Age- and Sex-Graded Data Evaluation of Vaccination Reactions after Initial Injection of the BNT162b2 mRNA Vaccine in a Local Vaccination Center in Germany
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Ethics
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johns Hopkins University, Center for Systems Science and Engineering (CSSE). COVID-19 Dashboard. 28 July 2021. Available online: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 (accessed on 28 July 2021).
- Robert Koch-Institut. COVID-19-Dashboard. 28 July 2021. Available online: https://experience.arcgis.com/experience/478220a4c454480e823b17327b2bf1d4/page/page_0/ (accessed on 28 July 2021).
- Robert Koch-Institut; Bundesministerium für Gesundheit. Impfdashboard. 28 July 2021. Available online: https://impfdashboard.de/ (accessed on 28 July 2021).
- Robert Koch-Institut. Aufklärungsmerkblatt, Schutzimpfung Gegen COVID-19 (Corona Virus Disease 2019)—Mit mRNA-Impfstoffen. 2021. Available online: www.rki.de/DE/Content/Infekt/Impfen/Materialien/Downloads-COVID-19/Aufklaerungsbogen-de.pdf?__blob=publicationFile (accessed on 21 June 2021).
- Bundesministerium für Gesundheit. Verordnung zum Anspruch auf Schutzimpfung Gegen das Coronavirus SARS-CoV-2 (Coronavirus-Impfverordnung—CoronaImpfV). 31 März 2021. Available online: www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/C/Coronavirus/Verordnungen/CoronaImpfV_BAnz_AT_01.04.2021_V1.pdf (accessed on 19 May 2021).
- Ministerium für Soziales, Arbeit, Gesundheit und Demografie des Landes Rheinland-Pfalz. Impfdokumentation Rheinland-Pfalz. 2021. Available online: https://impfdokumentation-rlp.de/ (accessed on 28 July 2021).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Deutsches Register Klinischer Studien. Registrierung. 2021. Available online: www.drks.de/drks_web/navigate.do?navigationId=start&messageDE=Home&messageEN=Home (accessed on 19 May 2021).
- Paul-Ehrlich-Institut; Bundesinstitut für Impfstoffe und Biomedizinische Arzneimittel. Sicherheitsbericht, Verdachtsfälle von Nebenwirkungen und Impfkomplikationen nach Impfung zum Schutz vor COVID-19 seit Beginn der Impfkampagne am 27.12.2020 bis zum 30.06.2021. 15 July 2021. Available online: https://www.pei.de/SharedDocs/Downloads/DE/newsroom/dossiers/sicherheitsberichte/sicherheitsbericht-27-12-bis-30-06-21.pdf?__blob=publicationFile&v=3. (accessed on 1 August 2021).
- Effros, R.B. Roy Walford and the immunologic theory of aging. Immun. Ageing 2005, 2, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagelkerken, L.; Hertogh-Huijbregts, A.; Dobber, R.; Dräger, A. Age-related changes in lymphokine production related to a decreased number of CD45RBhi CD4+ T cells. Eur. J. Immunol. 1991, 21, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Thalhammer, F.; Wenisch, C.; Burgmann, H.; Euler, C.; Fasching, P.; Grubeck-Loebenstein, B.; Mutz, I.; Popp, W.; Weiss, G.; Wiedermann-Schmidt, U. Impfen im Alter, Expertenstatement unter Patronanz der Österreichischen Gesellschaft für Infektionskrankheiten. ÖAZ 2008, 11 (Suppl. 11), 1–8. [Google Scholar]
- Hainz, U.; Jenewein, B.; Asch, E.; Pfeiffer, K.-P.; Berger, P.; Gruebeck-Loebenstein, B. Insufficient protection for healthy elderly adults by tetanus and TBE vaccines. Vaccine 2005, 23, 3232–3235. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, E.; Simpson, C.R.; Shi, T.; Kerr, S.; Agrawal, U.; Akbari, A.; Bedston, S.; Beggs, J.; Bradley, D.; Chuter, A.; et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: A national prospective cohort study. Lancet 2021, 397, 1646–1657. [Google Scholar] [CrossRef]
- Weinberger, B. Vaccines for the elderly: Current use and future challenges. Immun. Ageing 2018, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Gerbershagen, H.J.; Pogatzki-Zahn, E.; Aduckathil, S.; Peelen, L.M.; Kappen, T.H.; van Wijck, A.J.; Kalkman, C.J.; Meissner, W. Procedure-specific risk factor analysis for the development of severe postoperative pain. Anesthesiology 2014, 120, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, A.; Yahiaoui-Doktor, M.; Meissner, W.; Zahn, P.K.; Pogatzki-Zahn, E.M. Predicting poor postoperative acute pain outcome in adults: An international, multicentre database analysis of risk factors in 50,005 patients. Pain Rep. 2020, 5, e831. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M. Kognitive Defizite: Wie man Schmerzen auch bei Demenz erkennen kann. Dtsch. Arztebl. 2014, 111, 4. Available online: www.aerzteblatt.de/archiv/162727/Kognitive-Defizite-Wie-man-Schmerzen-auch-bei-Demenz-erkennen-kann (accessed on 28 July 2021).
- Warden, V.; Hurley, A.C.; Volicer, L. Development and psychometric evaluation of the pain assessment in advanced dementia (PAINAD) scale. J. Am. Med. Dir. Assoc. 2003, 4, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Statista. Umfrage zur Corona-Impfbereitschaft in Deutschland 2021. 6 May 2021. Available online: https://de.statista.com/statistik/daten/studie/1147628/umfrage/umfrage-zur-corona-impfbereitschaft-in-deutschland/#statisticContainer (accessed on 20 May 2021).
- Universität Erfurt; Robert Koch-Institut; Bundeszentrale für Gesundheitliche Aufklärung; Leibniz-Institut für Psychologie; Science Media Center; Bernhard Nocht Institut für Tropenmedizin; Yale Institute for Global Health. COVID-19 Snapshot Monitoring (Cosmo). 5 May 2021. Available online: https://projekte.uni-erfurt.de/cosmo2020/web/topic/impfung/10-impfungen/ (accessed on 20 May 2021).
- Lin, C.; Tu, P.; Beitsch, L.M. Confidence and Receptivity for COVID-19 Vaccines: A Rapid Systematic Review. Vaccines 2020, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Lenzen-Schulte, M. Atypische Gerinnungsstörungen nach COVID-19-Impfung: Vorgehen bei Hirnvenenthrombose. Dtsch. Arztebl. 2021, 118, A-780/B-653. Available online: https://www.aerzteblatt.de/archiv/218654/Atypische-Gerinnungsstoerungen-nach-COVID-19-Impfung-Vorgehen-bei-Hirnvenenthrombose (accessed on 28 July 2021).
- Abu Mouch, S.; Roguin, A.; Hellou, E.; Ishai, A.; Shoshan, U.; Mahamid, L.; Zoabi, M.; Aisman, M.; Goldschmid, N.; Yanay, N.B. Myocarditis following COVID-19 mRNA vaccination. Vaccine 2021, 39, 3790–3793. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K.; Barh, D.; Uhal, B.; Takayama, K.; Aljabali, A.; El-Aziz, T.A.; Lal, A.; Redwan, E.; Adadi, P.; Chauhan, G.; et al. COVID-19 Vaccines and Thrombosis—Roadblock or Dead-End Street? Biomolecules 2021, 11, 1020. [Google Scholar] [CrossRef] [PubMed]
- Bundeszentrale für Politische Bildung. GENESIS-Online: Bevölkerung: Altersjahre, Geschlecht (04/2020). 31 December 2018. Available online: www.bpb.de/nachschlagen/zahlen-und-fakten/soziale-situation-in-deutschland/61538/altersgruppen (accessed on 20 May 2021).
