Critical Assessment of Purification and Analytical Technologies for Enveloped Viral Vector and Vaccine Processing and Their Current Limitations in Resolving Co-Expressed Extracellular Vesicles
Abstract
:1. Introduction
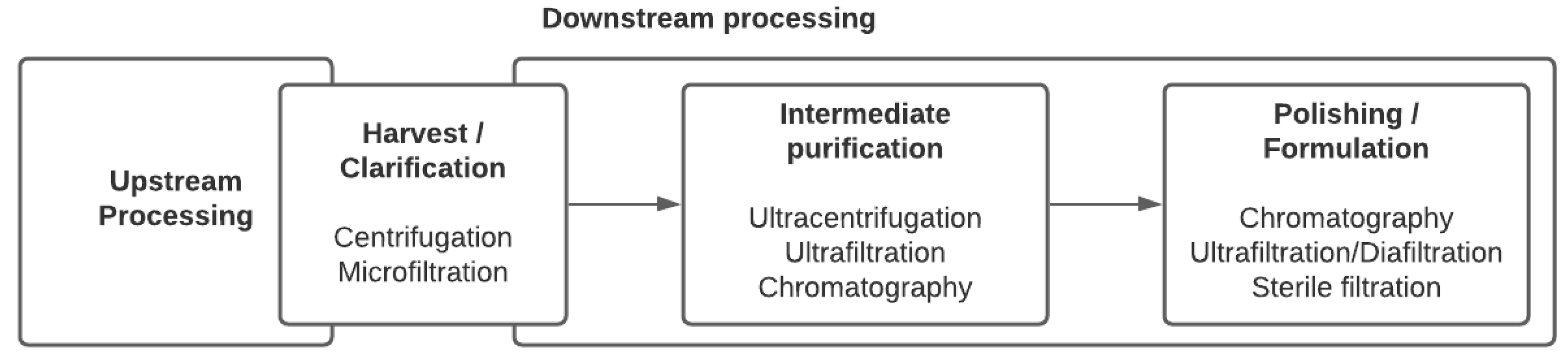
2. Viral Purification Processes
2.1. Harvest and Clarification
2.1.1. Centrifugation
2.1.2. Microfiltration
2.2. Concentration and Intermediate Purification
2.2.1. Traditional Ultracentrifugation
2.2.2. Ultrafiltration Tangential Flow Filtration
2.2.3. Chromatography
2.3. Polishing
3. Analytical Tools in Virus Production
3.1. Identity and Purity
3.2. Quantity and Potency
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- U.S. Food & Drug Administration. Vaccines Licensed for Use in the United States|FDA. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states (accessed on 7 June 2021).
- Wang, F.; Qin, Z.; Lu, H.; He, S.; Luo, J.; Jin, C.; Song, X. Clinical translation of gene medicine. J. Gene Med. 2019, 21, e3108. [Google Scholar] [CrossRef] [Green Version]
- GTCT. Gene Therapy Clinical Trials Worldwide. Available online: http://www.abedia.com/wiley (accessed on 19 May 2021).
- Moleirinho, M.G.; Silva, R.J.S.; Alves, P.M.; Carrondo, M.J.T.; Peixoto, C. Current challenges in biotherapeutic particles manufacturing. Expert Opin. Biol. Ther. 2020, 20, 451–465. [Google Scholar] [CrossRef] [Green Version]
- Suni, J.I.; Meurman, O.; Hirvonen, P.; Vaheri, A. Vaccination with a live RA 27/3 strain rubella vaccine (Rudivax), a follow-up study. Vaccine 1984, 2, 281–283. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Approved Cellular and Gene Therapy Products|FDA. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products (accessed on 7 June 2021).
- European Medicines Agency. Zynteglo. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zynteglo (accessed on 7 June 2021).
- European Medicines Agency. Strimvelis. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/strimvelis (accessed on 7 June 2021).
- European Medicines Agency. Zalmoxis. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zalmoxis (accessed on 7 June 2021).
