Regional Delivery of Anti-PD-1 Agent for Colorectal Liver Metastases Improves Therapeutic Index and Anti-Tumor Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Murine CRCLM Model
2.3. Bioluminescence Monitoring and Quantification
2.4. CPI Delivery
2.5. Cell Isolation
2.6. Flow Cytometry and Antibodies
2.7. Serum Studies
2.8. Western Blots
2.9. Statistics
3. Results
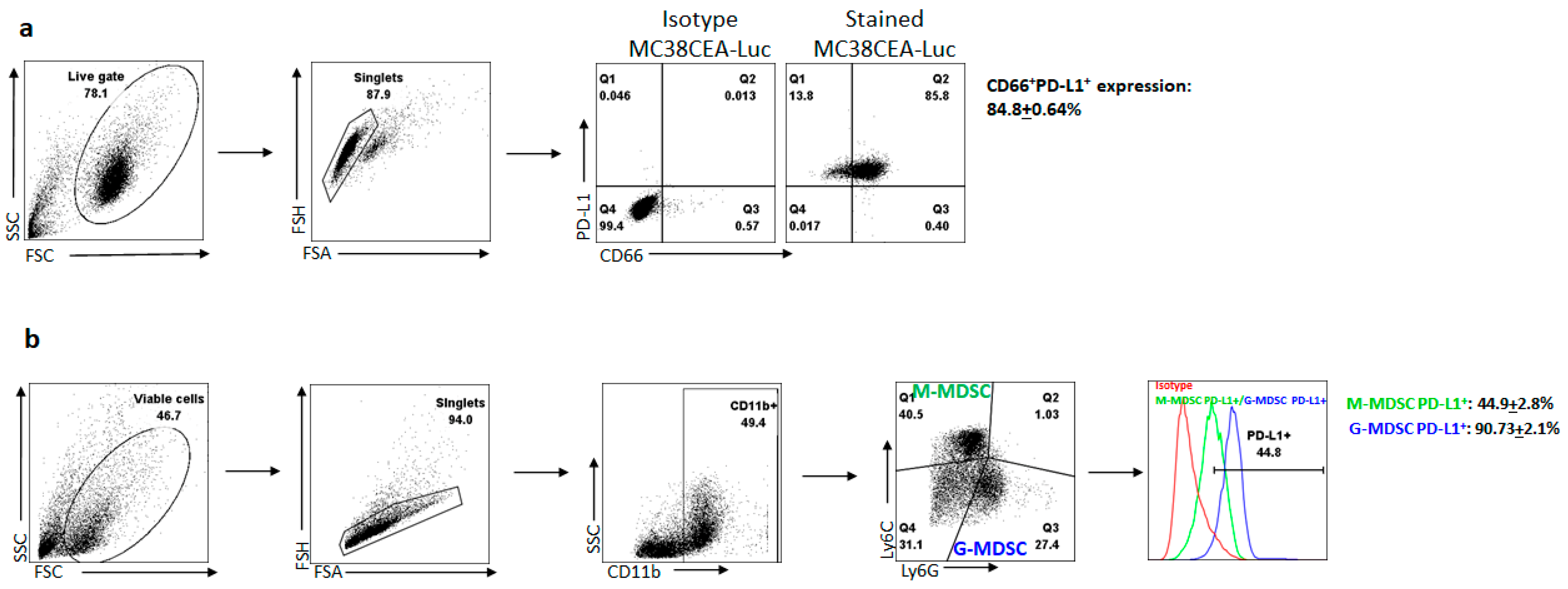
3.1. Liver Metastases Promote Immunosuppression in the Tumor Microenvironment via the PD-1/PD-L1 Axis
3.2. Anti-PD-1 Antibody Effective as In Vivo Checkpoint Inhibitor Therapy against Tumors
3.3. Reduction in Systemic Exposure with Low Dose Regional Delivery
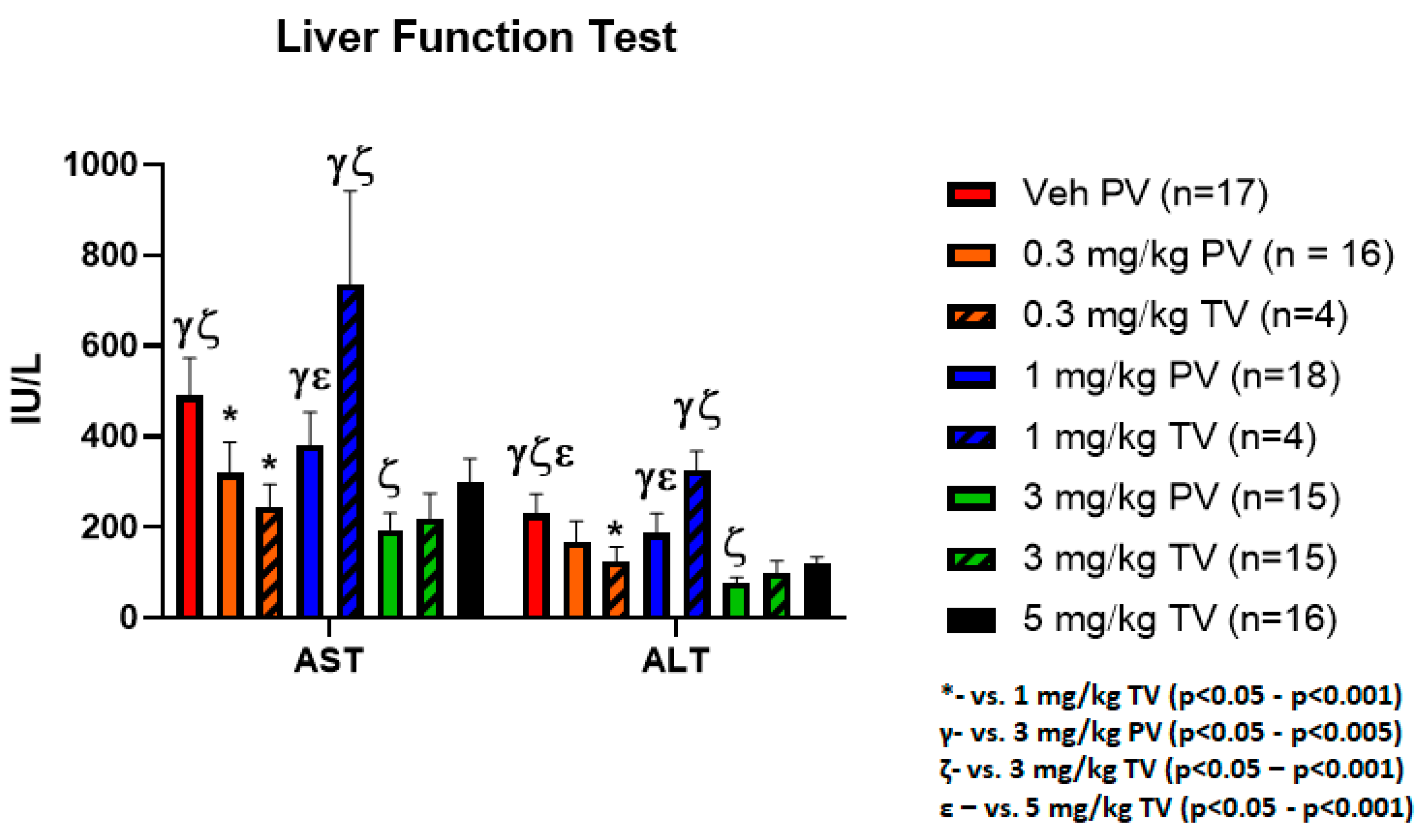
3.4. Equivalent Hepatic Toxicity Comparing Delivery Methods with Similar Doses
3.5. PD-1 Inhibition Promotes Apoptotic Signaling in Tumor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine transaminase |
| AST | Aspartate transaminase |
| CPI | Checkpoint inhibitor |
| CRCLM | Colorectal cancer liver metastases |
| ELISA | Enzyme-linked immunosorbent assay |
| FDA | Food and Drug Administration |
| G-MDSC | Granulocytic myeloid-derived suppressor cells |
| IACUC | Institutional Animal Care and Use Committee |
| IP | Intraperitoneal |
| irAE | Immune-related adverse events |
| LFT | Liver function tests |
| L-MDSC | Liver myeloid-derived suppressor cells |
| L-NPC | Liver non-parenchymal cells |
| MMR | Mismatch repair |
| M-MDSC | Monocytic myeloid-derived suppressor cells |
| PBS | Phosphate buffered saline |
| PTD | Post-treatment day |
| PV | Portal vein |
| RD | Regional delivery |
| RWMC | Roger Williams Medical Center |
| SD | Systemic delivery |
| SEM | Standard error of mean |
| TB | Tumor bioluminescence |
| TILs | Tumor infiltrating lymphocytes |
| TMB | Tumor mutational burden |
| TME | Tumor microenvironment |
| TV | Tail vein |
References
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef]
- Bang, Y.J.; Kang, Y.K.; Catenacci, D.V.; Muro, K.; Fuchs, C.S.; Geva, R.; Hara, H.; Golan, T.; Garrido, M.; Jalal, S.I.; et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: Results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer 2019, 22, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010, 28, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.F.; Prince, E.; Pillarisetty, V.G.; Katz, S.C. Challenges in assessing solid tumor responses to immunotherapy. Cancer Gene. Ther. 2019, 7, 528–538. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.; Choo, S.; Trojan, J.; Welling Rd, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Ferris, R.; Gillison, M.L. Nivolumab for Squamous-Cell Cancer of Head and Neck. N. Engl. J. Med. 2017, 376, 596. [Google Scholar] [PubMed]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients with Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Institute CR. Timeline of Progress: Treatment Approved; Institute CR: New York, NY, USA, 2020; Volume 2020. [Google Scholar]