- Nittner-Marszalska, M.; Rosiek-Biegus, M.; Kopeć, A.; Pawłowicz, R.; Kosińska, M.; Łata, A.; Szenborn, L. Pfizer-BioNTech COVID-19 Vaccine Tolerance in Allergic versus Non-Allergic Individuals. Vaccines 2021, 9, 553. [Google Scholar] [CrossRef] [PubMed]
- Gee, J.; Marquez, P.; Su, J.; Calvert, G.M.; Liu, R.; Myers, T.; Nair, N.; Martin, S.; Clark, T.; Markowitz, L.; et al. First month of COVID-19 vaccine safety monitoring—United States, 14 December 2020–13 January 2021. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Potluri, T.; Fink, A.L.; Sylvia, K.E.; Dhakal, S.; Vermillion, M.S.; Steeg, L.V.; Deshpande, S.; Narasimhan, H.; Klein, S.L. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPJ Vaccines 2019, 4, 29, Erratum in 2019, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Yale IMPACT Research Team; Ellingson, M.K.; Wong, P.; Israelow, B.; Lucas, C.; Klein, J.; Silva, J.; Mao, T.; Oh, J.E.; et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020, 588, 315–320. [Google Scholar] [CrossRef] [PubMed]

| Characteristics (n) | Total (n) | Females (n) | Males (n) | p/r |
|---|---|---|---|---|
| Number of vaccinated individuals | 1065 | 632 | 433 | |
| Age (y) (1065) | ||||
| Median | 82.4 | 82.5 | 82.3 | p = 0.345 * |
| Range | 21.0–99.3 | 21.3–99.3 | 21.0–98.1 | |
| Mean ± SD | 75.9 ± 17.4 | 75.7 ± 17.7 | 76.3 ± 16.9 | |
| Vaccination | 1065 | |||
| Vaccination reactions | 345 | 228 | 117 | p = 0.002 r = 0.095 |
| Local vaccination reactions | 274 | 178 | 96 | p = 0.032 * r = 0.067 |
| Mild | 221 | 142 | 79 | p = 0.450 * r = 0.052 n.s. |
| Moderate | 49 | 32 | 17 | |
| Severe | 4 | 4 | 0 | |
| Systemic vaccination reactions | 137 | 95 | 42 | p = 0.012 * r = 0.078 |
| Mild | 99 | 66 | 33 | p = 0.624 * r = 0.090 n.s. |
| Moderate | 29 | 22 | 7 | |
| Severe | 9 | 7 | 2 | |
| Allergic reactions | 0 | 0 | 0 | |
| Vaccination complications | 0 | 0 | 0 |
| COVID-19 Vaccination | ≤80 Years | >80 Years | p/r |
|---|---|---|---|
| Number of vaccinated individuals (n/1065) | 245 | 820 | |
| Vaccination reactions | 154 | 191 | p < 0.001 r = 0.356 |
| Local vaccination reactions | 139 | 135 | p < 0.001 r = 0.388 |
| Mild | 105 | 116 | p = 0.077 * r = 0.134 |
| Moderate | 31 | 18 | |
| Severe | 3 | 1 | |
