Oral Delivery of Novel Recombinant Lactobacillus Elicit High Protection against Staphylococcus aureus Pulmonary and Skin Infections
Abstract
:1. Introduction
2. Materials and Methods
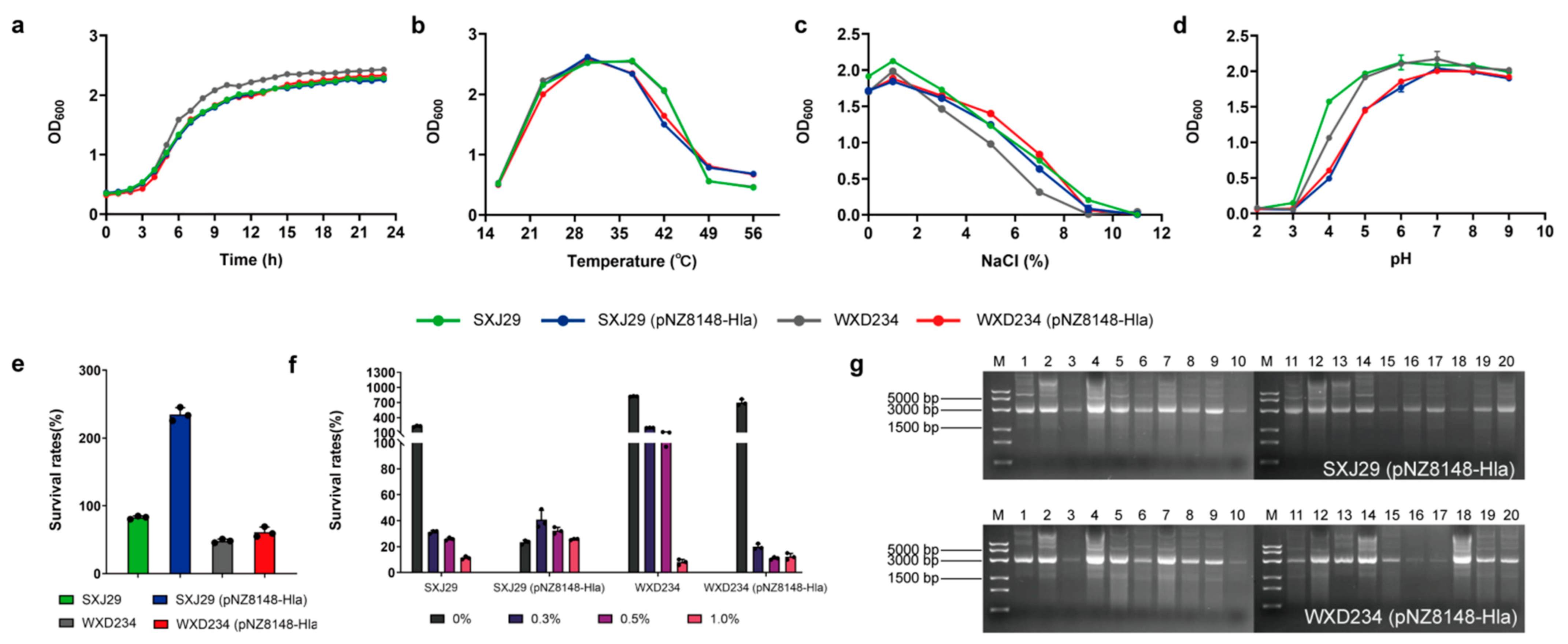
2.1. Bacterial Strains and Growth Conditions
2.2. Experimental Animals and Ethics Statement
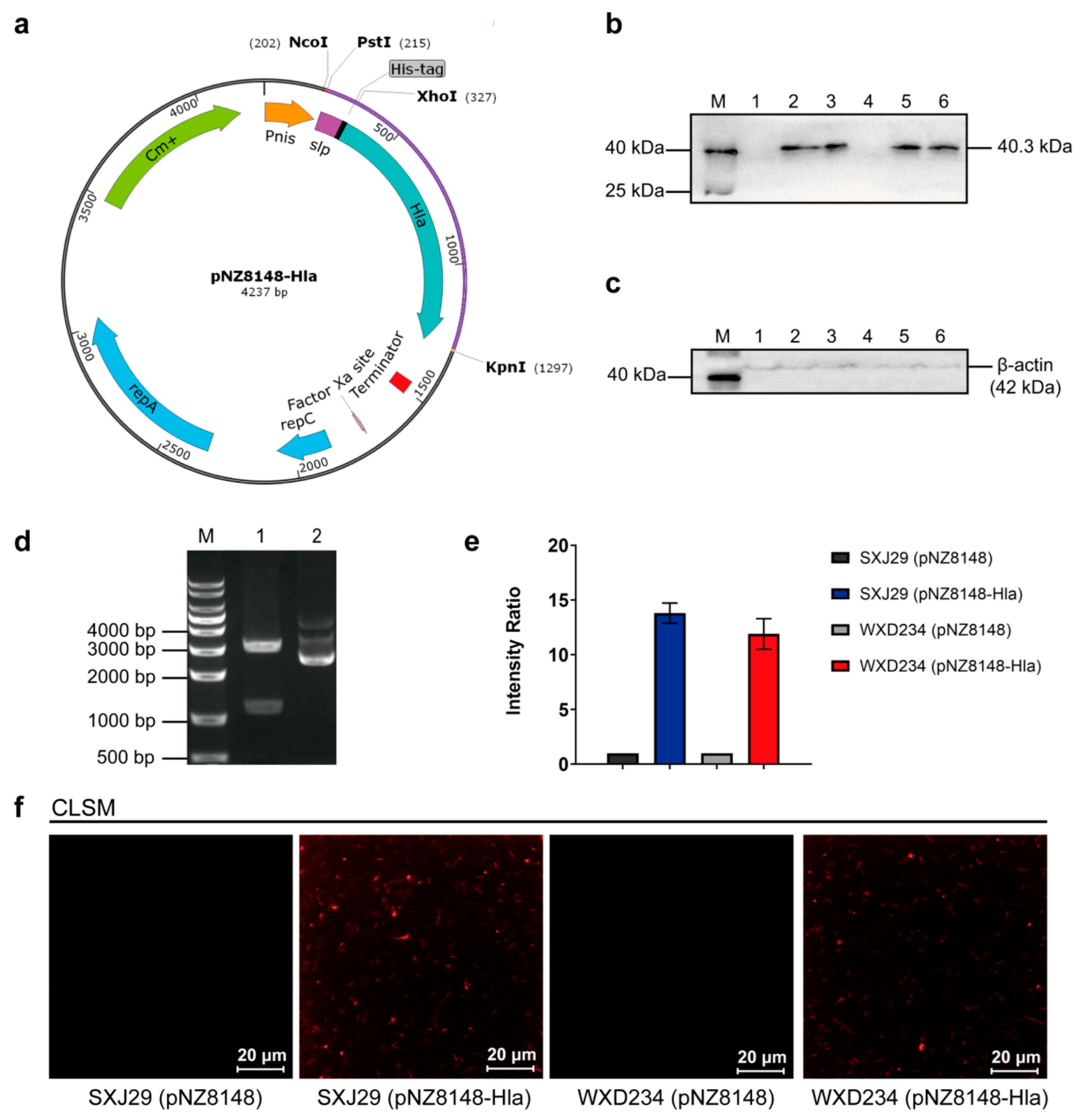
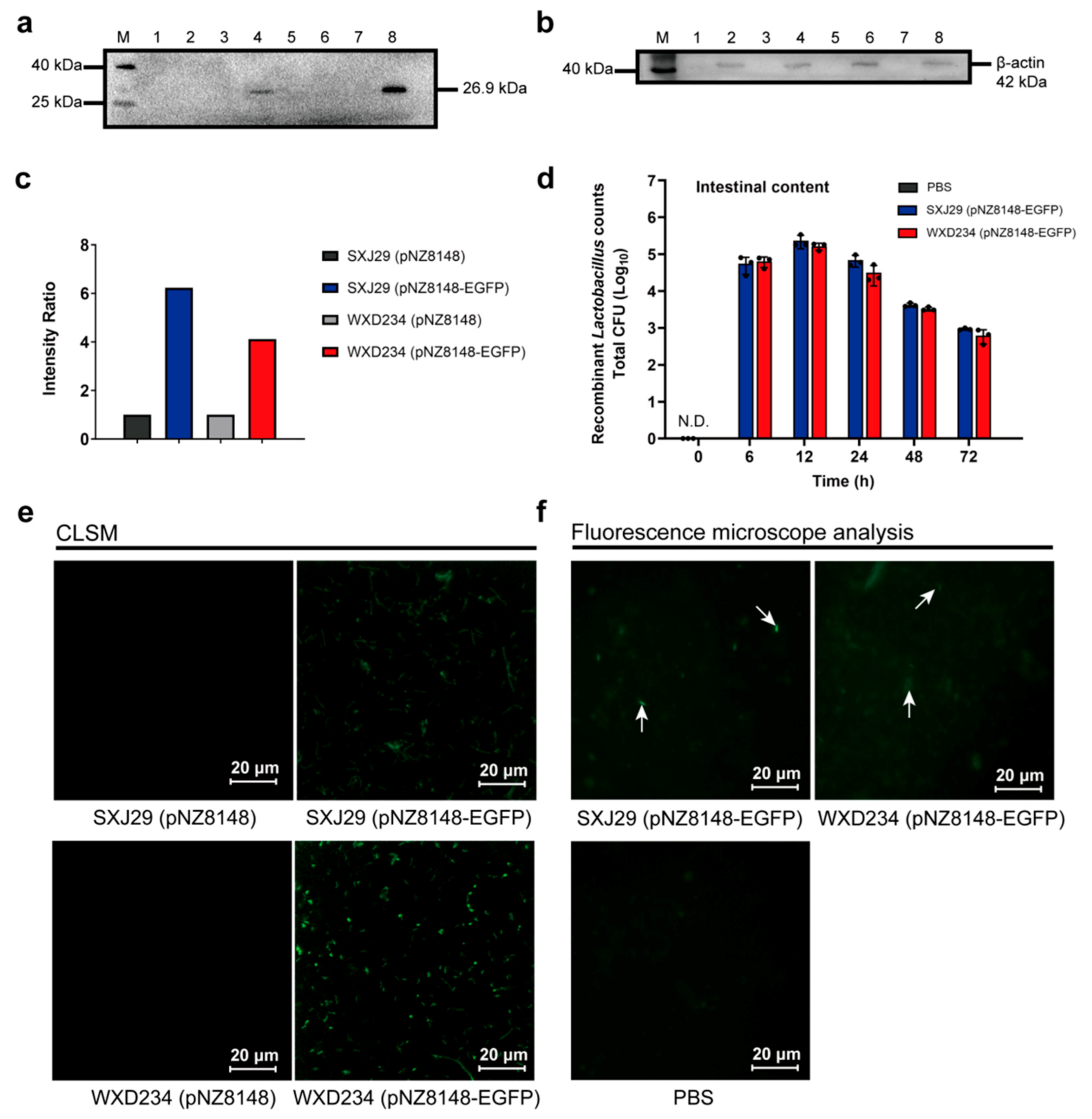
2.3. Construction of HlaH35L Cell Surface Display Lactobacillus Based on SXJ29 and WXD234
2.4. Colonization and Persistence of Recombinant Lactobacillus in the Intestine
2.5. Vaccination
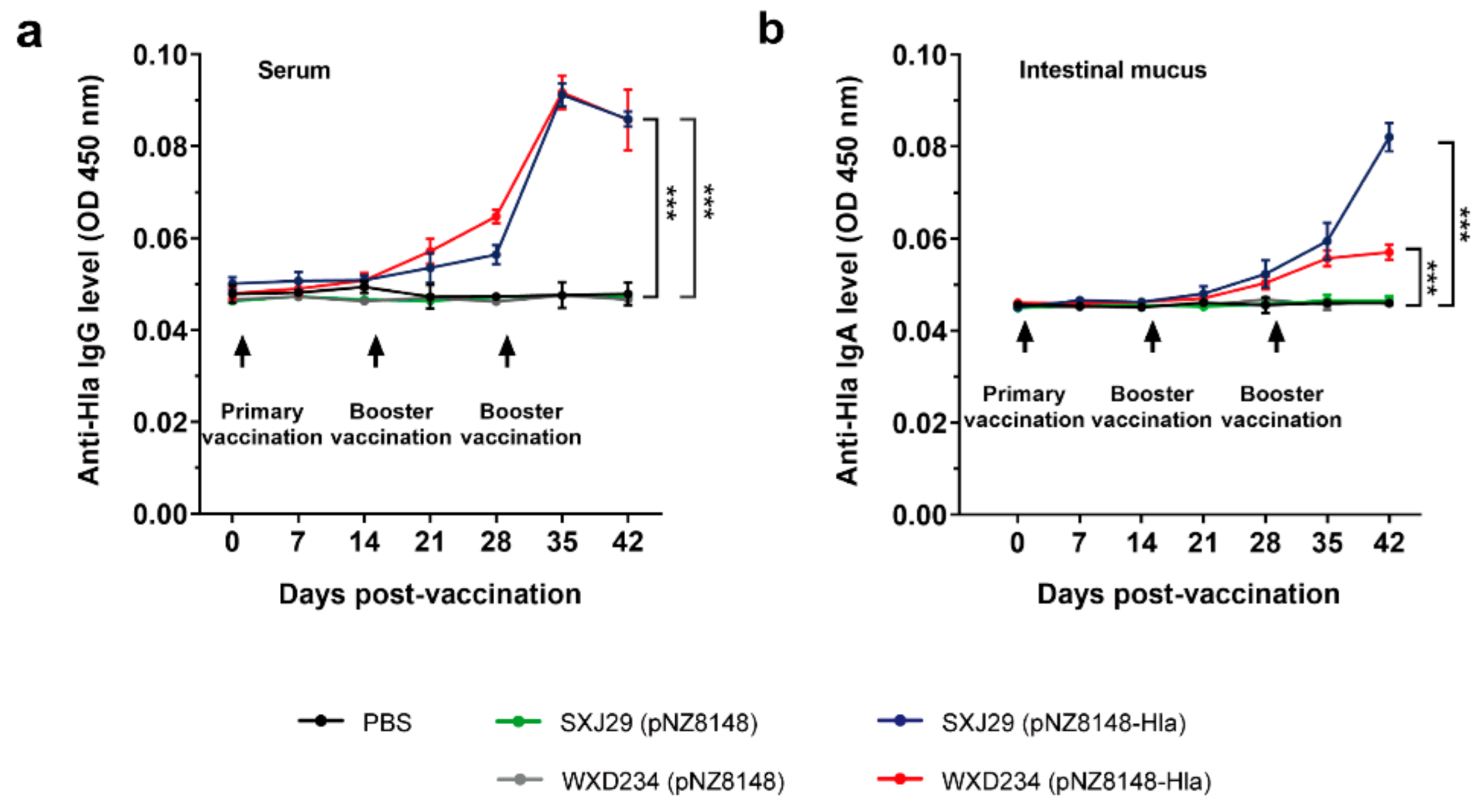
2.6. Determination of IgG and IgA Levels by ELISA
2.7. T Cell Proliferation Assay
2.8. Cytokine Level Assay
2.9. Mouse Model of S. aureus-Induced Pulmonary Infection
2.10. Mouse Model of S. aureus-Induced Skin Infection
2.11. Statistical Analysis
3. Results
3.1. Cell Surface Display System for HlaH35L Expression in Transformed Lactobacillus
3.2. Recombinant Lactobacillus Colonize and Express Heterologous Protein Stably in the Intestine
3.3. Recombinant Lactobacillus Elicited Intestinal Mucus IgA and Serum IgG-Mediated Immune Responses
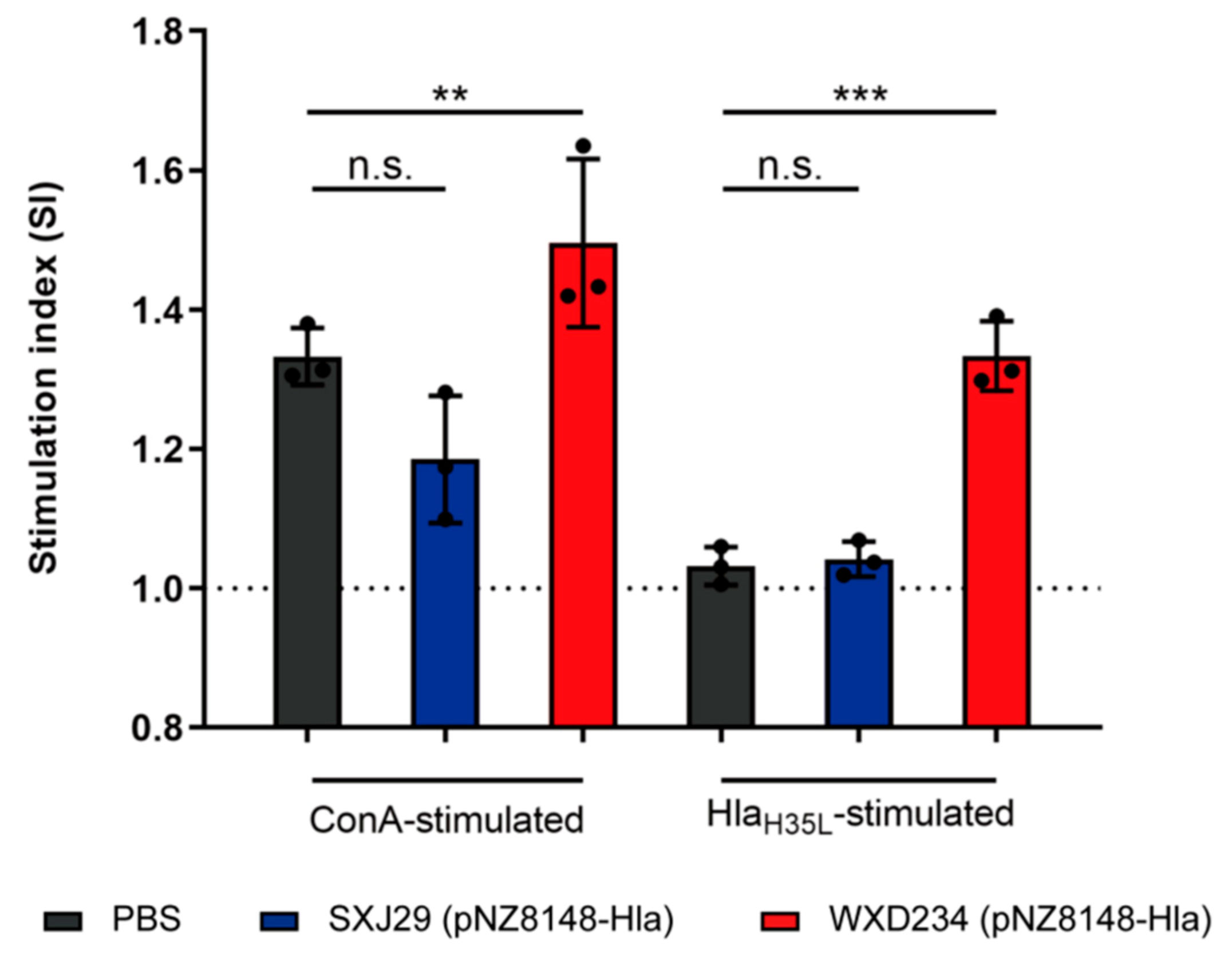
3.4. Recombinant Lactobacillus Enhanced Lymphocyte Proliferation in Peyer’s Patches
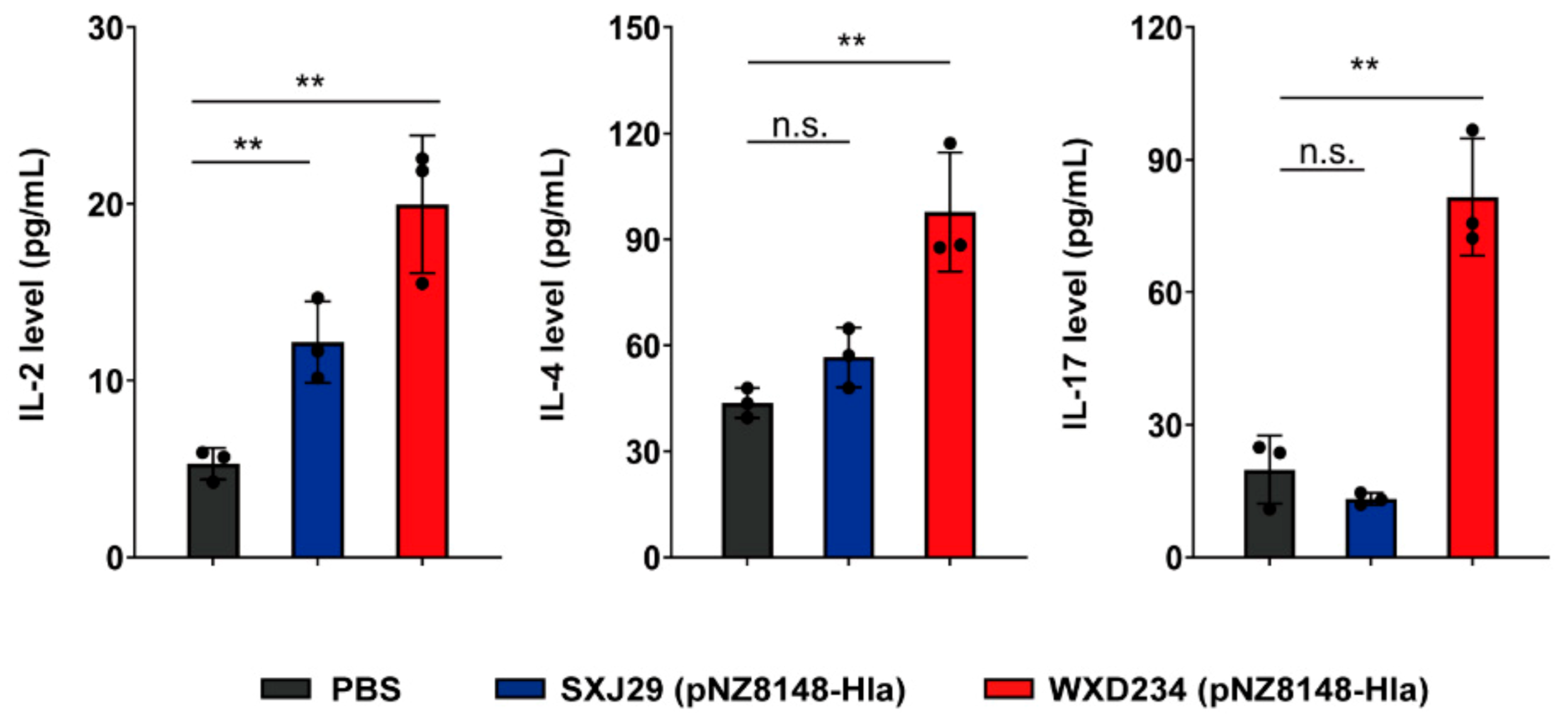
3.5. Recombinant Lactobacillus Enhanced Production of IL-2, IL-4, and IL-17 in Mesenteric Lymphatic Tissue
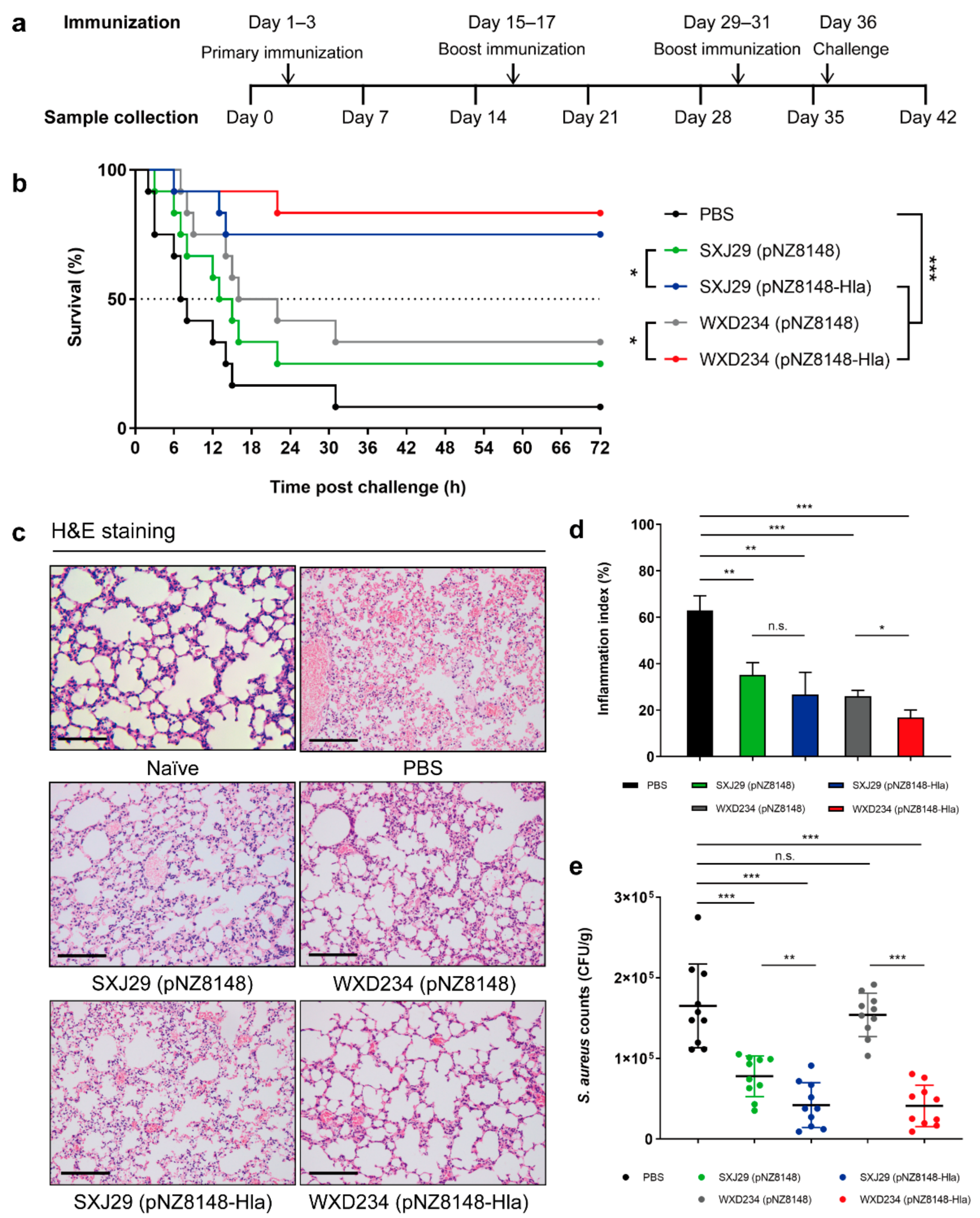
3.6. Recombinant Lactobacillus Effectively Protected Mice against S. aureus-Induced Pulmonary Infection
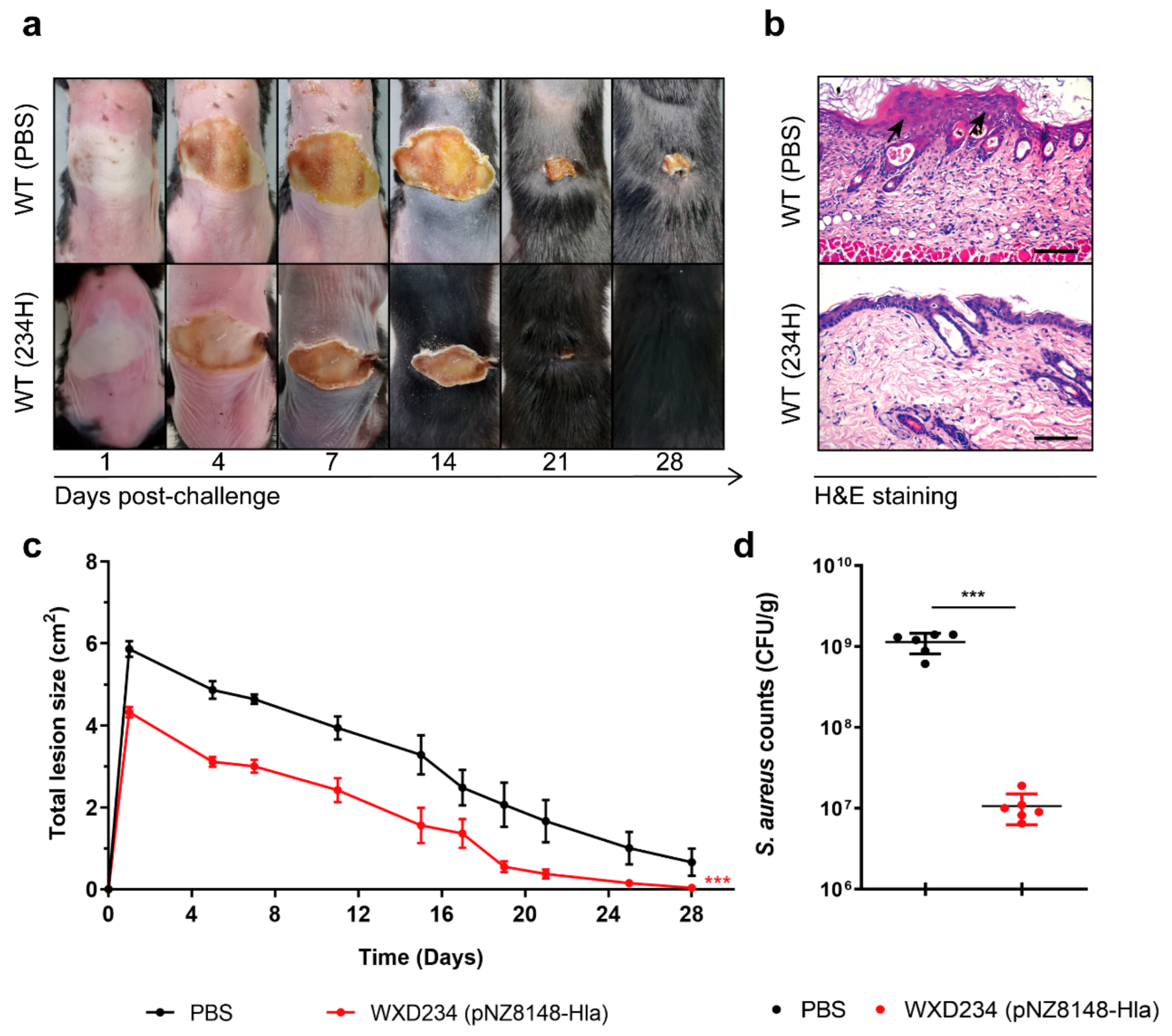
3.7. Recombinant Lactobacillus Promoted Resistance to S. aureus-Induced Skin Infection
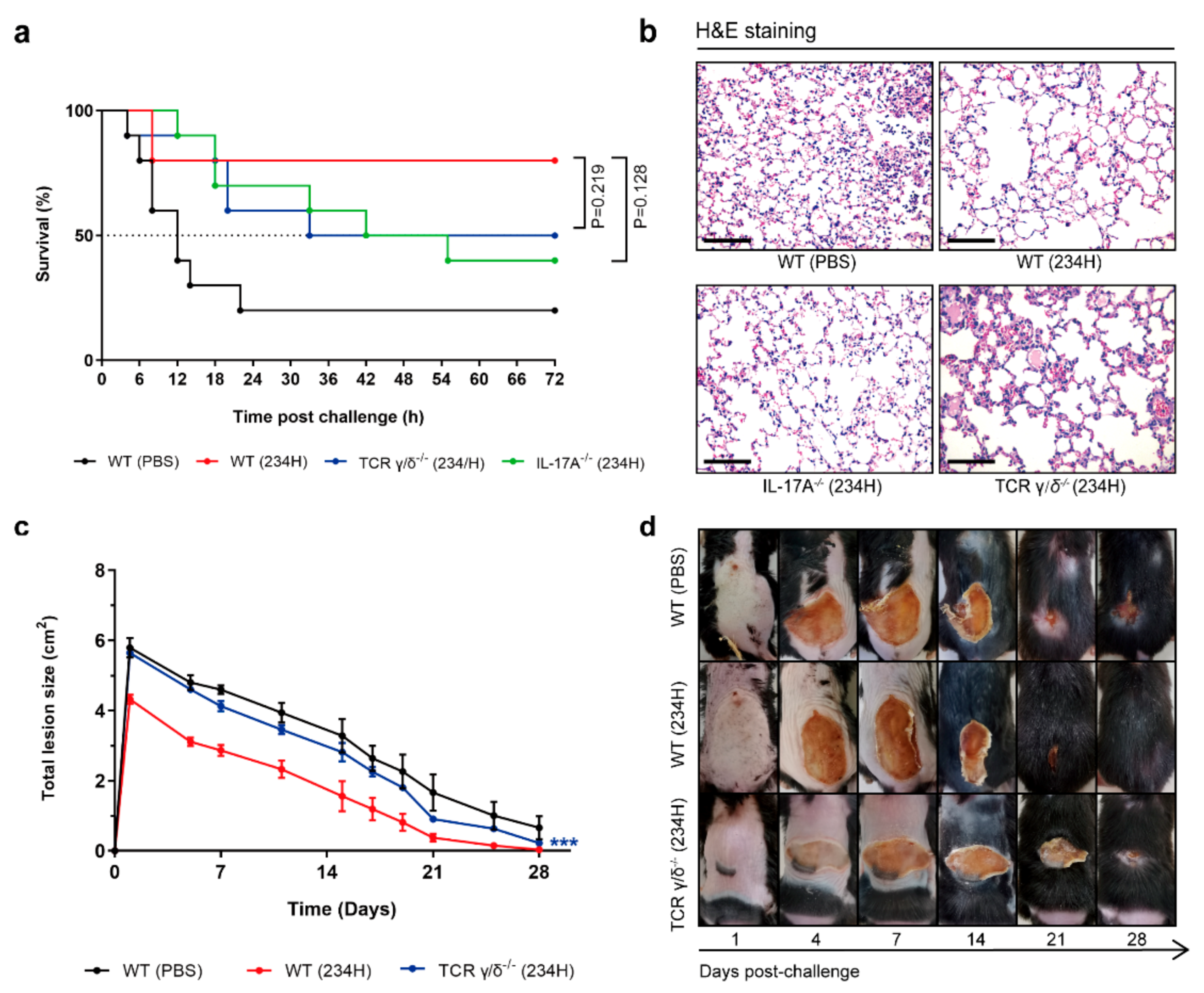
3.8. Recombinant Lactobacillus Lost Superior Protection Post Vaccination in TCR γ/δ-Deficient or IL-17A-Deficient Mice
| Strain or Plasmid | Description | Source or Reference |
|---|---|---|
| Strains | ||
| SXJ29 | WT Lactobacillus kefiri | Isolated from dairy products |
| WXD234 | WT Lactobacillus plantarum | Isolated from dairy products |
| SXJ29 (pNZ8148) | Derivative of SXJ29 carrying pNZ8148 | This study |
| WXD234 (pNZ8148) | Derivative of WXD234 carrying pNZ8148 | This study |
| SXJ29 (pNZ8148-Hla) | Derivative of SXJ29 carrying pNZ8148-Hla | This study |
| WXD234 (pNZ8148-Hla) | Derivative of WXD234 carrying pNZ8148-Hla | This study |
| SXJ29 (pNZ8148-EGFP) | Derivative of SXJ29 carrying pNZ8148-EGFP | This study |
| WXD234 (pNZ8148-EGFP) | Derivative of WXD234 carrying pNZ8148-EGFP | This study |
| S. aureus USA300 | S. aureus USA300 | American Type Culture Collection (Manassas, VA, USA) |
| Plasmids | ||
| pUC57-Hla | Ampr; pUC57::SPslpA::his-tag::hlaH35L | GenScript Bio Co. |
| pNZ8148 | Cmr; L. lactis expression vector; PnisA promoter | Nanjing ZFdows Bio Co. [44] |
| pNZ8148-Hla | Cmr; pNZ8148::SPslpA::his-tag::hlaH35L | This study |
| pNZ8148-EGFP | Cmr; pNZ8148::egfp | This study |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [Green Version]
- Lucero, C.A.; Hageman, J.; Zell, E.R.; Bulens, S.; Nadle, J.; Petit, S.; Gershman, K.; Ray, S.; Harrison, L.H.; Lynfield, R.; et al. Evaluating the potential public health impact of a Staphylococcus aureus vaccine through use of population-based surveillance for invasive methicillin-resistant S. aureus disease in the United States. Vaccine 2009, 27, 5061–5068. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Jha, R.K.; Mishra, S.K.; Tiwari, B.R.; Asaad, A.M. Recent advances in Staphylococcus aureus infection: Focus on vaccine development. Infect. Drug Resist. 2019, 12, 1243–1255. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, E.C.; McLoughlin, R.M. Considering the ’alternatives’ for next-generation anti-Staphylococcus aureus vaccine development. Trends Mol. Med. 2019, 25, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.S.; Fowler, V.G.; Shukla, S.K.; Rose, W.E.; Proctor, R.A. Development of a vaccine against Staphylococcus aureus invasive infections: Evidence based on human immunity, genetics and bacterial evasion mechanisms. FEMS Microbiol. Rev. 2020, 44, 123–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chumakov, K.; Ehrenfeld, E.; Wimmer, E.; Agol, V.I. Vaccination against polio should not be stopped. Nat. Rev. Microbiol. 2007, 5, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Longini, I.M., Jr.; Nizam, A.; Ali, M.; Yunus, M.; Shenvi, N.; Clemens, J.D. Controlling endemic cholera with oral vaccines. PLoS Med. 2007, 4, e336. [Google Scholar] [CrossRef]
- Avtushenko, S.S.; Sorokin, E.M.; Zoschenkova, N.Y.; Zacharova, N.G.; Naichin, A.N. Clinical and immunological characteristics of the emulsion form of inactivated influenza vaccine delivered by oral immunization. J. Biotechnol. 1996, 44, 21–28. [Google Scholar] [CrossRef]
- Lazzell, V.; Waldman, R.H.; Rose, C.; Khakoo, R.; Jacknowitz, A.; Howard, S. Immunization against influenza in humans using an oral enteric-coated killed virus vaccine. J. Biol. Stand. 1984, 12, 315–321. [Google Scholar] [CrossRef]
- Seo, S.U.; Kim, J.J.; Yang, H.; Kwon, H.J.; Yang, J.Y.; Curtiss Iii, R.; Kweon, M.N. Effective protection against secondary pneumococcal pneumonia by oral vaccination with attenuated Salmonella delivering PspA antigen in mice. Vaccine 2012, 30, 6816–6823. [Google Scholar] [CrossRef]
- García, V.E.; Iglesias, M.F.; Cerquetti, M.C.; Gómez, M.I.; Sordelli, D.O. Interaction between granulocytes and antibodies in the enhancement of lung defenses against Staphylococcus aureus after intranasal immunization of mice with live-attenuated bacteria. FEMS Immunol. Med. Microbiol. 1994, 9, 55–63. [Google Scholar] [CrossRef]
- Narita, K.; Hu, D.L.; Tsuji, T.; Nakane, A. Intranasal immunization of mutant toxic shock syndrome toxin 1 elicits systemic and mucosal immune response against Staphylococcus aureus infection. FEMS Immunol. Med. Microbiol. 2008, 52, 389–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castagliuolo, I.; Piccinini, R.; Beggiao, E.; Palù, G.; Mengoli, C.; Ditadi, F.; Vicenzoni, G.; Zecconi, A. Mucosal genetic immunization against four adhesins protects against Staphylococcus aureus-induced mastitis in mice. Vaccine 2006, 24, 4393–4402. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wei, C.; Liu, B.; Jing, H.; Feng, Q.; Tong, Y.; Yang, Y.; Yang, L.; Zuo, Q.; Zhang, Y.; et al. Induction of systemic and mucosal immunity against methicillin-resistant Staphylococcus aureus infection by a novel nanoemulsion adjuvant vaccine. Int. J. Nanomed. 2015, 10, 7275–7290. [Google Scholar] [CrossRef] [Green Version]
- Irene, C.; Fantappiè, L.; Caproni, E.; Zerbini, F.; Anesi, A.; Tomasi, M.; Zanella, I.; Stupia, S.; Prete, S.; Valensin, S.; et al. Bacterial outer membrane vesicles engineered with lipidated antigens as a platform for Staphylococcus aureus vaccine. Proc. Natl. Acad. Sci. USA 2019, 116, 21780–21788. [Google Scholar] [CrossRef] [Green Version]
- Guglielmotti, D.M.; Marcó, M.; Golowczyc, M.; Reinheimer, J.A.; Quiberoni, A. Probiotic potential of Lactobacillus delbrueckii strains and their phage resistant mutants. Int. Dairy J. 2007, 17, 916–925. [Google Scholar] [CrossRef]
- Oliveira, M.L.; Arêas, A.P.; Campos, I.B.; Monedero, V.; Perez-Martínez, G.; Miyaji, E.N.; Leite, L.C.; Aires, K.A.; Lee Ho, P. Induction of systemic and mucosal immune response and decrease in Streptococcus pneumoniae colonization by nasal inoculation of mice with recombinant lactic acid bacteria expressing pneumococcal surface antigen A. Microbes Infect. 2006, 8, 1016–1024. [Google Scholar] [CrossRef]
- Ho, P.S.; Kwang, J.; Lee, Y.K. Intragastric administration of Lactobacillus casei expressing transmissible gastroentritis coronavirus spike glycoprotein induced specific antibody production. Vaccine 2005, 23, 1335–1342. [Google Scholar] [CrossRef]
- Mohamadzadeh, M.; Duong, T.; Sandwick, S.J.; Hoover, T.; Klaenhammer, T.R. Dendritic cell targeting of Bacillus anthracis protective antigen expressed by Lactobacillus acidophilus protects mice from lethal challenge. Proc. Natl. Acad. Sci. USA 2009, 106, 4331–4336. [Google Scholar] [CrossRef] [Green Version]
- Zegers, N.D.; Kluter, E.; van Der Stap, H.; van Dura, E.; van Dalen, P.; Shaw, M.; Baillie, L. Expression of the protective antigen of Bacillus anthracis by Lactobacillus casei: Towards the development of an oral vaccine against anthrax. J. Appl. Microbiol. 1999, 87, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Pant, N.; Hultberg, A.; Zhao, Y.; Svensson, L.; Pan-Hammarstrom, Q.; Johansen, K.; Pouwels, P.H.; Ruggeri, F.M.; Hermans, P.; Frenken, L.; et al. Lactobacilli expressing variable domain of llama heavy-chain antibody fragments (lactobodies) confer protection against rotavirus-induced diarrhea. J. Infect. Dis. 2006, 194, 1580–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, D.M.; Gaerthé, B.; Leer, R.J.; Van Der Stap, J.G.; Smittenaar, C.; Heijne Den Bak-Glashouwer, M.; Thole, J.E.; Tielen, F.J.; Pouwels, P.H.; Havenith, C.E. Engineering the microflora to vaccinate the mucosa: Serum immunoglobulin G responses and activated draining cervical lymph nodes following mucosal application of tetanus toxin fragment C-expressing lactobacilli. Immunology 2000, 100, 510–518. [Google Scholar] [CrossRef]
- Xiu, L.; Sheng, S.; Hu, Z.; Liu, Y.; Li, J.; Zhang, H.; Liang, Y.; Du, R.; Wang, X. Exopolysaccharides from Lactobacillus kiferi as adjuvant enhanced the immuno-protective against Staphylococcus aureus infection. Int. J. Biol. Macromol. 2020, 161, 10–23. [Google Scholar] [CrossRef]
- Menzies, B.E.; Kernodle, D.S. Site-directed mutagenesis of the alpha-toxin gene of Staphylococcus aureus: Role of histidines in toxin activity in vitro and in a murine model. Infect. Immun. 1994, 62, 1843–1847. [Google Scholar] [CrossRef] [Green Version]
- Menzies, B.E.; Kernodle, D.S. Passive immunization with antiserum to a nontoxic alpha-toxin mutant from Staphylococcus aureus is protective in a murine model. Infect. Immun. 1996, 64, 1839–1841. [Google Scholar] [CrossRef] [Green Version]
- Vidgrén, G.; Palva, I.; Pakkanen, R.; Lounatmaa, K.; Palva, A. S-layer protein gene of Lactobacillus brevis: Cloning by polymerase chain reaction and determination of the nucleotide sequence. J. Bacteriol. 1992, 174, 7419–7427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maragkoudakis, P.A.; Zoumpopoulou, G.; Miaris, C.; Kalantzopoulos, G.; Pot, B.; Tsakalidou, E. Probiotic potential of Lactobacillus strains isolated from dairy products. Int. Dairy J. 2006, 16, 189–199. [Google Scholar] [CrossRef]
- Song, B.F.; Ju, L.Z.; Li, Y.J.; Tang, L.J. Chromosomal insertions in the Lactobacillus casei upp gene that are useful for vaccine expression. Appl. Environ. Microbiol. 2014, 80, 3321–3326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyons, A.B.; Parish, C.R. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 1994, 171, 131–137. [Google Scholar] [CrossRef]
- Han, Y.; Renu, S.; Patil, V.; Schrock, J.; Feliciano-Ruiz, N.; Selvaraj, R.; Renukaradhya, G.J. Mannose-Modified Chitosan-Nanoparticle-Based Salmonella Subunit OralVaccine-Induced Immune Response and Efficacy in a Challenge Trial in Broilers. Vaccines 2020, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. Th1/Th2 cells. Inflamm. Bowel Dis. 1999, 5, 285–294. [Google Scholar] [CrossRef]
- Bedoya, S.K.; Lam, B.; Lau, K.; Larkin, J., 3rd. Th17 cells in immunity and autoimmunity. Clin. Dev. Immunol. 2013, 2013, 986789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Gu, W.; Sun, B. TH1/TH2 cell differentiation and molecular signals. Adv. Exp. Med. Biol. 2014, 841, 15–44. [Google Scholar] [CrossRef]
- Gao, X.; Ma, Y.; Wang, Z.; Bai, J.; Jia, S.; Feng, B.; Jiang, Y.; Cui, W.; Tang, L.; Li, Y.; et al. Oral immunization of mice with a probiotic Lactobacillus casei constitutively expressing the α-toxoid induces protective immunity against Clostridium perfringens α-toxin. Virulence 2019, 10, 166–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations (*). Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef] [Green Version]
- Lalor, S.J.; McLoughlin, R.M. Memory γδ T cells-newly appreciated protagonists in infection and immunity. Trends Immunol. 2016, 37, 690–702. [Google Scholar] [CrossRef]
- Holtmeier, W. Compartmentalization gamma/delta T cells and their putative role in mucosal immunity. Crit Rev. Immunol. 2003, 23, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Dillen, C.A.; Pinsker, B.L.; Marusina, A.I.; Merleev, A.A.; Farber, O.N.; Liu, H.; Archer, N.K.; Lee, D.B.; Wang, Y.; Ortines, R.V.; et al. Clonally expanded γδ T cells protect against Staphylococcus aureus skin reinfection. J. Clin. Investig. 2018, 128, 1026–1042. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Pietras, E.M.; Garcia, N.C.; Ramos, R.I.; Farzam, D.M.; Monroe, H.R.; Magorien, J.E.; Blauvelt, A.; Kolls, J.K.; Cheung, A.L.; et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Investig. 2010, 120, 1762–1773. [Google Scholar] [CrossRef] [Green Version]
- Mierau, I.; Kleerebezem, M. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl. Environ. Microbiol. 2005, 68, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, A.; Wincker, P.; Mauger, S.; Jaillon, O.; Malarme, K.; Weissenbach, J.; Ehrlich, S.D.; Sorokin, A. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 2001, 11, 731–753. [Google Scholar] [CrossRef] [Green Version]
- Duwat, P.; Cesselin, B.; Sourice, S.; Gruss, A. Lactococcus lactis, a bacterial model for stress responses and survival. Int. J. Food Microbiol. 2000, 55, 83–86. [Google Scholar] [CrossRef]
- Poquet, I.; Ehrlich, S.D.; Gruss, A. An export-specific reporter designed for gram-positive bacteria: Application to Lactococcus lactis. J. Bacteriol. 1998, 180, 1904–1912. [Google Scholar] [CrossRef] [Green Version]
- Ravn, P.; Arnau, J.; Madsen, S.M.; Vrang, A.; Israelsen, H. The development of TnNuc and its use for the isolation of novel secretion signals in Lactococcus lactis. Gene 2000, 242, 347–356. [Google Scholar] [CrossRef]
- Yu, M.; Wang, L.; Ma, S.; Wang, X.; Wang, Y.; Xiao, Y.; Jiang, Y.; Qiao, X.; Tang, L.; Xu, Y.; et al. Immunogenicity of eGFP-marked recombinant Lactobacillus casei against transmissible gastroenteritis virus and porcine epidemic diarrhea virus. Viruses 2017, 9, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, L.; Huang, X.; Ma, S.; Yu, M.; Shi, W.; Qiao, X.; Tang, L.; Xu, Y.; Li, Y. Oral delivery of probiotics expressing dendritic cell-targeting peptide fused with porcine epidemic diarrhea virus COE Antigen: A promising vaccine strategy against PEDV. Viruses 2017, 9, 312. [Google Scholar] [CrossRef]
- Ma, S.; Wang, L.; Huang, X.; Wang, X.; Chen, S.; Shi, W.; Qiao, X.; Jiang, Y.; Tang, L.; Xu, Y.; et al. Oral recombinant Lactobacillus vaccine targeting the intestinal microfold cells and dendritic cells for delivering the core neutralizing epitope of porcine epidemic diarrhea virus. Microb. Cell Fact. 2018, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M.; Hatano, Y.; Williams, M.L. Basis for the barrier abnormality in atopic dermatitis: Outside-inside-outside pathogenic mechanisms. J. Allergy Clin. Immunol. 2008, 121, 1337–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.W.; Choi, E.B.; Min, T.K.; Kim, J.H.; Kim, M.H.; Jeon, S.G.; Lee, B.J.; Gho, Y.S.; Jee, Y.K.; Pyun, B.Y.; et al. An important role of α-hemolysin in extracellular vesicles on the development of atopic dermatitis induced by Staphylococcus aureus. PLoS ONE 2014, 9, e100499. [Google Scholar] [CrossRef]
- Song, L.; Hobaugh, M.R.; Shustak, C.; Cheley, S.; Bayley, H.; Gouaux, J.E. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science 1996, 274, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.D.; Bubeck Wardenburg, J.; Gardner, D.J.; Long, D.; Whitney, A.R.; Braughton, K.R.; Schneewind, O.; DeLeo, F.R. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J. Infect. Dis. 2010, 202, 1050–1058. [Google Scholar] [CrossRef] [Green Version]
- Bubeck Wardenburg, J.; Schneewind, O. Vaccine protection against Staphylococcus aureus pneumonia. J. Exp. Med. 2008, 205, 287–294. [Google Scholar] [CrossRef]
- Seegers, J.F. Lactobacilli as live vaccine delivery vectors: Progress and prospects. Trends Biotechnol. 2002, 20, 508–515. [Google Scholar] [CrossRef]
- Mao, R.; Zhou, K.; Han, Z.; Wang, Y. Subtilisin QK-2: Secretory expression in Lactococcus lactis and surface display onto gram-positive enhancer matrix (GEM) particles. Microb. Cell Fact. 2016, 15, 80. [Google Scholar] [CrossRef] [Green Version]
- Mao, R.; Wu, D.; Hu, S.; Zhou, K.; Wang, M.; Wang, Y. Secretory expression and surface display of a new and biologically active single-chain insulin (SCI-59) analog by lactic acid bacteria. Appl. Environ. Microbiol. 2017, 101, 3259–3271. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhong, J.; Liang, X.; Liu, W.; Huan, L. Improvement of human interferon alpha secretion by Lactococcus lactis. Biotechnol. Lett. 2010, 32, 1271–1277. [Google Scholar] [CrossRef] [Green Version]
- Kidd, P. Th1/Th2 balance: The hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 2003, 8, 223–246. [Google Scholar]
- Magram, J.; Connaughton, S.E.; Warrier, R.R.; Carvajal, D.M.; Wu, C.-y.; Ferrante, J.; Stewart, C.; Sarmiento, U.; Faherty, D.A.; Gately, M.K. IL-12-deficient mice are defective in IFN-γ production and type 1 cytokine responses. Immunity 1996, 4, 471–481. [Google Scholar] [CrossRef] [Green Version]
- VanCott, J.L.; Staats, H.F.; Pascual, D.W.; Roberts, M.; Chatfield, S.N.; Yamamoto, M.; Coste, M.; Carter, P.B.; Kiyono, H.; McGhee, J.R. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J. Immunol. 1996, 156, 1504–1514. [Google Scholar] [PubMed]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.S.; Compans, R.W.; Kang, S.M. Oral vaccination with inactivated influenza vaccine induces cross-protective immunity. Vaccine 2012, 30, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Worbs, T.; Bode, U.; Yan, S.; Hoffmann, M.W.; Hintzen, G.; Bernhardt, G.; Förster, R.; Pabst, O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J. Exp. Med. 2006, 203, 519–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licciardi, P.V.; Tang, M.L. Vaccine adjuvant properties of probiotic bacteria. Discov. Med. 2011, 12, 525–533. [Google Scholar] [PubMed]
- Zhang, Z.; Lv, J.; Pan, L.; Zhang, Y. Roles and applications of probiotic Lactobacillus strains. Appl. Environ. Microbiol. 2018, 102, 8135–8143. [Google Scholar] [CrossRef] [PubMed]
- Aktas, B.; De Wolfe, T.J.; Safdar, N.; Darien, B.J.; Steele, J.L. The impact of Lactobacillus casei on the composition of the cecal microbiota and innate immune system is strain specific. PLoS ONE 2016, 11, e0156374. [Google Scholar] [CrossRef]
- Sisto, A.; Luongo, D.; Treppiccione, L.; De Bellis, P.; