Characterization of Membrane-Associated Progesterone Receptor Component-2 (MAPRC2) from Trichinella spiralis and Its Interaction with Progesterone and Mifepristone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Parasites Preservation
2.2. Bioinformatics Analysis and Molecular Modeling
2.3. Cloning of Ts-MAPRC2
2.4. Development of Recombinant Ts-MAPRC2 (rTs-MAPRC2)
2.5. Generation of the Rat-Polyclonal Antibody of rTs-MAPRC2
2.6. Immuno-Blot Assay of rTs-MAPRC2
2.7. Identification of Native Ts-MAPRC2 Protein by Western Blot Assay
2.8. Determine the Binding Ability of rTs-MAPRC2 Protein with Progesterone
2.9. Immunofluorescent Assay of rTs-MAPRC2 at Different Developmental Stages
2.10. Relative mRNA Expression of Ts-MAPRC2 Gene at Different Stages Incubated with Progesterone (P4) and Mifepristone (RU486)
2.11. In Vitro Phenotypic Effect of P4, RU486, and rTs-MAPRC2-Ab on F-AL and ML Stages
2.12. The Phenotypic Effect and Relative mRNA Expression of Mifepristone on F-AL Stage by In Vivo
2.13. Statistical Analysis
3. Results
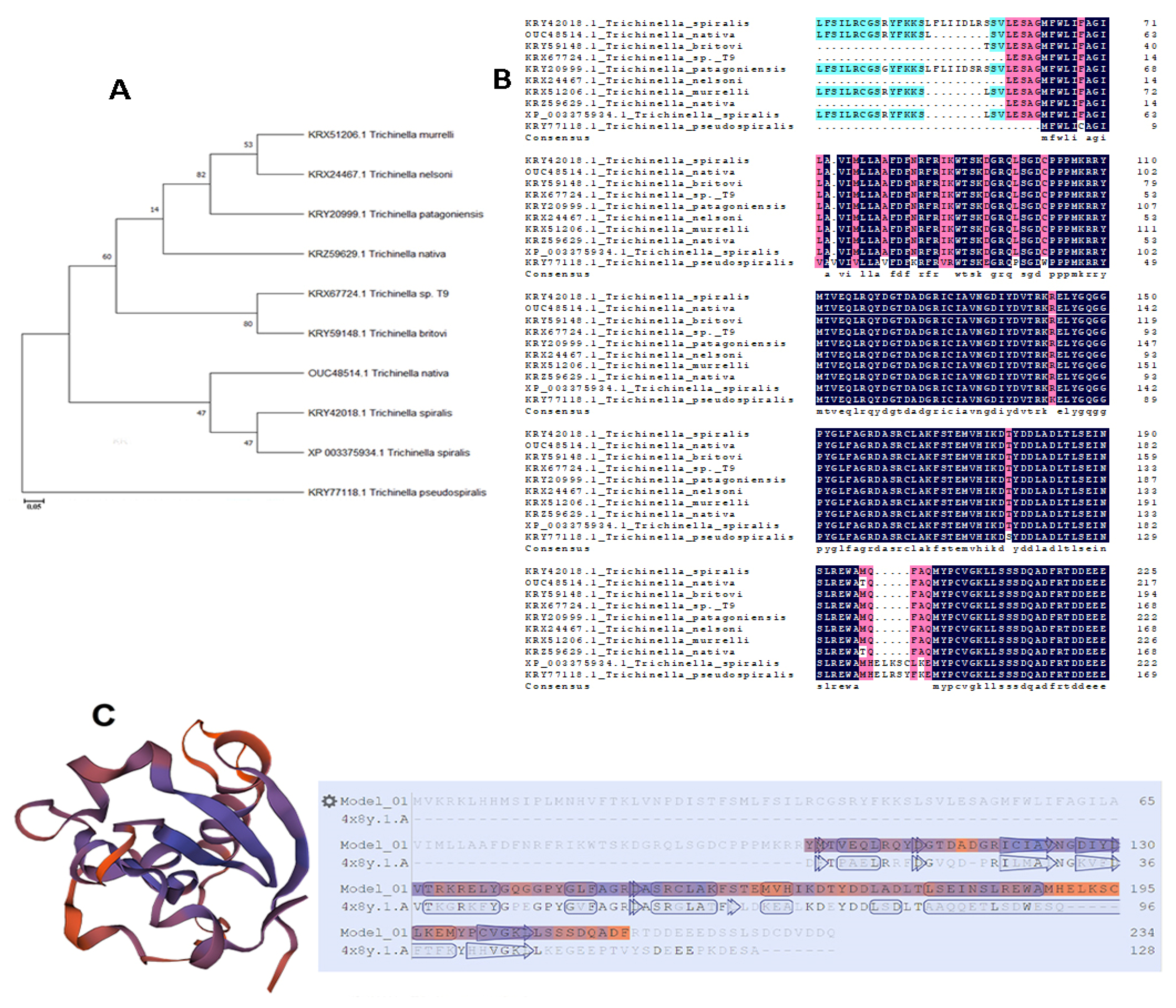
3.1. Sequence Analysis of Ts-MAPRC2
3.2. Cloning, Expression, Purification, Immunoblot of rTs-MAPRC2 and Native Ts-MAPRC2 Protein
3.3. Binding Ability of rTs-MAPRC2 Protein by Sandwich ELISA
3.4. Immunofluorescent Assay (IFA) of Ts-MAPRC2 Gene at Various Developmental Stages
3.5. Relative mRNA Expression of Ts-MAPRC2 at all Stages by In Vitro Treatment of P4 and RU486
3.5.1. Comparison Between the Same Stage and Same Concentration of P4
3.5.2. Comparison Among the Same Stage and Same Concentration of Mifepristone (RU486)
3.5.3. Comparison Among the P4 and RU486 at F-AL Stage Using P30 and M30 as Controls
3.6. In Vitro Phenotypic Effect of P4, RU486, and rTs-MAPRC2-Ab on F-AL and ML Stages
3.7. Phenotypic Effect and Relative mRNA Expression of Mifepristone on F-AL Stage by In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Bai, X.; Li, C.; Tong, M.; Zhang, P.; Cai, W.; Liu, X.; Liu, M. Molecular characterization of fructose-1,6-bisphosphate aldolase from Trichinella spiralis and its potential in inducing immune protection. Front. Cell. Infect. Microbiol. 2019, 9, 122. [Google Scholar] [CrossRef]
- Cui, J.; Wang, Z. An epidemiological overview of swine trichinellosis in China. Veter. J. 2011, 190, 323–328. [Google Scholar] [CrossRef]
- Murrell, K.D.; Pozio, E. Worldwide occurrence and impact of human trichinellosis, 1986–2009. Emerg. Infect. Dis. 2011, 17, 2194–2202. [Google Scholar] [CrossRef] [PubMed]
- WHO. Multicriteria-Based Ranking for Risk Management of Food-Borne Parasites; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Cui, J.; Jiang, P.; Na Liu, L.; Wang, Z.Q. Survey of Trichinella infections in domestic pigs from northern and eastern Henan, China. Veter. Parasitol. 2013, 194, 133–135. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, X.; Wang, L.A.; Han, L.H.; Yang, M.; Duan, J.Y.; Sun, G.G.; Qi, X.; Liu, R.D.; Wang, Z.Q.; et al. Survey of Trichinella infection from domestic pigs in the historical endemic areas of Henan province, central China. Parasitol. Res. 2016, 115, 4707–4709. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Hu, X.; Liu, X.; Tang, B.; Liu, M. Current research of Trichinellosis in China. Front. Microbiol. 2017, 8, 1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Li, W.; Fu, B. Vaccines against Trichinella spiralis: Progress, challenges and future prospects. Transbound. Emerg. Dis. 2018, 65, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Wu, X.; Wang, X.; Bai, X.; Shi, H.; Tang, B.; Liu, X.; Song, Y.; Boireau, P.; Wang, F.; et al. Vaccination of mice with an antigenic serine protease-like protein elicits a protective immune response against Trichinella spiralis infection. J. Parasitol. 2013, 99, 426–432. [Google Scholar] [CrossRef]
- Gu, Y.; Sun, X.; Li, B.; Huang, J.; Zhan, B.; Zhu, X. Vaccination with a paramyosin-based multi-epitope vaccine elicits significant protective immunity against Trichinella spiralis infection in mice. Front. Microbiol. 2017, 8, 1475. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.Y.; Zhang, Y.; Yang, D.; Ren, H.N.; Sun, G.G.; Jiang, P.; Liu, R.D.; Zhang, X.; Cui, J.; Wang, Z.Q. The immune protection induced by a serine protease inhibitor from the foodborne parasite Trichinella spiralis. Front. Microbiol. 2018, 9, 1544. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Li, W.; Yang, Z.; Pan, A.; Liao, W.; Zhou, X. A novel antigenic cathepsin B protease induces protective immunity in Trichinella-infected mice. Vaccine 2018, 36, 248–255. [Google Scholar] [CrossRef]
- Strauss, J.F.; Barbieri, R.L.; Gargiulo, A.R. Yen & Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management: Eighth Edition; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 8, ISBN 9780323582322. [Google Scholar] [CrossRef] [Green Version]
- Piccinni, M.P.; Giudizi, M.G.; Biagiotti, R.; Beloni, L.; Giannarini, L.; Sampognaro, S.; Parronchi, P.; Manetti, R.; Annunziato, F.; Livi, C. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J. Immunol. 1995, 155, 128–133. [Google Scholar]
- Miyaura, H.; Iwata, M. Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. J. Immunol. 2002, 168, 1087–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anzaldúa, S.R.; Camacho-Arroyo, I.; Cerbón, M.A. Histomorphological changes in the oviduct epithelium of the rabbit during early pregnancy. Anat. Histol. Embryol. 2002, 31, 308–312. [Google Scholar] [CrossRef]
- Nuñez, G.G.; Gentile, T.; Costantino, S.N.; Sarchi, M.I.; Venturiello, S.M. In vitro and in vivo effects of progesterone on Trichinella spiralis newborn larvae. Parasitology 2005, 131, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Hlaka, L.; Chitanga, S.; Masola, B.; Mukaratirwa, S. Host pregnancy influences the establishment of Trichinella zimbabwensis in Balb C mice. J. Parasit. Dis. 2017, 41, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Shao, J.; Gao, Y.; Xu, J.; Yu, S.; Liu, Z.; Jia, L. The unique pharmacological characteristics of mifepristone (RU486): From terminating pregnancy to preventing cancer metastasis. Med. Res. Rev. 2014, 34, 979–1000. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Diaz, H.; Nava-Castro, K.E.; Escobedo, G.; Dominguez-Ramirez, L.; García-Varela, M.; Del Río-Araiza, V.H.; Palacios-Arreola, M.I.; Morales-Montor, J. A novel progesterone receptor membrane component (PGRMC) in the human and swine parasite Taenia solium: Implications to the host-parasite relationship. Parasites Vectors 2018, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Kapp, N.; Whyte, P.; Tang, J.; Jackson, E.; Brahmi, D. A review of evidence for safe abortion care. Contraception 2013, 88, 350–363. [Google Scholar] [CrossRef]