- Ohyama, C.; Kojima, T.; Kondo, T.; Naya, Y.; Inoue, T.; Tomita, Y.; Eto, M.; Hisasue, S.; Uemura, H.; Obara, W.; et al. Nivolumab in patients with unresectable locally advanced or metastatic urothelial carcinoma: CheckMate 275 2-year global and Japanese patient population analyses. Int J. Clin. Oncol. 2019, 24, 1089–1098. [Google Scholar] [CrossRef]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.R.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef]
- Sharma, P.; Callahan, M.K.; Bono, P.; Kim, J.; Spiliopoulou, P.; Calvo, E.; Pillai, R.N.; Ott, P.A.; de Braud, F.; Morse, M.; et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): A multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016, 17, 1590–1598. [Google Scholar] [CrossRef]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef]
- Sharma, P.; Siefker-Radtke, A.; de Braud, F.; Basso, U.; Calvo, E.; Bono, P.; Morse, M.A.; Ascierto, P.A.; Lopez-Martin, J.; Brossart, P.; et al. Nivolumab Alone and With Ipilimumab in Previously Treated Metastatic Urothelial Carcinoma: CheckMate 032 Nivolumab 1 mg/kg Plus Ipilimumab 3 mg/kg Expansion Cohort Results. J. Clin. Oncol. 2019, 37, 1608–1616. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.; Arvis, C.D.; Ahn, M.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Schachter, J.; Ribas, A.; Long, G.V.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017, 390, 1853–1862. [Google Scholar] [CrossRef]
- Schadendorf, D.; Hodi, F.S.; Robert, C.; Weber, J.S.; Margolin, K.; Hamid, O.; Patt, D.; Chen, T.; Berman, D.M.; Wolchok, J.D. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. 2015, 33, 1889. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.R.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Trinh, S.; Le, A.; Gowani, S.; La-Beck, N.M. Management of Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitor Therapy: A Minireview of Current Clinical Guidelines. Asia Pac. J. Oncol. Nurs. 2019, 6, 154–160. [Google Scholar] [PubMed]
- Xing, P.; Zhang, F.; Wang, G.; Xu, Y.; Li, C.; Wang, S.; Guo, Y.; Cai, S.; Wang, Y.; Li, J. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: A systematic review and meta-analysis. J. Immunother Cancer 2019, 7, 341. [Google Scholar] [CrossRef] [PubMed]