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raposo, G.; Stahl, P.D. Extracellular vesicles: A new communication paradigm? Nat. Rev. Mol. Cell Biol. 2019, 20, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Phillips, W.; Willms, E.; Hill, A.F. Understanding extracellular vesicle and nanoparticle heterogeneity: Novel methods and considerations. Proteomics 2021, e2000118. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Andreu, Z.; Yanez-Mo, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Nabhan, J.F.; Hu, R.; Oh, R.S.; Cohen, S.N.; Lu, Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc. Natl. Acad. Sci. USA 2012, 109, 4146–4151. [Google Scholar] [CrossRef] [Green Version]
- McNamara, R.P.; Costantini, L.M.; Myers, T.A.; Schouest, B.; Maness, N.J.; Griffith, J.D.; Damania, B.A.; MacLean, A.G.; Dittmer, D.P. Nef Secretion into Extracellular Vesicles or Exosomes Is Conserved across Human and Simian Immunodeficiency Viruses. mBio 2018, 9, e02344-17. [Google Scholar] [CrossRef] [Green Version]
- Sampey, G.C.; Saifuddin, M.; Schwab, A.; Barclay, R.; Punya, S.; Chung, M.C.; Hakami, R.M.; Zadeh, M.A.; Lepene, B.; Klase, Z.A.; et al. Exosomes from HIV-1-infected Cells Stimulate Production of Pro-inflammatory Cytokines through Trans-activating Response (TAR) RNA. J. Biol. Chem. 2016, 291, 1251–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, S.J.; Booth, A.M.; Hildreth, J.E. The Trojan exosome hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 10592–10597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolte-‘t Hoen, E.; Cremer, T.; Gallo, R.C.; Margolis, L.B. Extracellular vesicles and viruses: Are they close relatives? Proc. Natl. Acad. Sci. USA 2016, 113, 9155–9161. [Google Scholar] [CrossRef] [Green Version]
- Sviben, D.; Forcic, D.; Halassy, B.; Allmaier, G.; Marchetti-Deschmann, M.; Brgles, M. Mass spectrometry-based investigation of measles and mumps virus proteome. Virol. J. 2018, 15, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do Minh, A.; Star, A.T.; Stupak, J.; Fulton, K.M.; Haqqani, A.S.; Gélinas, J.-F.; Li, J.; Twine, S.M.; Kamen, A.A. Characterization of Extracellular Vesicles Secreted in Lentiviral Producing HEK293SF Cell Cultures. Viruses 2021, 13, 797. [Google Scholar] [CrossRef]
- Segura, M.M.; Garnier, A.; Di Falco, M.R.; Whissell, G.; Meneses-Acosta, A.; Arcand, N.; Kamen, A. Identification of host proteins associated with retroviral vector particles by proteomic analysis of highly purified vector preparations. J. Virol. 2008, 82, 1107–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 2015, 219, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Konadu, K.A.; Huang, M.B.; Roth, W.; Armstrong, W.; Powell, M.; Villinger, F.; Bond, V. Isolation of Exosomes from the Plasma of HIV-1 Positive Individuals. J. Vis. Exp. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantin, R.; Diou, J.; Belanger, D.; Tremblay, A.M.; Gilbert, C. Discrimination between exosomes and HIV-1: Purification of both vesicles from cell-free supernatants. J. Immunol. Methods 2008, 338, 21–30. [Google Scholar] [CrossRef]