| Systemic vaccination reactions | 54 | 83 | p < 0.001 r = 0.150 |
| Mild | 37 | 62 | p = 0.717 * r = 0.062 n.s. |
| Moderate | 13 | 16 | |
| Severe | 4 | 5 | |
| Allergic reactions | 0 | 0 | |
| Vaccination complications | 0 | 0 |
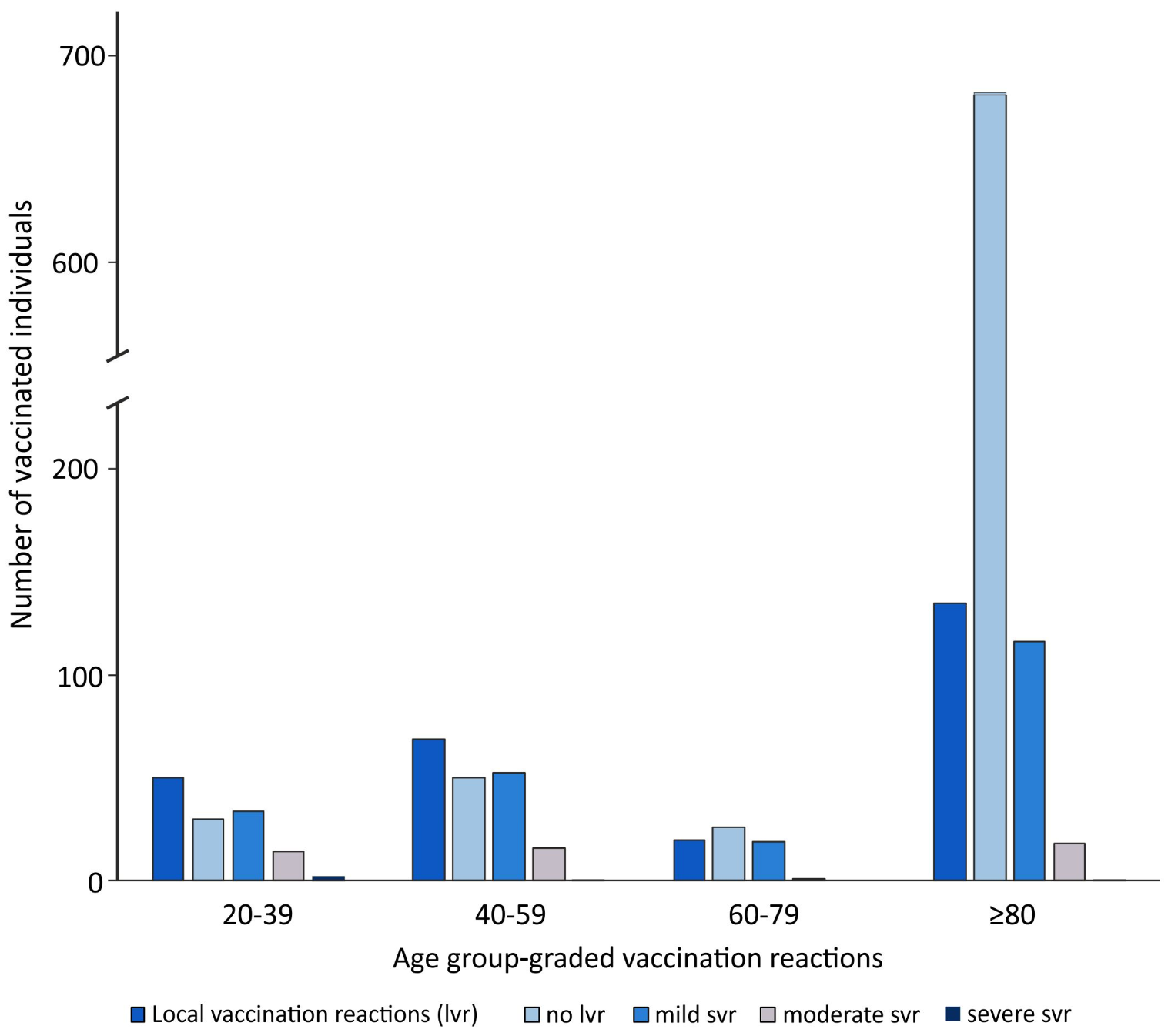
| COVID-19 Vaccination | 20–39 Years | 40–59 Years | 60–80 Years | >80 Years | p/r |
|---|---|---|---|---|---|
| Number of the vaccinated individuals (n/1065) | 80 | 119 | 46 | 820 | |
| Vaccination reactions | 56 | 76 | 22 | 191 | p < 0.001 r = 0.360 |
| Local vaccination reactions | 50 | 69 | 20 | 135 | p < 0.001 r = 0.387 |
| Mild | 34 | 52 | 19 | 116 | p = 0.033 * r = 0.185 |
| Moderate | 14 | 16 | 1 | 18 | |
| Severe | 2 | 1 | 0 | 1 | |
| Systemic vaccination reactions | 22 | 23 | 9 | 83 | p < 0.001 r = 0.156 |
| Mild | 17 | 12 | 8 | 62 | p = 0.179 * r = 0.046 n.s. |
| Moderate | 4 | 9 | 0 | 16 | |
| Severe | 1 | 2 | 1 | 5 | |
| Allergic reactions | 0 | 0 | 0 | 0 | |
| Vaccination complications | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, M.A.; Wieler, H.J.; Enders, P.; Buchholz, H.-G.; Plachter, B. Age- and Sex-Graded Data Evaluation of Vaccination Reactions after Initial Injection of the BNT162b2 mRNA Vaccine in a Local Vaccination Center in Germany. Vaccines 2021, 9, 911. https://doi.org/10.3390/vaccines9080911
Hoffmann MA, Wieler HJ, Enders P, Buchholz H-G, Plachter B. Age- and Sex-Graded Data Evaluation of Vaccination Reactions after Initial Injection of the BNT162b2 mRNA Vaccine in a Local Vaccination Center in Germany. Vaccines. 2021; 9(8):911. https://doi.org/10.3390/vaccines9080911
Chicago/Turabian StyleHoffmann, Manuela A., Helmut J. Wieler, Peter Enders, Hans-Georg Buchholz, and Bodo Plachter. 2021. "Age- and Sex-Graded Data Evaluation of Vaccination Reactions after Initial Injection of the BNT162b2 mRNA Vaccine in a Local Vaccination Center in Germany" Vaccines 9, no. 8: 911. https://doi.org/10.3390/vaccines9080911
APA StyleHoffmann, M. A., Wieler, H. J., Enders, P., Buchholz, H.-G., & Plachter, B. (2021). Age- and Sex-Graded Data Evaluation of Vaccination Reactions after Initial Injection of the BNT162b2 mRNA Vaccine in a Local Vaccination Center in Germany. Vaccines, 9(8), 911. https://doi.org/10.3390/vaccines9080911