- Burga, R.A.; Thorn, M.; Point, G.R.; Guha, P.; Nguyen, C.T.; Licata, L.A.; DeMatteo, R.P.; Ayala, A.; Espat, N.J.; Junghands, R.P.; et al. Liver myeloid-derived suppressor cells expand in response to liver metastases in mice and inhibit the anti-tumor efficacy of anti-CEA CAR-T. Cancer Immunol. Immunother. 2015, 64, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Guha, P.; Gardell, J.; Rabinowitz, B.; Lopes, M.; DaSilva, N.A.; Rowley, D.; Katz, S.C. Monocytic and granulocytic myeloid-derived suppressor cell plasticity and differentiation are organ-specific. Oncogene 2021, 40, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Guha, P.; Cunetta, M.; Somasundar, P.; Espat, N.J.; Junghans, R.P.; Katz, S.C. Frontline Science: Functionally impaired geriatric CAR-T cells rescued by increased alpha5beta1 integrin expression. J. Leukoc. Biol. 2017, 102, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Guha, P.; Gardell, J.; Darpolor, J.; Cunetta, M.; Lima, M.; Miller, G.; Espat, N.J.; Junghans, R.P.; Katz, S.C. STAT3 inhibition induces Bax-dependent apoptosis in liver tumor myeloid-derived suppressor cells. Oncogene 2018, 38, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Thorn, M.; Guha, P.; Cunetta, M.; Espat, N.J.; Miller, G.; Junghans, R.P. Tumor-associated GM-CSF overexpression induces immunoinhibitory molecules via STAT3 in myeloid-suppressor cells infiltrating liver metastases. Cancer Gene. Ther. 2016, 23, 188–198. [Google Scholar] [CrossRef]
- Huang, P.Y.; Guo, S.S.; Zhang, Y.; Lu, J.B.; Chen, Q.Y.; Tang, L.Q.; Zhang, L.; Liu, L.T.; Zhang, L.; Mai, H.Q. Tumor CTLA-4 overexpression predicts poor survival in patients with nasopharyngeal carcinoma. Oncotarget 2016, 7, 13060–13068. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, E.-E.; Kilvaer, T.K.; Rakaee, M.; Richardsen, E.; Hald, S.M.; Andersen, S.; Busund, L.; Bremnes, R.M.; Donnem, T. CTLA-4 expression in the non-small cell lung cancer patient tumor microenvironment: Diverging prognostic impact in primary tumors and lymph node metastases. Cancer Immunol. Immunother. CII 2017, 66, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.W.; Ngiow, S.F.; Ribas, A.; Smyth, M.J. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015, 75, 2139–2145. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, W.; Huang, Y.; Cui, R.; Li, X.; Li, B. Evolving Roles for Targeting CTLA-4 in Cancer Immunotherapy. Cell. Physiol. Biochem. 2018, 47, 721–734. [Google Scholar] [CrossRef]
- Collins, J.M. Pharmacologic rationale for regional drug delivery. J. Clin. Oncol. 1984, 2, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Bracco, S.; Leonini, S.; De Francesco, S.; Cioni, S.; Gennari, P.; Vallone, I.M. Intra-arterial chemotherapy with melphalan for intraocular retinoblastoma. Br. J. Ophthalmol. 2013, 97, 1219–1221. [Google Scholar] [CrossRef]
- Grootenboers, M.J.; Heeren, J.; van Putte, B.P.; Hendriks, J.M.; van Boven, W.J.; Van Schil, P.E.; Schramel, F.M. Isolated lung perfusion for pulmonary metastases, a review and work in progress. Perfusion 2006, 21, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.C.; Burga, R.A.; McCormack, E.; Wang, L.J.; Mooring, W.; Point, G.R.; Khara, P.D.; Thorn, M.; Ma, Q.; Stainken, B.F.; et al. Phase I Hepatic Immunotherapy for Metastases Study of Intra-Arterial Chimeric Antigen Receptor-Modified T-cell Therapy for CEA+ Liver Metastases. Clin. Cancer Res. 2015, 21, 3149–3159. [Google Scholar] [CrossRef]