- DeMarino, C.; Barclay, R.A.; Pleet, M.L.; Pinto, D.O.; Branscome, H.; Paul, S.; Lepene, B.; El-Hage, N.; Kashanchi, F. Purification of High Yield Extracellular Vesicle Preparations Away from Virus. J. Vis. Exp. 2019. [Google Scholar] [CrossRef] [PubMed]
- Besnard, L.; Fabre, V.; Fettig, M.; Gousseinov, E.; Kawakami, Y.; Laroudie, N.; Scanlan, C.; Pattnaik, P. Clarification of vaccines: An overview of filter based technology trends and best practices. Biotechnol. Adv. 2016, 34, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.R.; Wieschalka, S.; Wagner, R. Single-Use Depth Filters: Application in Clarifying Industrial Cell Cultures—BioProcess International. BioProcess Int. 2017, 16, 6–11. [Google Scholar]
- Segura, M.M.; Kamen, A.; Trudel, P.; Garnier, A. A novel purification strategy for retrovirus gene therapy vectors using heparin affinity chromatography. Biotechnol. Bioeng. 2005, 90, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.M.; Hanssen, H.; Gerba, C.P. Simple method for the concentration of influenza virus from allantoic fluid on microporous filters. Appl. Environ. Microbiol. 1980, 39, 500. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food & Drug Administration. Package Insert—JYNNEOS; Bavarian Nordic A/S: Kvistgaard, Denmark, 2019. [Google Scholar]
- Moreira, A.S.; Cavaco, D.G.; Faria, T.Q.; Alves, P.M.; Carrondo, M.J.T.; Peixoto, C. Advances in Lentivirus Purification. Biotechnol. J. 2021, 16, e2000019. [Google Scholar] [CrossRef]
- Kishishita, N.; Takeda, N.; Anuegoonpipat, A.; Anantapreecha, S. Development of a pseudotyped-lentiviral-vector-based neutralization assay for chikungunya virus infection. J. Clin. Microbiol. 2013, 51, 1389–1395. [Google Scholar] [CrossRef] [Green Version]
- Olgun, H.B.; Tasyurek, H.M.; Sanlioglu, A.D.; Sanlioglu, S. High-Grade Purification of Third-Generation HIV-Based Lentiviral Vectors by Anion Exchange Chromatography for Experimental Gene and Stem Cell Therapy Applications. In Skin Stem Cells: Methods and Protocols; Turksen, K., Ed.; Springer: New York, NY, USA, 2019; pp. 347–365. [Google Scholar] [CrossRef]
- Rodrigues, T.; Carrondo, M.J.T.; Alves, P.M.; Cruz, P.E. Purification of retroviral vectors for clinical application: Biological implications and technological challenges. J. Biotechnol. 2007, 127, 520–541. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Package Insert and Patient Information—IXIARO; Valneva Austria GmbH: Vienna, Austria, 2018. [Google Scholar]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef]
- Mol, E.A.; Goumans, M.-J.; Doevendans, P.A.; Sluijter, J.P.G.; Vader, P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2061–2065. [Google Scholar] [CrossRef]
- Van Deun, J.; Mestdagh, P.; Sormunen, R.; Cocquyt, V.; Vermaelen, K.; Vandesompele, J.; Bracke, M.; De Wever, O.; Hendrix, A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- Perry, C.; Rayat, A. Lentiviral Vector Bioprocessing. Viruses 2021, 13, 268. [Google Scholar] [CrossRef]
- Wolff, M.W.; Reichl, U. Downstream Processing: From Egg to Cell Culture-Derived Influenza Virus Particles. Chem. Eng. Technol. 2008, 31, 846–857. [Google Scholar] [CrossRef]
- Rodrigues, T.; Carvalho, A.; Carmo, M.; Carrondo, M.J.T.; Alves, P.M.; Cruz, P.E. Scaleable purification process for gene therapy retroviral vectors. J. Gene Med. 2007, 9, 233–243. [Google Scholar] [CrossRef]
- Geraerts, M.; Michiels, M.; Baekelandt, V.; Debyser, Z.; Gijsbers, R. Upscaling of lentiviral vector production by tangential flow filtration. J. Gene Med. 2005, 7, 1299–1310. [Google Scholar] [CrossRef] [Green Version]
- Lobb, R.J.; Becker, M.; Wen Wen, S.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Orr, V.; Zhong, L.; Moo-Young, M.; Chou, C.P. Recent advances in bioprocessing application of membrane chromatography. Biotechnol. Adv. 2013, 31, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, K.; Scheibe, O.; Kocourek, A.; Muelich, J.; Jurkiewicz, E.; Pfeifer, A. Highly efficient concentration of lenti- and retroviral vector preparations by membrane adsorbers and ultrafiltration. BMC Biotechnol. 2011, 11, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, K.B.; Ren, J.; Beckner, M.A.; He, C.; Liu, S. Monolith columns for liquid chromatographic separations of intact proteins: A review of recent advances and applications. Anal. Chim. Acta 2019, 1046, 48–68. [Google Scholar] [CrossRef]
- Kramberger, P.; Urbas, L.; Štrancar, A. Downstream processing and chromatography based analytical methods for production of vaccines, gene therapy vectors, and bacteriophages. Hum. Vaccines Immunother. 2015, 11, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Forcic, D.; Brgles, M.; Ivancic-Jelecki, J.; Santak, M.; Halassy, B.; Barut, M.; Jug, R.; Markušić, M.; Strancar, A. Concentration and purification of rubella virus using monolithic chromatographic support. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 981–986. [Google Scholar] [CrossRef]
- Hansen, S.P.; Rene, F.; Udo, R.; Michael, W.; Gram, A.P. Purification of Vaccinia Viruses Using Hydrophobic Interaction Chromatography. U.S. Patent US20110306114A1, 23 August 2011. [Google Scholar]
- Njayou, M.; Quash, G. Purification of measles virus by affinity chromatography and by ultracentrifugation: A comparative study. J. Virol. Methods 1991, 32, 67–77. [Google Scholar] [CrossRef]
- Brgles, M.; Sviben, D.; Forčić, D.; Halassy, B. Nonspecific native elution of proteins and mumps virus in immunoaffinity chromatography. J. Chromatogr. A 2016, 1447, 107–114. [Google Scholar] [CrossRef]
- Opitz, L.; Salaklang, J.; Büttner, H.; Reichl, U.; Wolff, M.W. Lectin-affinity chromatography for downstream processing of MDCK cell culture derived human influenza A viruses. Vaccine 2007, 25, 939–947. [Google Scholar] [CrossRef]
- Olofsson, S.; Jeansson, S.; Lycke, E. Unusual lectin-binding properties of a herpes simplex virus type 1-specific glycoprotein. J. Virol. 1981, 38, 564–570. [Google Scholar] [CrossRef] [Green Version]
- Opitz, L.; Hohlweg, J.; Reichl, U.; Wolff, M.W. Purification of cell culture-derived influenza virus A/Puerto Rico/8/34 by membrane-based immobilized metal affinity chromatography. J. Virol. Methods 2009, 161, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wechuck, J.B.; Goins, W.F.; Krisky, D.M.; Wolfe, D.; Ataai, M.M.; Glorioso, J.C. Immobilized Cobalt Affinity Chromatography Provides a Novel, Efficient Method for Herpes Simplex Virus Type 1 Gene Vector Purification. J. Virol. 2004, 78, 8994–9006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, K.; Jin, S.; Ataai, M.M.; Schultz, J.S.; Ibeh, J. Tagging Retrovirus Vectors with a Metal Binding Peptide and One-Step Purification by Immobilized Metal Affinity Chromatography. J. Virol. 2004, 78, 9820–9827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheeks, M.C.; Kamal, N.; Sorrell, A.; Darling, D.; Farzaneh, F.; Slater, N.K.H. Immobilized metal affinity chromatography of histidine-tagged lentiviral vectors using monolithic adsorbents. J. Chromatogr. A 2009, 1216, 2705–2711. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Vandersluis, M.; Stout, J.; Haupts, U.; Sanders, M.; Jacquemart, R. Affinity chromatography for vaccines manufacturing: Finally ready for prime time? Vaccine 2019, 37, 5491–5503. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, M.; Sanches, R.M.; Walford, J.A.; Slater, N.K. Purification of a functional gene therapy vector derived from Moloney murine leukaemia virus using membrane filtration and ceramic hydroxyapatite chromatography. Biotechnol. Bioeng. 2002, 80, 445–453. [Google Scholar] [CrossRef]
- Heath, N.; Grant, L.; De Oliveira, T.M.; Rowlinson, R.; Osteikoetxea, X.; Dekker, N.; Overman, R. Rapid isolation and enrichment of extracellular vesicle preparations using anion exchange chromatography. Sci. Rep. 2018, 8, 5730. [Google Scholar] [CrossRef]
- Joshi, P.R.H.; Bernier, A.; Moço, P.D.; Schrag, J.; Chahal, P.S.; Kamen, A. Development of a scalable and robust AEX method for enriched rAAV preparations in genome-containing VCs of serotypes 5, 6, 8, and 9. Mol. Methods Clin. Dev. 2021, 21, 341–356. [Google Scholar] [CrossRef]
- Liangsupree, T.; Multia, E.; Riekkola, M.-L. Modern isolation and separation techniques for extracellular vesicles. J. Chromatogr. A 2021, 1636, 461773. [Google Scholar] [CrossRef]
- Boudeffa, D.; Bertin, B.; Biek, A.; Mormin, M.; Leseigneur, F.; Galy, A.; Merten, O.W. Toward a Scalable Purification Protocol of GaLV-TR-Pseudotyped Lentiviral Vectors. Hum. Gene Ther. Methods 2019, 30, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Blom, H.; Åkerblom, A.; Kon, T.; Shaker, S.; van der Pol, L.; Lundgren, M. Efficient chromatographic reduction of ovalbumin for egg-based influenza virus purification. Vaccine 2014, 32, 3721–3724. [Google Scholar] [CrossRef]
- Corso, G.; Mäger, I.; Lee, Y.; Görgens, A.; Bultema, J.; Giebel, B.; Wood, M.J.A.; Nordin, J.Z.; Andaloussi, S.E. Reproducible and scalable purification of extracellular vesicles using combined bind-elute and size exclusion chromatography. Sci. Rep. 2017, 7, 11561. [Google Scholar] [CrossRef] [PubMed]
- Kotrbová, A.; Štěpka, K.; Maška, M.; Pálenik, J.J.; Ilkovics, L.; Klemová, D.; Kravec, M.; Hubatka, F.; Dave, Z.; Hampl, A.; et al. TEM ExosomeAnalyzer: A computer-assisted software tool for quantitative evaluation of extracellular vesicles in transmission electron microscopy images. J. Extracell. Vesicles 2019, 8, 1560808. [Google Scholar] [CrossRef] [Green Version]
- Rikkert, L.G.; Nieuwland, R.; Terstappen, L.W.M.M.; Coumans, F.A.W. Quality of extracellular vesicle images by transmission electron microscopy is operator and protocol dependent. J. Extracell. Vesicles 2019, 8, 1555419. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zhao, X.; Zou, X.; Zhu, W.; Chen, Y.; Wang, D.; Shu, Y. Droplet Digital Polymerase Chain Reaction Method for Absolute Quantification of Influenza A Viruses. Bing Du Xue Bao 2017, 33, 1–5. [Google Scholar]
- Wang, Y.; Bergelson, S.; Feschenko, M. Determination of Lentiviral Infectious Titer by a Novel Droplet Digital PCR Method. Hum. Gene Ther. Methods 2018, 29, 96–103. [Google Scholar] [CrossRef]
- Gélinas, J.F.; Kiesslich, S.; Gilbert, R.; Kamen, A.A. Titration methods for rVSV-based vaccine manufacturing. MethodsX 2020, 7, 100806. [Google Scholar] [CrossRef]
- Yang, L.; Yamamoto, T. Quantification of Virus Particles Using Nanopore-Based Resistive-Pulse Sensing Techniques. Front. Microbiol. 2016, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Bousse, T.; Shore, D.A.; Goldsmith, C.S.; Hossain, M.J.; Jang, Y.; Davis, C.T.; Donis, R.O.; Stevens, J. Quantitation of influenza virus using field flow fractionation and multi-angle light scattering for quantifying influenza A particles. J. Virol. Methods 2013, 193, 589–596. [Google Scholar] [CrossRef] [Green Version]
- van der Pol, E.; Hoekstra, A.G.; Sturk, A.; Otto, C.; van Leeuwen, T.G.; Nieuwland, R. Optical and non-optical methods for detection and characterization of microparticles and exosomes. J Thromb Haemost 2010, 8, 2596–2607. [Google Scholar] [CrossRef] [PubMed]
- Lorbetskie, B.; Wang, J.; Gravel, C.; Allen, C.; Walsh, M.; Rinfret, A.; Li, X.; Girard, M. Optimization and qualification of a quantitative reversed-phase HPLC method for hemagglutinin in influenza preparations and its comparative evaluation with biochemical assays. Vaccine 2011, 29, 3377–3389. [Google Scholar] [CrossRef]
- Transfiguracion, J.; Coelho, H.; Kamen, A. High-performance liquid chromatographic total particles quantification of retroviral vectors pseudotyped with vesicular stomatitis virus-G glycoprotein. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 813, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Transfiguracion, J.; Tran, M.Y.; Lanthier, S.; Tremblay, S.; Coulombe, N.; Acchione, M.; Kamen, A.A. Rapid In-Process Monitoring of Lentiviral Vector Particles by High-Performance Liquid Chromatography. Mol. Ther. Methods Clin. Dev. 2020, 18, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.R.H.; Bernier, A.; Chahal, P.S.; Kamen, A. Development and Validation of an Anion-Exchange High-Performance Liquid Chromatography Method for Analysis of Empty Capsids and Capsids Encapsidating Genetic Material in a Purified Preparation of Recombinant Adeno-Associated Virus Serotype 5. Hum. Gene Ther. 2021. [Google Scholar] [CrossRef]
- Rossi, C.A.; Kearney, B.J.; Olschner, S.P.; Williams, P.L.; Robinson, C.G.; Heinrich, M.L.; Zovanyi, A.M.; Ingram, M.F.; Norwood, D.A.; Schoepp, R.J. Evaluation of ViroCyt® Virus Counter for rapid filovirus quantitation. Viruses 2015, 7, 857–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Americo, J.L.; Earl, P.L.; Moss, B. Droplet digital PCR for rapid enumeration of viral genomes and particles from cells and animals infected with orthopoxviruses. Virology 2017, 511, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Lippe, R. Flow Virometry: A Powerful Tool To Functionally Characterize Viruses. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Bilali, N.; Duron, J.; Gingras, D.; Lippé, R. Quantitative Evaluation of Protein Heterogeneity within Herpes Simplex Virus 1 Particles. J. Virol. 2017, 91, e00320-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, V.A.; Renner, T.M.; Varette, O.; Le Boeuf, F.; Wang, J.; Diallo, J.-S.; Bell, J.C.; Langlois, M.-A. Single-particle characterization of oncolytic vaccinia virus by flow virometry. Vaccine 2016, 34, 5082–5089. [Google Scholar] [CrossRef]
- Tang, V.A.; Renner, T.M.; Fritzsche, A.K.; Burger, D.; Langlois, M.A. Single-Particle Discrimination of Retroviruses from Extracellular Vesicles by Nanoscale Flow Cytometry. Sci. Rep. 2017, 7, 17769. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Duggan, E. Analysis of Individual Extracellular Vesicles by Flow Cytometry. In Flow Cytometry Protocols; Hawley, T.S., Hawley, R.G., Eds.; Springer: New York, NY, USA, 2018; pp. 79–92. [Google Scholar] [CrossRef]
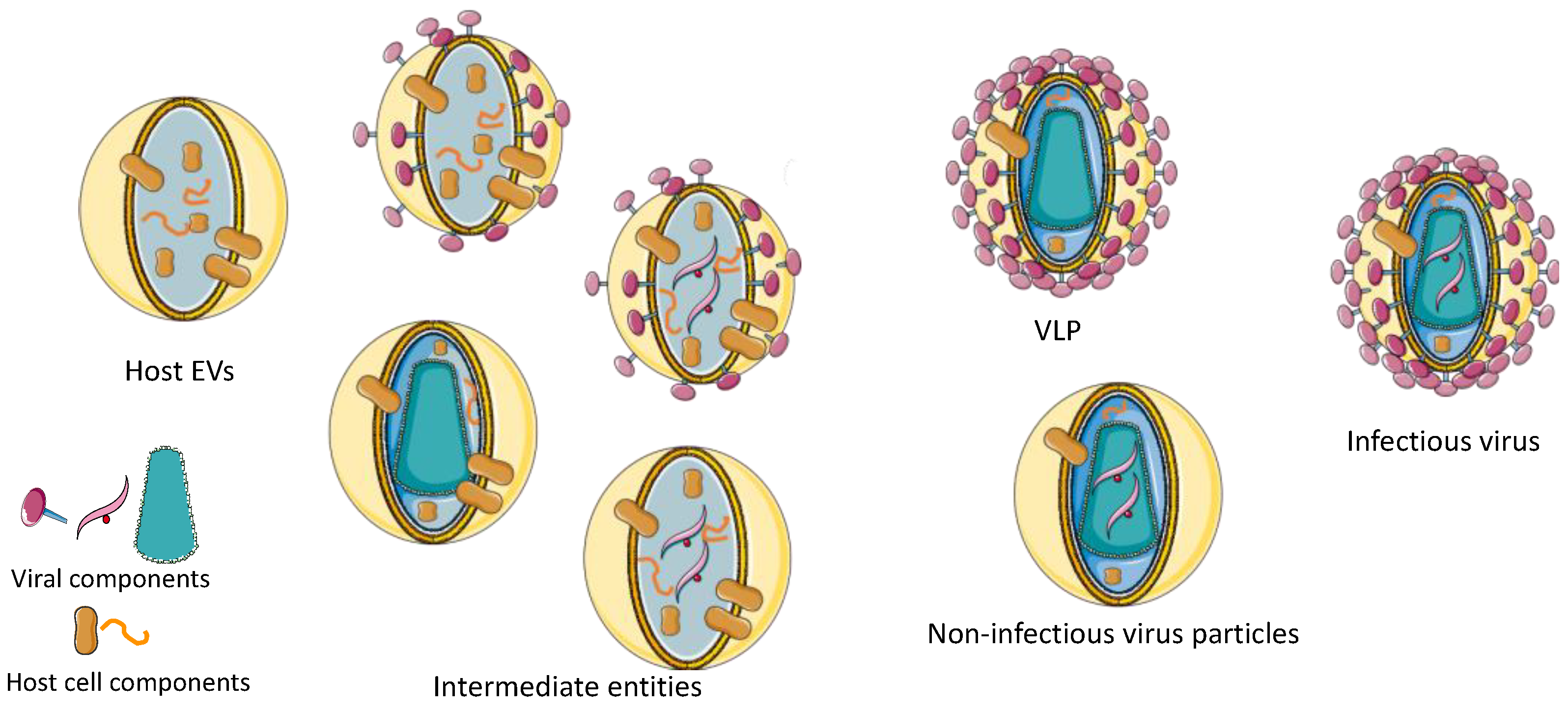
| Virus | Trade Name | Manufacturer | Production System | Target Disease/Indication | Reference | |
|---|---|---|---|---|---|---|
| Viral vaccine | VSV | ERVEBO | Merck Sharp & Dohme (MSD) | Vero cells | Ebola | [1] |
| Influenza virus | FluMist | Medimmune | Specific pathogen-free (SPF) eggs | Influenza | [1] | |
| JEV | Ixiaro | Valneva Austria GmbH | Vero cells | Japanese encephalitis | [1] | |
| Measles virus | M-M-R II ProQuad | MSD | Chick embryo cell culture | Measles | [1] | |
| Mumps virus | M-M-R II ProQuad | MSD | Chick embryo cell culture | Mumps | [1] | |
| Rubella virus | M-M-R II | MSD | WI-38 human diploid lung fibroblasts MRC-5 cells | Rubella | ||
| ProQuad | [1,5] | |||||
| Rudivax | Sanofi Pasteur MSD | |||||
| VZV | ProQuad | MSD | MRC-5 cells | Varicella | [1] | |
| ZOSTAVAX | ||||||
| VARIVAX | WI-38 human diploid lung fibroblasts | |||||
| Vaccinia virus | JYNNEOS | Bavarian Nordic A/S Emergent Product Development Gaithersburg, Inc. | Primary chicken embryo fibroblast cells Vero cells | Smallpox | [1] | |
| ACAM2000 | ||||||
| YFV | YF-Vax | Sanofi Pasteur, Inc | Avian leukosis virus-free chicken embryos | Yellow fever | [1] | |
| Gene therapy | Lentivirus | KYMRIAH | Novartis | HEK293-derived cells | Precursor B-cell lymphoblastic leukemia-lymphomaBeta-thalassemia | [6] |
| Zynteglo | Bluebird bio | HEK293-derived cells | [7] | |||
| Retrovirus | Strimvelis | HEK293-derived cells | Severe combined immunodeficiency | [8] | ||
| Zalmoxis | HEK293-derived cells | Adjunctive treatment in haploidentical HSC transplantation | [9] | |||
| YESCARTA | HEK293-derived cells | Lymphoma | [6] | |||
| HSV-1 | IMLYGIC | HEK293-derived cells | Melanoma | [6] |
| Particle | Size Range | Density | |
|---|---|---|---|
| EVs | Exosome | 30–150 nm | 1.13–1.21 g·ml−1 |
| Microvesicle | 100–1000 nm | 1.03–1.08 g·ml−1 | |
| Apoptotic body | 50–5000 nm | 1.16–1.28 g·ml−1 | |
| Enveloped viruses | VSV | 70–170 nm | 1.19–1.20 g·ml−1 |
| Influenza A virus | 80–120 nm | 1.2 g·ml−1 | |
| Lentivirus | 80–100 nm | 1.16–1.18 g·ml−1 | |
| γ-retrovirus | 80–120 nm | 1.15–1.17 g·ml−1 | |
| HSV-1 | 155–240 nm | 1.27 g·ml−1 |
| Critical Quality Attribute | Assay (Parameter) |
|---|---|
| Identity | PCR-based assay (genomic DNA) Western blot (viral protein) Sequencing (genomic DNA) Mass spectrometry (viral protein) Isoelectric focusing (isoelectric point) |
| Purity | Electron microscopy (viral structure) ELISA (residual HCPs) Mass spectrometry (residual HCPs) PCR-based assay (residual HC-DNA) |
| Safety | Bioburden (microbial contaminants) Sterility test (microbial contaminants) Endotoxin assay (endotoxin) Mycoplasma testing (mycoplasma) |
| Quantity | PCR-based assay (vector genome particles) Plaque assay (infectious particles) TCID50 (infectious particles) ELISA (total vector particles) NTA (total vector particles) TRPS (total vector particles) FFF-MALS (total vector particles) Flow virometry (total vector particles) |
| Potency | Cell-based assay (functional activity) In vivo assay (functional activity) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minh, A.D.; Kamen, A.A. Critical Assessment of Purification and Analytical Technologies for Enveloped Viral Vector and Vaccine Processing and Their Current Limitations in Resolving Co-Expressed Extracellular Vesicles. Vaccines 2021, 9, 823. https://doi.org/10.3390/vaccines9080823
Minh AD, Kamen AA. Critical Assessment of Purification and Analytical Technologies for Enveloped Viral Vector and Vaccine Processing and Their Current Limitations in Resolving Co-Expressed Extracellular Vesicles. Vaccines. 2021; 9(8):823. https://doi.org/10.3390/vaccines9080823
Chicago/Turabian StyleMinh, Aline Do, and Amine A. Kamen. 2021. "Critical Assessment of Purification and Analytical Technologies for Enveloped Viral Vector and Vaccine Processing and Their Current Limitations in Resolving Co-Expressed Extracellular Vesicles" Vaccines 9, no. 8: 823. https://doi.org/10.3390/vaccines9080823
APA StyleMinh, A. D., & Kamen, A. A. (2021). Critical Assessment of Purification and Analytical Technologies for Enveloped Viral Vector and Vaccine Processing and Their Current Limitations in Resolving Co-Expressed Extracellular Vesicles. Vaccines, 9(8), 823. https://doi.org/10.3390/vaccines9080823