- Yr, Q.Y.Y. Progress in treatment and prevention of Trichinellosis. J. Infect. Dis. Ther. 2015, 3, 251. [Google Scholar] [CrossRef]
- Wang, L.; Cui, J.; Hu, D.D.; Liu, R.D.; Wang, Z.Q. Identification of early diagnostic antigens from major excretory-secretory proteins of Trichinella spiralis muscle larvae using immunoproteomics. Parasites Vectors 2014, 7, 40. [Google Scholar] [CrossRef]
- Yang, Y.; Lacour, S.A.; Lainé-Prade, V.; Versillé, N.; Grasset-Chevillot, A.; Feng, S.; Liu, M.Y.; Boireau, P.; Vallee, I. Trichinella spiralis newborn larvae: Characterization of a stage specific serine proteinase expression, NBL1, using monoclonal antibodies. Parasitology 2015, 142, 783–790. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, Y.; Liu, Z.; Aleem, M.; Liu, J.; Luo, J.; Yan, R.; Xu, L.; Song, X.; Li, X. Recombinant Toxoplasma gondii ribosomal protein P2 modulates the functions of murine macrophages in vitro and provides immunity against acute toxoplasmosis in vivo. Vaccines 2021, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Aimulajiang, K.; Cao, M.; Liao, S.; Naqvi, M.A.-U.-H.; Tian, X.; Li, Z.; Lu, M.; Lakho, S.A.; Li, X.; Xu, L.; et al. Development and potential application of ras domain containing protein from Haemonchus contortus for diagnosis of goat infection. Animals 2020, 10, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virgo, B.B.; Bellward, G.D. Serum progesterone levels in the pregnant and postpartum laboratory mouse. Endocrinology 1974, 95, 1486–1490. [Google Scholar] [CrossRef] [PubMed]
- González, C.G.; Garcia, F.D.; Ferníndez, S.F.; Patterson, A.M. Role of 17-β-estradiol and progesterone on glucose homeostasis: Effects of food restriction (50%) in pregnant and non pregnant rats. J. Endocrinol. Investig. 1997, 20, 397–403. [Google Scholar] [CrossRef]
- Anderson, L.L. Reproductive biology of pigs. In Reproductive Biology of Pigs; Iowa State University: Ames, IA, USA, 2009. [Google Scholar]
- Ikoma, D.; Hulteen, L.; Holden, J. Threshold progesterone level of 25 ng/mL to sustain pregnancy in first trimester in women with history of infertility or miscarriage. Clin. Obstet. Gynecol. Reprod. Med. 2018, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.C.; Pu, H.F.; Wang, P.S. Unimpaired postreceptor regulation of luteinizing hormone secretion by gonadotropin-releasing hormone and estrogen in aged rat anterior pituitary cells. Endocrinology 1993, 132, 1189–1194. [Google Scholar] [CrossRef]
- Liu, R.D.; Wang, Z.Q.; Wang, L.; Long, S.R.; Ren, H.J.; Cui, J. Analysis of differentially expressed genes of Trichinella spiralis larvae activated by bile and cultured with intestinal epithelial cells using real-time PCR. Parasitol. Res. 2013, 112, 4113–4120. [Google Scholar] [CrossRef] [PubMed]
- Cahill, M.A. Progesterone receptor membrane component 1: An integrative review. J. Steroid Biochem. Mol. Biol. 2007, 105, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Lösel, R.M.; Besong, D.; Peluso, J.J.; Wehling, M. Progesterone receptor membrane component 1—Many tasks for a versatile protein. Steroids 2008, 73, 929–934. [Google Scholar] [CrossRef]
- Falkenstein, E.; Meyer, C.; Eisen, C.; Scriba, P.C.; Wehling, M. Full-length cDNA sequence of a progesterone membrane-binding protein from porcine vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1996, 229, 86–89. [Google Scholar] [CrossRef]
- Peluso, J.J. Multiplicity of progesterone’s actions and receptors in the mammalian ovary. Biol. Reprod. 2006, 75, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.; Johnston, D.A. Mining the schistosome DNA sequence database. Trends Parasitol. 2001, 17, 501–503. [Google Scholar] [CrossRef]
- Hu, W.; Yan, Q.; Shen, D.-K.; Liu, F.; Zhu, Z.-D.; Song, H.-D.; Xu, X.-R.; Wang, Z.-J.; Rong, Y.-P.; Zeng, L.-C.; et al. Evolutionary and biomedical implications of a Schistosoma japonicum complementary DNA resource. Nat. Genet. 2003, 35, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.; Hunt, J.S. Sex steroid hormones and macrophage function. Life Sci. 1996, 59, 1–14. [Google Scholar] [CrossRef]
- Piccinni, M.P. Role of hormone-controlled Th1- and Th2-type cytokines in successful pregnancy. J. Neuroimmunol. 2000, 109, 30–33. [Google Scholar] [CrossRef]
- Roberts, C.W.; Walker, W.; Alexander, J. Sex-associated hormones and immunity to protozoan parasites. Clin. Microbiol. Rev. 2001, 14, 476–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagliardo, L.F.; McVay, C.S.; Appleton, J.A. Molting, ecdysis, and reproduction of Trichinella spiralis are supported in vitro by intestinal epithelial cells. Infect. Immun. 2002, 70, 1853–1859. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleem, M.T.; Shi, J.; Yu, Z.; Wen, Z.; Zhang, Y.; Liang, M.; Lakho, S.A.; Haseeb, M.; Ali, H.; Hassan, M.W.; et al. Characterization of Membrane-Associated Progesterone Receptor Component-2 (MAPRC2) from Trichinella spiralis and Its Interaction with Progesterone and Mifepristone. Vaccines 2021, 9, 934. https://doi.org/10.3390/vaccines9080934
Aleem MT, Shi J, Yu Z, Wen Z, Zhang Y, Liang M, Lakho SA, Haseeb M, Ali H, Hassan MW, et al. Characterization of Membrane-Associated Progesterone Receptor Component-2 (MAPRC2) from Trichinella spiralis and Its Interaction with Progesterone and Mifepristone. Vaccines. 2021; 9(8):934. https://doi.org/10.3390/vaccines9080934
Chicago/Turabian StyleAleem, Muhammad Tahir, Jiawen Shi, Zhengqing Yu, Zhaohai Wen, Yang Zhang, Meng Liang, Shakeel Ahmed Lakho, Muhammad Haseeb, Haider Ali, Muhammad Waqas Hassan, and et al. 2021. "Characterization of Membrane-Associated Progesterone Receptor Component-2 (MAPRC2) from Trichinella spiralis and Its Interaction with Progesterone and Mifepristone" Vaccines 9, no. 8: 934. https://doi.org/10.3390/vaccines9080934
APA StyleAleem, M. T., Shi, J., Yu, Z., Wen, Z., Zhang, Y., Liang, M., Lakho, S. A., Haseeb, M., Ali, H., Hassan, M. W., Song, X., Li, X., Xu, L., & Yan, R. (2021). Characterization of Membrane-Associated Progesterone Receptor Component-2 (MAPRC2) from Trichinella spiralis and Its Interaction with Progesterone and Mifepristone. Vaccines, 9(8), 934. https://doi.org/10.3390/vaccines9080934









