Incorporating the Cluster A and V1V2 Targets into a Minimal Structural Unit of the HIV-1 Envelope to Elicit a Cross-Clade Response with Potent Fc-Effector Functions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Immunogen Design and Preparation
2.2. Surface Plasmon Resonance (SPR)
2.3. Mice and Immunization
2.4. Immunogen-Specific ELISA
2.5. Competition ELISA
2.6. Rapid Fluorometric Antibody-Dependent Cellular Cytotoxicity
2.7. Phagocytosis
2.8. Complement Deposition
2.9. Statistical Analysis
3. Results
3.1. V1V2 Is Presented in the Native Conformation When Co-Expressed with ID2
3.2. ID2, ID2-V1V2 Mix Elicits More Antigen-Specific Antibodies than ID2 Alone
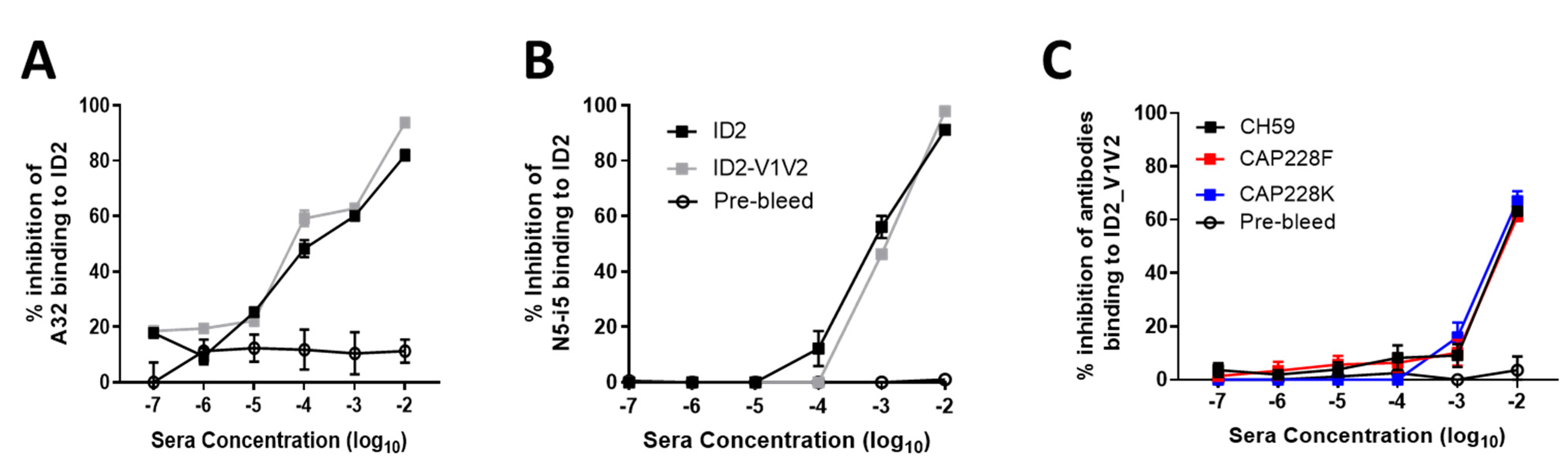
3.3. ID2-V1V2 Immunization Generates Sera Capable of Blocking the Binding of V1V2 Antibodies
3.4. V1V2 Enhances ADCC against Cross-Clade gp120-Coated Target Cells
3.5. ID2-V1V2 Immunization Elicits FLSC-Specific Antibodies Capable of Phagocytosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Disclaimer
References
- Dahabieh, M.S.; Battivelli, E.; Verdin, E. Understanding HIV latency: The road to an HIV cure. Annu. Rev. Med. 2015, 66, 407–421. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Finzi, A.; Sodroski, J. The conformational states of the HIV-1 envelope glycoproteins. Trends Microbiol. 2020, 28, 655–667. [Google Scholar] [CrossRef]
- Richard, J.; Prévost, J.; Alsahafi, N.; Ding, S.; Finzi, A. Impact of HIV-1 envelope conformation on ADCC responses. Trends Microbiol. 2018, 26, 253–265. [Google Scholar] [CrossRef]
- Pegu, A.; Hessell, A.J.; Mascola, J.R.; Haigwood, N.L. Use of broadly neutralizing antibodies for HIV-1 prevention. Immunol. Rev. 2017, 275, 296–312. [Google Scholar] [CrossRef]
- Garber, D.A.; Adams, D.R.; Guenthner, P.; Mitchell, J.; Kelley, K.; Schoofs, T.; Gazumyan, A.; Nason, M.; Seaman, M.S.; McNicholl, J.; et al. Durable protection against repeated penile exposures to simian-human immunodeficiency virus by broadly neutralizing antibodies. Nat. Commun. 2020, 11, 3195. [Google Scholar] [CrossRef]
- Julg, B.; Tartaglia, L.J.; Keele, B.F.; Wagh, K.; Pegu, A.; Sok, D.; Abbink, P.; Schmidt, S.D.; Wang, K.; Chen, X.; et al. Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer protection against a clade C SHIV challenge. Sci. Transl. Med. 2017, 9, eaal1321. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, Y.; Gautam, R.; Chun, T.-W.; Sadjadpour, R.; Foulds, K.E.; Shingai, M.; Klein, F.; Gazumyan, A.; Golijanin, J.; Donaldson, M.; et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nat. Cell Biol. 2017, 543, 559–563. [Google Scholar] [CrossRef]
- Corey, L.; Gilbert, P.B.; Juraska, M.; Montefiori, D.C.; Morris, L.; Karuna, S.T.; Edupuganti, S.; Mgodi, N.M.; Decamp, A.C.; Rudnicki, E.; et al. Two randomized trials of neutralizing antibodies to prevent HIV-1 acquisition. N. Engl. J. Med. 2021, 384, 1003–1014. [Google Scholar] [CrossRef]
- Burton, D.R.; Mascola, J.R. Antibody responses to envelope glycoproteins in HIV-1 infection. Nat. Immunol. 2015, 16, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Kwong, P.D.; Mascola, J.R.; Nabel, G.J. Broadly neutralizing antibodies and the search for an HIV-1 vaccine: The end of the beginning. Nat. Rev. Immunol. 2013, 13, 693–701. [Google Scholar] [CrossRef]
- Burton, D.R.; Hessell, A.J.; Keele, B.F.; Klasse, P.J.; Ketas, T.A.; Moldt, B.; Dunlop, D.C.; Poignard, P.; Doyle, L.A.; Cavacini, L.; et al. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc. Natl. Acad. Sci. USA 2011, 108, 11181–11186. [Google Scholar] [CrossRef] [Green Version]
- Dugast, A.-S.; Chan, Y.; Hoffner, M.; Licht, A.; Nkolola, J.; Li, H.; Streeck, H.; Suscovich, T.J.; Ghebremichael, M.; Ackerman, M.E.; et al. Lack of protection following passive transfer of polyclonal highly functional low-dose non-neutralizing antibodies. PLoS ONE 2014, 9, e97229. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; Pazgier, M.; Sajadi, M.M.; Kamin-Lewis, R.; Al-Darmarki, S.; Flinko, R.; Lovo, E.; Wu, X.; Robinson, J.E.; Seaman, M.S.; et al. Diverse specificity and effector function among human antibodies to HIV-1 envelope glycoprotein epitopes exposed by CD4 binding. Proc. Natl. Acad. Sci. USA 2013, 110, E69–E78. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, G.; Pollara, J.; Kozink, D.; Harms, T.; Drinker, M.; Freel, S.; Moody, M.A.; Alam, S.M.; Tomaras, G.D.; Ochsenbauer, C.; et al. An HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent antibody-dependent cellular cytotoxicity (ADCC) activity and defines a common ADCC epitope in human HIV-1 serum. J. Virol. 2011, 85, 7029–7036. [Google Scholar] [CrossRef] [Green Version]
- Veillette, M.; Coutu, M.; Richard, J.; Batraville, L.-A.; Dagher, O.; Bernard, N.; Tremblay, C.; Kaufmann, D.E.; Roger, M.; Finzi, A. The HIV-1 gp120 CD4-bound conformation is preferentially targeted by antibody-dependent cellular cytotoxicity-mediating antibodies in sera from HIV-1-infected individuals. J. Virol. 2014, 89, 545–551. [Google Scholar] [CrossRef] [Green Version]
- Williams, K.L.; Cortez, V.; Dingens, A.; Gach, J.; Rainwater, S.; Weis, J.F.; Chen, X.; Spearman, P.; Forthal, D.N.; Overbaugh, J. HIV-specific CD4-induced antibodies mediate broad and potent antibody-dependent cellular cytotoxicity activity and are commonly detected in plasma from HIV-infected humans. EBioMedicine 2015, 2, 1464–1477. [Google Scholar] [CrossRef] [Green Version]
- Dupuy, F.P.; Kant, S.; Barbé, A.; Routy, J.-P.; Bruneau, J.; Lebouché, B.; Tremblay, C.; Pazgier, M.; Finzi, A.; Bernard, N.F. Antibody-dependent cellular cytotoxicity-competent antibodies against HIV-1-infected cells in plasma from HIV-infected subjects. mBio 2019, 10, e02690-19. [Google Scholar] [CrossRef] [Green Version]
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; De Souza, M.; Adams, E.; et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar] [CrossRef]
- Bonsignori, M.; Pollara, J.; Moody, M.A.; Alpert, M.D.; Chen, X.; Hwang, K.-K.; Gilbert, P.B.; Huang, Y.; Gurley, T.C.; Kozink, D.M.; et al. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J. Virol. 2012, 86, 11521–11532. [Google Scholar] [CrossRef] [Green Version]
- Haynes, B.F.; Gilbert, P.B.; McElrath, M.J.; Zolla-Pazner, S.; Tomaras, G.D.; Alam, S.M.; Evans, D.T.; Montefiori, D.C.; Karnasuta, C.; Sutthent, R.; et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 2012, 366, 1275–1286. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.-X.; Bonsignori, M.; Alam, S.M.; McLellan, J.; Tomaras, G.D.; Moody, T.; Kozink, D.M.; Hwang, K.-K.; Chen, X.; Tsao, C.-Y.; et al. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 2013, 38, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Yates, N.L.; Shen, X.; Bonsignori, M.; Moody, M.A.; Liao, H.-X.; Fong, Y.; Alam, S.M.; Overman, R.G.; Denny, T.; et al. Infectious virion capture by HIV-1 gp120-specific IgG from RV144 vaccinees. J. Virol. 2013, 87, 7828–7836. [Google Scholar] [CrossRef] [Green Version]
- Pollara, J.; Bonsignori, M.; Moody, M.A.; Liu, P.; Alam, S.M.; Hwang, K.-K.; Gurley, T.C.; Kozink, D.M.; Armand, L.C.; Marshall, D.J.; et al. HIV-1 Vaccine-induced C1 and V2 env-specific antibodies synergize for increased antiviral activities. J. Virol. 2014, 88, 7715–7726. [Google Scholar] [CrossRef] [Green Version]
- Chung, A.; Kumar, M.P.; Arnold, K.; Yu, W.H.; Schoen, M.K.; Dunphy, L.; Suscovich, T.J.; Frahm, N.; Linde, C.; Mahan, A.E.; et al. Dissecting polyclonal vaccine-induced humoral immunity against HIV using systems serology. Cell 2015, 163, 988–998. [Google Scholar] [CrossRef] [Green Version]
- Barouch, D.H.; Stephenson, K.; Borducchi, E.N.; Smith, K.; Stanley, K.; McNally, A.G.; Liu, J.; Abbink, P.; Maxfield, L.F.; Seaman, M.S.; et al. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell 2013, 155, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Tolbert, W.; Gohain, N.; Veillette, M.; Chapleau, J.-P.; Orlandi, C.; Visciano, M.L.; Ebadi, M.; DeVico, A.L.; Fouts, T.; Finzi, A.; et al. Paring Down HIV Env: Design and crystal structure of a stabilized inner domain of HIV-1 gp120 displaying a major ADCC target of the A32 region. Structure 2016, 24, 697–709. [Google Scholar] [CrossRef] [Green Version]
- Sherburn, R.; Tolbert, W.D.; Gottumukkala, S.; Beaudoin-Bussières, G.; Finzi, A.; Pazgier, M. Effects of gp120 inner domain (ID2) immunogen doses on elicitation of anti-HIV-1 functional Fc-effector response to C1/C2 (Cluster A) epitopes in mice. Microorganisms 2020, 8, 1490. [Google Scholar] [CrossRef]
- Tolbert, W.D.; Sherburn, R.; Gohain, N.; Ding, S.; Flinko, R.; Orlandi, C.; Ray, K.; Finzi, A.; Lewis, G.K.; Pazgier, M. Defining rules governing recognition and Fc-mediated effector functions to the HIV-1 co-receptor binding site. BMC Biol. 2020, 18, 91. [Google Scholar] [CrossRef]
- Tolbert, W.D.; Sherburn, R.T.; Van, V.; Pazgier, M. Structural basis for epitopes in the gp120 cluster a region that invokes potent effector cell activity. Viruses 2019, 11, 69. [Google Scholar] [CrossRef] [Green Version]
- Tolbert, W.D.; Van, V.; Sherburn, R.; Tuyishime, M.; Yan, F.; Nguyen, D.N.; Stanfield-Oakley, S.; Easterhoff, D.; Bonsignori, M.; Haynes, B.F.; et al. Recognition patterns of the C1/C2 epitopes involved in Fc-mediated response in HIV-1 natural infection and the RV114 vaccine trial. mBio 2020, 11, e00208-20. [Google Scholar] [CrossRef]
- Orlandi, C.; Flinko, R.; Lewis, G.K. A new cell line for high throughput HIV-specific antibody-dependent cellular cytotoxicity (ADCC) and cell-to-cell virus transmission studies. J. Immunol. Methods 2016, 433, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Ackerman, M.E.; Moldt, B.; Wyatt, R.T.; Dugast, A.-S.; McAndrew, E.; Tsoukas, S.; Jost, S.; Berger, C.T.; Sciaranghella, G.; Liu, Q.; et al. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J. Immunol. Methods 2011, 366, 8–19. [Google Scholar] [CrossRef] [Green Version]
- Fischinger, S.; Fallon, J.K.; Michell, A.; Broge, T.; Suscovich, T.J.; Streeck, H.; Alter, G. A high-throughput, bead-based, antigen-specific assay to assess the ability of antibodies to induce complement activation. J. Immunol. Methods 2019, 473, 112630. [Google Scholar] [CrossRef] [PubMed]
- Rolland, M.; Edlefsen, P.T.; Larsen, B.B.; Tovanabutra, S.; Sanders-Buell, E.; Hertz, T.; Decamp, A.C.; Carrico, C.; Menis, S.; Magaret, C.A.; et al. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nat. Cell Biol. 2012, 490, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Karasavvas, N.; Billings, E.; Rao, M.; Williams, C.; Zolla-Pazner, S.; Bailer, R.T.; Koup, R.A.; Madnote, S.; Arworn, D.; Shen, X.; et al. The thai phase iii hiv type 1 vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res. Hum. Retrovir. 2012, 28, 1444–1457. [Google Scholar] [CrossRef] [PubMed]
- DeVico, A.; Fouts, T.; Lewis, G.K.; Gallo, R.C.; Godfrey, K.; Charurat, M.; Harris, I.; Galmin, L.; Pal, R. Antibodies to CD4-induced sites in HIV gp120 correlate with the control of SHIV challenge in macaques vaccinated with subunit immunogens. Proc. Natl. Acad. Sci. USA 2007, 104, 17477–17482. [Google Scholar] [CrossRef] [Green Version]
- DeVico, A.L. CD4-induced epitopes in the HIV envelope glycoprotein, gp120. Curr. HIV Res. 2007, 5, 561–571. [Google Scholar] [CrossRef]
- van Eeden, C.; Wibmer, C.K.; Scheepers, C.; Richardson, S.; Nonyane, M.; Lambson, B.; Mkhize, N.N.; Vijayakumar, B.; Sheng, Z.; Stanfield-Oakley, S.; et al. V2-directed vaccine-like antibodies from HIV-1 infection identify an additional K169-binding light chain motif with broad ADCC activity. Cell Rep. 2018, 25, 3123–3135. [Google Scholar] [CrossRef] [Green Version]
- Visciano, M.L.; Gohain, N.; Sherburn, R.; Orlandi, C.; Flinko, R.; Dashti, A.; Lewis, G.K.; Tolbert, W.D.; Pazgier, M. Induction of Fc-mediated effector functions against a stabilized inner domain of HIV-1 gp120 designed to selectively harbor the A32 epitope region. Front. Immunol. 2019, 10, 677. [Google Scholar] [CrossRef] [Green Version]
- Fouts, T.; Tuskan, R.; Godfrey, K.; Reitz, M.; Hone, D.; Lewis, G.K.; DeVico, A.L. Expression and characterization of a single-chain polypeptide analogue of the human immunodeficiency virus type 1 gp120-CD4 receptor complex. J. Virol. 2000, 74, 10245–10248. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, M.; Mills, J.; Kohl, S. Natural killer cytotoxicity and antibody-dependent cellular cytotoxicity of human immunodeficiency virus–infected cells by leukocytes from human neonates and adults. Pediatr. Res. 1993, 33, 469–474. [Google Scholar] [CrossRef] [Green Version]
- Duchemin, M.; Tudor, D.; Cottignies-Calamarte, A.; Bomsel, M. Antibody-dependent cellular phagocytosis of HIV-1-infected cells is efficiently triggered by IgA targeting HIV-1 envelope subunit gp41. Front. Immunol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Fischinger, S.; Dolatshahi, S.; Jennewein, M.F.; Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Michael, N.; Vasan, S.; Ackerman, M.E.; Streeck, H.; et al. IgG3 collaborates with IgG1 and IgA to recruit effector function in RV144 vaccinees. JCI Insight 2020, 5, e140925. [Google Scholar] [CrossRef]
- Anand, S.P.; Prevost, J.; Baril, S.; Richard, J.; Medjahed, H.; Chapleau, J.P.; Tolbert, W.D.; Kirk, S.; Smith, A.B., III; Wines, B.D.; et al. Two families of env antibodies efficiently engage Fc-gamma receptors and eliminate HIV-1-infected cells. J. Virol. 2019, 93, e01823-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Easterhoff, D.; Pollara, J.; Luo, K.; Tolbert, W.D.; Young, B.; Mielke, D.; Jha, S.; O’Connell, R.J.; Vasan, S.; Kim, J.; et al. Boosting with AIDSVAX B/E enhances env constant region 1 and 2 antibody-dependent cellular cytotoxicity breadth and potency. J. Virol. 2020, 94, e01120-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zolla-Pazner, S.; DeCamp, A.; Gilbert, P.B.; Williams, C.; Yates, N.L.; Williams, W.T.; Howington, R.; Fong, Y.; Morris, D.E.; Soderberg, K.A.; et al. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS ONE 2014, 9, e87572. [Google Scholar] [CrossRef]
- Powell, R.L.; Weiss, S.; Fox, A.; Liu, X.; Itri, V.; Jiang, X.; Luo, C.C.; Spencer, D.A.; Pandey, S.; Cheever, T.; et al. An HIV vaccine targeting the V2 region of the HIV envelope induces a highly durable polyfunctional Fc-mediated antibody response in rhesus macaques. J. Virol. 2020, 94, e01175-20. [Google Scholar] [CrossRef]
- Mengistu, M.; Ray, K.; Lewis, G.K.; DeVico, A.L. Antigenic properties of the human immunodeficiency virus envelope glycoprotein gp120 on virions bound to target cells. PLoS Pathog. 2015, 11, e1004772. [Google Scholar] [CrossRef] [Green Version]
- Pancera, M.; Majeed, S.; Ban, Y.-E.A.; Chen, L.; Huang, C.-C.; Kong, L.; Kwon, Y.D.; Stuckey, J.; Zhou, T.; Robinson, J.E.; et al. Structure of HIV-1 gp120 with gp41-interactive region reveals layered envelope architecture and basis of conformational mobility. Proc. Natl. Acad. Sci. USA 2010, 107, 1166–1171. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.P.; Sodroski, J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J. Virol. 1996, 70, 1863–1872. [Google Scholar] [CrossRef] [Green Version]
- Mengistu, M.; Tang, A.H.; Foulke, J.S., Jr.; Blanpied, T.A.; Gonzalez, M.W.; Spouge, J.L.; Gallo, R.C.; Lewis, G.K.; DeVico, A.L. Patterns of conserved gp120 epitope presentation on attached HIV-1 virions. Proc. Natl. Acad. Sci. USA 2017, 114, E9893–E9902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, K.; Mengistu, M.; Lewis, G.K.; Lakowicz, J.R.; DeVico, A.L. Antigenic properties of the HIV envelope on virions in solution. J. Virol. 2013, 88, 1795–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, G.K.; Guan, Y.; Kamin-Lewis, R.; Sajadi, M.; Pazgier, M.; DeVico, A.L. Epitope target structures of Fc-mediated effector function during HIV-1 acquisition. Curr. Opin. HIV AIDS 2014, 9, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.K.; Pazgier, M.; Evans, D.T.; Ferrari, G.; Bournazos, S.; Parsons, M.S.; Bernard, N.F.; Finzi, A. Beyond viral neutralization. AIDS Res. Hum. Retroviruses 2017, 33, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Pollara, J.; Bonsignori, M.; Moody, M.A.; Pazgier, M.; Haynes, B.F.; Ferrari, G. Epitope specificity of human immunodeficiency virus-1 antibody dependent cellular cytotoxicity [ADCC] responses. Curr. HIV Res. 2013, 11, 378–387. [Google Scholar] [CrossRef] [Green Version]
- Veillette, M.; Désormeaux, A.; Medjahed, H.; Gharsallah, N.-E.; Coutu, M.; Baalwa, J.; Guan, Y.; Lewis, G.; Ferrari, G.; Hahn, B.; et al. Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J. Virol. 2014, 88, 2633–2644. [Google Scholar] [CrossRef] [Green Version]
- Veillette, M.; Richard, J.; Pazgier, M.; Lewis, G.K.; Parsons, M.S.; Finzi, A. Role of HIV-1 envelope glycoproteins conformation and accessory proteins on ADCC responses. Curr. HIV Res. 2015, 14, 9–23. [Google Scholar] [CrossRef]
- Prévost, J.; Richard, J.; Medjahed, H.; Alexander, A.; Jones, J.; Kappes, J.C.; Ochsenbauer, C.; Finzi, A. Incomplete downregulation of CD4 expression affects HIV-1 env conformation and antibody-dependent cellular cytotoxicity responses. J. Virol. 2018, 92, e00484-18. [Google Scholar] [CrossRef] [Green Version]
- Finnegan, C.M.; Berg, W.; Lewis, G.K.; DeVico, A.L. Antigenic properties of the human immunodeficiency virus envelope during cell-cell fusion. J. Virol. 2001, 75, 11096–11105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finnegan, C.M.; Berg, W.; Lewis, G.K.; DeVico, A.L. Antigenic properties of the human immunodeficiency virus transmembrane glycoprotein during cell-cell fusion. J. Virol. 2002, 76, 12123–12134. [Google Scholar] [CrossRef] [Green Version]
- Lubeck, M.D.; Steplewski, Z.; Baglia, F.; Klein, M.H.; Dorrington, K.J.; Koprowski, H. The interaction of murine IgG subclass proteins with human monocyte Fc receptors. J. Immunol. 1985, 135, 1299–1304. [Google Scholar] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sherburn, R.; Tolbert, W.D.; Gottumukkala, S.; Hederman, A.P.; Beaudoin-Bussières, G.; Stanfield-Oakley, S.; Tuyishime, M.; Ferrari, G.; Finzi, A.; Ackerman, M.E.; et al. Incorporating the Cluster A and V1V2 Targets into a Minimal Structural Unit of the HIV-1 Envelope to Elicit a Cross-Clade Response with Potent Fc-Effector Functions. Vaccines 2021, 9, 975. https://doi.org/10.3390/vaccines9090975
Sherburn R, Tolbert WD, Gottumukkala S, Hederman AP, Beaudoin-Bussières G, Stanfield-Oakley S, Tuyishime M, Ferrari G, Finzi A, Ackerman ME, et al. Incorporating the Cluster A and V1V2 Targets into a Minimal Structural Unit of the HIV-1 Envelope to Elicit a Cross-Clade Response with Potent Fc-Effector Functions. Vaccines. 2021; 9(9):975. https://doi.org/10.3390/vaccines9090975
Chicago/Turabian StyleSherburn, Rebekah, William D. Tolbert, Suneetha Gottumukkala, Andrew P. Hederman, Guillaume Beaudoin-Bussières, Sherry Stanfield-Oakley, Marina Tuyishime, Guido Ferrari, Andrés Finzi, Margaret E. Ackerman, and et al. 2021. "Incorporating the Cluster A and V1V2 Targets into a Minimal Structural Unit of the HIV-1 Envelope to Elicit a Cross-Clade Response with Potent Fc-Effector Functions" Vaccines 9, no. 9: 975. https://doi.org/10.3390/vaccines9090975
APA StyleSherburn, R., Tolbert, W. D., Gottumukkala, S., Hederman, A. P., Beaudoin-Bussières, G., Stanfield-Oakley, S., Tuyishime, M., Ferrari, G., Finzi, A., Ackerman, M. E., & Pazgier, M. (2021). Incorporating the Cluster A and V1V2 Targets into a Minimal Structural Unit of the HIV-1 Envelope to Elicit a Cross-Clade Response with Potent Fc-Effector Functions. Vaccines, 9(9), 975. https://doi.org/10.3390/vaccines9090975