- Katz, S.C.; Point, G.R.; Cunetta, M.; Thorn, M.; Guha, P.; Espat, N.J.; Boutros, C.; Hanna, N.; Junghans, R.P. Regional CAR-T cell infusions for peritoneal carcinomatosis are superior to systemic delivery. Cancer. Gene. Ther. 2016, 23, 142–148. [Google Scholar] [CrossRef]
- Katz, S.C.; Hardaway, J.; Prince, E.; Guha, P.; Cunetta, M.; Moody, A.; Wang, L.J.; Armenio, V.; Espat, N.J.; Junghans, R.P. HITM-SIR: Phase Ib trial of intraarterial chimeric antigen receptor T-cell therapy and selective internal radiation therapy for CEA(+) liver metastases. Cancer. Gene. Ther. 2019, 27, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Kroon, H.M.; Thompson, J.F. Isolated Limb Infusion and Isolated Limb Perfusion for Melanoma: Can the Outcomes of these Procedures be Compared? Ann. Surg. Oncol. 2019, 26, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Tang, Y.; Sun, J.; Yuan, Z.; Li, S.; Sheng, J.; Ren, H.; Hao, J. Regional intra-arterial vs. systemic chemotherapy for advanced pancreatic cancer: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2012, 7, e40847. [Google Scholar] [CrossRef] [PubMed]
- Schrump, D.S.; Zhai, S.; Nguyen, D.M.; Weiser, T.S.; Fisher, B.A.; Terrill, R.E.; Flynn, B.M.; Duray, P.H.; Figg, W.D. Pharmacokinetics of paclitaxel administered by hyperthermic retrograde isolated lung perfusion techniques. J. Thorac. Cardiovasc. Surg. 2002, 123, 686–694. [Google Scholar] [CrossRef][Green Version]
- Sridhar, P.; Petrocca, F. Regional Delivery of Chimeric Antigen Receptor (CAR) T-Cells for Cancer Therapy. Cancers 2017, 9, 92. [Google Scholar] [CrossRef]
- Sugarbaker, P.; Gianola, F.; Speyer, J.; Wesley, R.; Barofsky, I.; Meyers, C. Prospective, randomized trial of intravenous versus intraperitoneal 5-fluorouracil in patients with advanced primary colon or rectal cancer. Surgery 1985, 98, 414–422. [Google Scholar]
- Sugarbaker, P.H.; Landy, D.; Pascal, R. Intraperitoneal chemotherapy for peritoneal carcinomatosis from colonic or appendiceal cystadenocarcinoma: Rationale and results of treatment. Prog. Clin. Biol. Res. 1990, 354, 141–170. [Google Scholar]
- Uemura, K.; Nishihara, K.; Hayashi, T.; Tomiyasu, K.; Matsuoka, K. Improvement in urinary retention due to recurrent anastomotic prostate cancer treated with various therapies by intra-arterial infusion of cisplatin and ifosfamide. J. Infect. Chemother. 2012, 18, 753–755. [Google Scholar] [CrossRef][Green Version]
