 and
Table 4 Hamburg is not unambiguously more efficient than Thuringia.
By the same token, our results do not seem to be largely driven by the federal states’ geography. For instance, Bremen (HB), the most efficient federal state, and Saarland (SL), among the federal states with the lowest efficiency scores, are both relatively small federal states. However, the scores of the federal states with the lowest population density, Mecklenburg Western Pomerania (MV) and Brandenburg (BB) might be driven by the countries’ geographies at least to some extent. Note that Mecklenburg Western Pomerania is assigned relatively high scores in model T (see
Table 1 and
Table 2 in the main text), which means that the federal state performs relatively well despite its dispersed population, anyway. In these countries one would expect that it is relatively difficult for people in rural areas to reach vaccination centers and that doctor’s offices are more efficient distributors of vaccination than large vaccination centers. Indeed, our analyses presented in
Section 3.4 provide evidence that the integration of doctor’s offices into those country’s vaccination campaigns might have substantially increased efficiency. A fruitful extension would be to cluster the federal states. This would allow the researcher to identify the most suitable candidates for benchmarking.
A DEA with a different input is presented to demonstrate the robustness of the results presented in the main text.
Table A1 and
Table A2 present the average DEA scores of CRS and VRS DEAs computed for the period 27 December 2020 to 16 May 2021 with only vaccine reserves in week
as the input variable (rather than vaccine reserves in
plus vaccine deliveries in week
t). The results remain largely unchanged.
Table A1.
Average efficiency scores for the period 27 December 2020 to 16 May 2021 for DEAs performed on a weekly basis under the CRS assumption using vaccine reserves at the end of the previous week as the input variable. Models T, 1S and 2S use total, first and second shots given as the respective output variable.
Table A1.
Average efficiency scores for the period 27 December 2020 to 16 May 2021 for DEAs performed on a weekly basis under the CRS assumption using vaccine reserves at the end of the previous week as the input variable. Models T, 1S and 2S use total, first and second shots given as the respective output variable.
| Federal State | T | 1S | 2S |
|---|
| BB | 0.4286 | 0.3988 | 0.3399 |
| BE | 0.5822 | 0.562 | 0.6169 |
| BW | 0.4186 | 0.4276 | 0.3587 |
| BY | 0.609 | 0.6221 | 0.5104 |
| HB | 0.7961 | 0.7846 | 0.7055 |
| HE | 0.4081 | 0.4175 | 0.3945 |
| HH | 0.509 | 0.5238 | 0.4301 |
| MV | 0.6064 | 0.5613 | 0.4875 |
| NI | 0.3868 | 0.4055 | 0.3063 |
| NW | 0.4773 | 0.5112 | 0.3465 |
| RP | 0.6069 | 0.5752 | 0.4555 |
| SH | 0.5925 | 0.5711 | 0.43 |
| SL | 0.3999 | 0.4243 | 0.3289 |
| SN | 0.3788 | 0.3543 | 0.4116 |
| ST | 0.3934 | 0.3979 | 0.353 |
| TH | 0.5383 | 0.5257 | 0.5536 |
Table A2.
Weekly average DEA efficiency scores for the period 27 December 2020 to 16 May 2021 under the VRS assumption using the vaccine stock at the end of the previous week as the input variable. Models T, 1S and 2S uses total, first and second shots given as the respective output variable.
Table A2.
Weekly average DEA efficiency scores for the period 27 December 2020 to 16 May 2021 under the VRS assumption using the vaccine stock at the end of the previous week as the input variable. Models T, 1S and 2S uses total, first and second shots given as the respective output variable.
| Federal State | T | 1S | 2S |
|---|
| BB | 0.5351 | 0.513 | 0.4278 |
| BE | 0.7466 | 0.7067 | 0.7372 |
| BW | 0.6372 | 0.6644 | 0.5538 |
| BY | 0.9306 | 0.902 | 0.7484 |
| HB | 0.9765 | 0.9765 | 0.9753 |
| HE | 0.5624 | 0.558 | 0.4916 |
| HH | 0.6115 | 0.6341 | 0.5095 |
| MV | 0.7152 | 0.7076 | 0.5812 |
| NI | 0.588 | 0.5977 | 0.4124 |
| NW | 1 | 0.947 | 0.8212 |
| RP | 0.7748 | 0.7257 | 0.6129 |
| SH | 0.7314 | 0.7187 | 0.5911 |
| SL | 0.4928 | 0.5245 | 0.4633 |
| SN | 0.4922 | 0.4505 | 0.5259 |
| ST | 0.4948 | 0.5052 | 0.4366 |
| TH | 0.643 | 0.6179 | 0.6849 |
Appendix B. Estimations
In the remainder of the Appendix, additional information to
Section 3.4 are presented. First, we present an alternative estimation to analyze the effect of the integration of doctor’s offices into Germany’s vaccination campaign. The following fixed effects regression is estimated:
In Equation (
A1), the share
is explained by a time trend, a federal state dummy
interacted with the time trend and the dummy variable
which takes the value 1 in the period starting on 5 April 2021 and 0 otherwise. Federal state-specific fixed effects are denoted
. With this specification, it is possible to disentangle how the share of vaccinations given in week
t in relation to deliveries in week
t and reserves in week
evolves over time in the different federal states. The fixed effects are chosen such that the effect of the interaction term
has to be interpreted relative to the federal state Bremen (HB), as will be explained in more detail below. The results are presented in
Table A3.
The results indicate that the share is significantly higher (1%-level) in the period after 5 April, which means that, on average, there is a structural break. The order of magnitude of the effect is the same as that presented in the main text. Note that this effect can be driven by doctor’s offices being integrated into Germany’s vaccination campaign, even though we cannot exclude that other factors also affected the results (see the discussion in the main text).
According to the results presented in
Table A3 the share
evolves over time on average. Every week, the share
increases by
plus the coefficient of the federal state dummy interacted with the time trend. This follows from
based on Equation (
A1). For instance, e.g., in Brandenburg (BB) the share of vaccinations given in week
t in relation to deliveries in week
t and reserves in week
decreases by
per week (see
Figure 3 in the main text).
Table A3.
Output table for Equation (
A1).
Table A3.
Output table for Equation (
A1).
| | Dep. Var. |
|---|
| 0.116 ** | (3.98) |
| Time trend | 0.0108 *** | (5.86) |
| BB × Time trend | −0.0157 *** | () |
| BE × Time trend | −0.0152 *** | () |
| BW × Time trend | −0.00465 *** | () |
| BY × Time trend | −0.0138 *** | () |
| HE × Time trend | −0.00836 *** | () |
| HH × Time trend | −0.0111 *** | () |
| MV × Time trend | −0.0209 *** | () |
| NI × Time trend | −0.00235 *** | () |
| NW × Time trend | −0.000310 *** | () |
| RP × Time trend | −0.0168 *** | () |
| SH × Time trend | −0.0167 *** | () |
| SL × Time trend | −0.0105 *** | () |
| SN × Time trend | −0.00858 *** | () |
| ST × Time trend | −0.00943 *** | () |
| TH × Time trend | −0.00311 *** | () |
| Constant | 0.333 *** | (26.80) |
| Observations | 320 |
| 0.424 |
| adjusted | 0.360 |
To completely interpret the results, consider the federal state Bremen. The constant can be interpreted as the starting value of the share for the federal state. Week in our sample is the last week of December 2020. Subsequently, i.e., in calender weeks of 2021, that share increases by per week. For weeks 14–19, i.e., the calender weeks starting on 5 April 2021, the level of increases further by . Thus, in the last week, the average effect assigned to Bremen would be .
Whereas we observe a clear time trend in the data for Bremen and, for instance, for Thuringia (TH) (see
Figure 3 in the main text), other states do not exhibit such an obvious trend. In Equation (
A1), we, therefore, still allow for a general time trend but do not impose such a trend for each federal state. In contrast, as a next step we approach the problem differently by allowing at the same time the analysis of whether the potential effect of the integration of doctor’s offices into Germany’s vaccination campaign differs between federal states.
In Equation (
A2),
is explained by a time trend
t, the variable
that takes the value 1 for the period after 5 April 2021, and 0 otherwise as well as a federal state specific fixed effect
. The federal state dummy
is also interacted with
to investigate whether a change in
potentially triggered by the integration of doctor’s offices into the vaccination campaign differs between federal states. This approach enables an investigation of whether the integration of doctor’s offices into the vaccination campaign potentially improved efficiency of the vaccination campaign on average and whether this effects differs between federal states at the same time. The interaction term
is defined such that the results of each federal state have to be interpreted relative to Bremen. The results of the regression are presented in
Table A4.
Table A4.
Output table for Equation (
A2).
Table A4.
Output table for Equation (
A2).
| | Dep. Var. |
|---|
| 0.247 *** | (8.87) |
| × BB | −0.206 *** | () |
| × BE | −0.185 *** | () |
| × BW | −0.0927 *** | () |
| × BY | −0.181 *** | () |
| × HE | −0.124 *** | () |
| × HH | −0.158 *** | () |
| × MV | −0.142 *** | () |
| × NI | −0.0713 *** | () |
| × NW | 0.0120 *** | () |
| × RP | −0.186 *** | () |
| × SH | −0.183 *** | () |
| × SL | −0.198 *** | () |
| × SN | −0.136 *** | () |
| × ST | −0.108 *** | () |
| × TH | −0.147 *** | () |
| Time trend | 0.000941 | (0.34) |
| Constant | 0.333 *** | (14.03) |
| Observations | 320 |
| 0.40 |
| adjusted | 0.332 |
One can see that the coefficient of the dummy
is significant at the 0.1%-level. The coefficient of
reported in
Table A4 implies that, all else equal, in the period after 5 April,
moreout of 100 available doses in a given week were vaccinated on average, or, in other words,
less doses were held back as reserves in Bremen. In interpreting the results, the potential effect of the integration of doctor’s offices into the vaccination campaign is a jump in
by 24.7% for Bremen. That jump is lower for all other federal states except for Northrhine-Westphalia, where the jump is
. The lowest increase occurs in Brandenburg (BB) where the apparent effect of the integration of doctor’s offices into the vaccination campaign is
.
A more detailed discussion on the differences between Regressions (
A1) and (
A2) is appropriate. The main difference is that (
A1) allows the time trend to vary between federal states whereas (
A2) allows the effect of the dummy
to be federal state-specific. In contrast to (
A1), in (
A2) all differences in the time dimension between federal states is absorbed by the interaction term
so that the time trend eventually becomes insignificant. Based on a graphical analysis of
Figure 3 in the main text one cannot clearly state which approach is more appropriate. For instance, Lower-Saxony (NI) and Hesse (HE) show similar patterns after 5 April, however, Lower-Saxony shows a clearer time trend. This indicates that Equation (
A1) appears to be a more suitable approach to identify differences in the time dimension between federal states. On the other hand, comparing Bremen and Lower-Saxony, one can see that Bremen has a more pronounced effect of
. One could argue that there is a more visible structural break in Bremen than in Lower-Saxony. In that case, Equation (
A2) seems more appropriate to identify differences in the time dimension. Based on these observations, it seems appropriate to discuss both variations. In any case, both estimations indicate structural breaks after 5 April, so that the results presented here confirm the findings outlined in the main text.
Finally, it can be analyzed which federal states show a time trend and for whom a structural break after 5 April can be diagnosed. In doing so, we estimate the following regression for each federal state:
In Equation (
A3), we test whether each federal state has a time trend, a structural break after April 5 and whether the time trend changes after April 5. The results are presented in
Table A5.
Table A5.
Time trend and structural break after 5 April 2021, and the interaction between the time trend and the structural break in for each federal state. The number of stars indicates the p-values of the respective t-statistics. In contrast to the previous analyses, *** indicates the , ** and * .
Table A5.
Time trend and structural break after 5 April 2021, and the interaction between the time trend and the structural break in for each federal state. The number of stars indicates the p-values of the respective t-statistics. In contrast to the previous analyses, *** indicates the , ** and * .
| Federal State | Time Trend (t) | Structural Break () | Interaction () |
|---|
| BB | none | positive ** | none |
| BE | none | positive *** | negative *** |
| BW | none | positive ** | none |
| BY | none | positive * | none |
| HB | none | positive *** | negative ** |
| HE | none | none | none |
| HH | none | positive * | none |
| MV | negative *** | none | positive ** |
| NI | none | none | none |
| NW | none | positive *** | none |
| RP | none | none | none |
| SH | none | none | none |
| SL | none | none | none |
| SN | none | positive *** | negative *** |
| ST | none | positive *** | negative ** |
| TH | positive ** | positive *** | negative *** |
Table A5 shows that 10 out of 16 federal states exhibit a structural break after 5 April 2021. That structural break is positive, i.e., an upward jump in
is observed. However, starting from that higher level, one can see that in 5 of these federal states (HB, BE, SN, ST, TH) the shares
start to decline again, which follows from the negative sign of the coefficient
of the interaction term
. This indicates that in these federal states there was a strong initial impetus on
at the beginning of 5 April, which began to level out afterwards. In the remaining 5 federal states (BB, BW, BY, HH, NW) the shares remain at a higher level. For Mecklenburg Western Pomerania (MV), we do not find a structural break, however, a negative time trend is found with an opposing positive trend after 5 April (positive coefficient of the interaction term). No effect of the integration of doctor’s offices into the vaccination campaign is found for 5 federal states (HE, NI, RP, SH, SL).
As a further robustness check, a Zivot-Andrews structural break test [
38] was performed. This can be seen as a conservative approach because the test identifies endogenously at most one structural break. Based on the data, a structural break was identified in calender weeks 13 and 14 (i.e., the weeks around 5 April) in 6 federal states (including, in particular, Bremen and Northrhine-Westphalia) despite the scarcity of observations. These findings are consistent with the interpretation outlined in the main text that the identified effect is driven by those federal states where the integration of general practitioners into the vaccination campaign had a particularly strong effect.