- Vicente, D.; Narayanan, J.S.; Ray, P.; Chai, L.F.; Erdem, S.; Carr, M.; Capacio, B.; Cox, B.; Jaroch, D.; Katz, S.C.; et al. Comparison of gemcitabine delivery and tumor response in a pressurized pancreatic retrograde venous infusion versus systemic infusion in an orthotopic murine model. J. Clin. Oncol. 2020, 38, 737. [Google Scholar] [CrossRef]
- Agrawal, S.; Feng, Y.; Roy, A.; Kollia, G.; Lestini, B. Nivolumab dose selection: Challenges, opportunities, and lessons learned for cancer immunotherapy. J. Immunother. Cancer 2016, 4, 72. [Google Scholar] [CrossRef]
- Bajaj, G.; Wang, X.; Agrawal, S.; Gupta, M.; Roy, A.; Feng, Y. Model-based population pharmacokinetic analysis of nivolumab in patients with solid tumors. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 58–66. [Google Scholar] [CrossRef]
- Chatterjee, M.S.; Elassaiss-Schaap, J.; Lindauer, A.; Turner, D.C.; Sostelly, A.; Freshwater, T.; Mayawala, K.; Ahamadi, M.; Stone, J.A.; de Greef, R.; et al. Population pharmacokinetic/pharmacodynamic modeling of tumor size dynamics in pembrolizumab-treated advanced melanoma. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 29–39. [Google Scholar] [CrossRef]
- Elassaiss-Schaap, J.; Rossenu, S.; Lindauer, A.; Kang, S.P.; De Greef, R.; Sachs, J.R.; de Alwis, D.P. Using model-based “learn and confirm” to reveal the pharmacokinetics-pharmacodynamics relationship of pembrolizumab in the KEYNOTE-001 Trial. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 21–28. [Google Scholar] [CrossRef]
- Goldstein, D.A.; Gordon, N.; Davidescu, M.; Leshno, M.; Steuer, C.E.; Patel, N.; Stemmer, S.M.; Zer, A. A Phamacoeconomic analysis of personalized dosing vs. fixed dosing of pembrolizumab in Firstline PD-L1-positive Non–Small cell lung cancer. JNCI J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- Li, H.; Yu, J.; Liu, C.; Liu, J.; Subramaniam, S.; Zhao, H.; Blumenthal, G.M.; Turner, D.C.; Li, C.; Ahamadi, M.; et al. Time dependent pharmacokinetics of pembrolizumab in patients with solid tumor and its correlation with best overall response. J. Pharmacokinet. Pharmacodyn. 2017, 44, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Hamid, O.; Daud, A.; Hodi, F.S.; Wolchok, J.D.; Kefford, R.; Joshua, A.M.; Patnaik, A.; Hwu, W.; Weber, J.S.; et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 2016, 315, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Ribas, A.; Wolchok, J.D.; Hodi, F.S.; Hamid, O.; Kefford, R.; Weber, J.S.; Joshua, A.M.; Hwu, W.; Gangadhar, T.C.; et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet 2014, 384, 1109–1117. [Google Scholar] [CrossRef]
- Centanni, M.; Moes, D.J.A.R.; Trocóniz, I.F.; Ciccolini, J.; van Hasselt, J.G.C. Clinical Pharmacokinetics and Pharmacodynamics of Immune Checkpoint Inhibitors. Clin. Pharmacokinet. 2019, 58, 835–857. [Google Scholar] [CrossRef] [PubMed]
- Fessas, P.; Lee, H.; Ikemizu, S.; Janowitz, T. A molecular and preclinical comparison of the PD-1-targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab. Semin. Oncol. 2017, 44, 136–140. [Google Scholar] [CrossRef]
- Fu, J.; Wang, F.; Dong, L.H.; Xing, M.J.; Cheng, X.; Wei, S.; Xu, J.; Han, M.; Dong, K.; Song, H. Receptor occupancy measurement of anti-PD-1 antibody drugs in support of clinical trials. Bioanalysis 2019, 11, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Schwickart, M.; Schneider, A.K.; Vainshtein, I.; Del Nagro, C.; Standifer, N.; Roskos, L.K. Receptor occupancy assessment by flow cytometry as a pharmacodynamic biomarker in biopharmaceutical development. Cytom. B Clin. Cytom. 2016, 90, 117–127. [Google Scholar] [CrossRef]
- Mo, H.; Huang, J.; Xu, J.; Chen, X.; Wu, D.; Qu, D.; Wang, X.; Lan, B.; Wang, X.; Xu, J.; et al. Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: A dose-escalation, phase 1 study. Br. J. Cancer 2018, 119, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Renner, A.; Burotto, M.; Rojas, C. Immune Checkpoint Inhibitor Dosing: Can We Go Lower Without Compromising Clinical Efficacy? J. Glob. Oncol. 2019, 5, 1–5. [Google Scholar] [CrossRef]
- Sheng, J.; Srivastava, S.; Sanghavi, K.; Lu, Z.; Schmidt, B.J.; Bello, A.; Gupta, M. Clinical Pharmacology Considerations for the Development of Immune Checkpoint Inhibitors. J. Clin. Pharmacol. 2017, 57 (Suppl. 10), S26–S42. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fei, K.; Jing, H.; Wu, Z.; Wu, W.; Zhou, S.; Ni, H.; Chen, B.; Xiong, Y.; Liu, Y.; et al. Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. In MAbs; Taylor & Francis: New York, NY, USA, 2016; Volume 11, pp. 1443–1451. [Google Scholar]
- Akbari, O.; Stock, P.; Singh, A.K.; Lombardi, V.; Lee, W.L.; Freeman, G.J.; Sharpe, A.H.; Umetsu, D.T.; Dekruyff, R.H. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal. Immunol. 2010, 3, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Butte, M.J.; Keir, M.E.; Phamduy, T.B.; Sharpe, A.H.; Freeman, G.J. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007, 27, 111–122. [Google Scholar] [CrossRef]
- Iwama, S.; De Remigis, A.; Callahan, M.K.; Slovin, S.F.; Wolchok, J.D.; Caturegli, P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci. Transl. Med. 2014, 6, 230ra245. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; Warshauer, J.T.; Bluestone, J.A. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat. Med. 2017, 23, 540–547. [Google Scholar] [CrossRef]
- Khan, Z.; Hammer, C.; Guardino, E.; Chandler, G.S.; Albert, M.L. Mechanisms of immune-related adverse events associated with immune checkpoint blockade: Using germline genetics to develop a personalized approach. Genome Med. 2019, 11, 39. [Google Scholar] [CrossRef]
- Theofilopoulos, A.N.; Kono, D.H.; Baccala, R. The multiple pathways to autoimmunity. Nat. Immunol. 2017, 18, 716–724. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Cuevas, C.; Chang, A.E.; Goel, V.K.; Von Hoff, D.D.; Hingorani, S.R. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012, 21, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Vo, T.T.T.; Wu, C.Z.; Chi, M.C.; Lin, C.M.; Fang, M.L.; Lee, I.T. Release Notice—Canadian Cancer Statistics: A 2020 special report on lung cancer. Health Promot. Chronic. Dis. Prev. Can. 2020, 40, 325. [Google Scholar]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Liu, M.; Su, J.; Zhang, P.; Tang, F.; Ye, P.; Devenport, M.; Wang, X.; Zhang, Y.; Liu, Y.; et al. Uncoupling therapeutic from immunotherapy-related adverse effects for safer and effective anti-CTLA-4 antibodies in CTLA4 humanized mice. Cell Res. 2018, 28, 433–447. [Google Scholar] [CrossRef]
- Gyorki, D.E.; Callahan, M.; Wolchok, J.D.; Ariyan, C.E. The delicate balance of melanoma immunotherapy. Clin. Transl. Immunol. 2013, 2, e5. [Google Scholar] [CrossRef]
- Liu, J.; Blake, S.J.; Smyth, M.J.; Teng, M.W. Improved mouse models to assess tumour immunity and irAEs after combination cancer immunotherapies. Clin. Transl. Immunol. 2014, 3, e22. [Google Scholar] [CrossRef] [PubMed]
- Olson, B.; Li, Y.; Lin, Y.; Liu, E.T.; Patnaik, A. Mouse Models for Cancer Immunotherapy Research. Cancer Discov. 2018, 8, 1358–1365. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, L.F.; Hardaway, J.C.; Heatherton, K.R.; O’Connell, K.P.; Lopes, M.C.; Rabinowitz, B.A.; Ghosh, C.C.; Guha, P.; Jaroch, D.; Cox, B.F.; et al. Regional Delivery of Anti-PD-1 Agent for Colorectal Liver Metastases Improves Therapeutic Index and Anti-Tumor Activity. Vaccines 2021, 9, 807. https://doi.org/10.3390/vaccines9080807
Chai LF, Hardaway JC, Heatherton KR, O’Connell KP, Lopes MC, Rabinowitz BA, Ghosh CC, Guha P, Jaroch D, Cox BF, et al. Regional Delivery of Anti-PD-1 Agent for Colorectal Liver Metastases Improves Therapeutic Index and Anti-Tumor Activity. Vaccines. 2021; 9(8):807. https://doi.org/10.3390/vaccines9080807
Chicago/Turabian StyleChai, Louis F., John C. Hardaway, Kara R. Heatherton, Kyle P. O’Connell, Mikayla C. Lopes, Benjamin A. Rabinowitz, Chandra C. Ghosh, Prajna Guha, David Jaroch, Bryan F. Cox, and et al. 2021. "Regional Delivery of Anti-PD-1 Agent for Colorectal Liver Metastases Improves Therapeutic Index and Anti-Tumor Activity" Vaccines 9, no. 8: 807. https://doi.org/10.3390/vaccines9080807
APA StyleChai, L. F., Hardaway, J. C., Heatherton, K. R., O’Connell, K. P., Lopes, M. C., Rabinowitz, B. A., Ghosh, C. C., Guha, P., Jaroch, D., Cox, B. F., & Katz, S. C. (2021). Regional Delivery of Anti-PD-1 Agent for Colorectal Liver Metastases Improves Therapeutic Index and Anti-Tumor Activity. Vaccines, 9(8), 807. https://doi.org/10.3390/vaccines9080807




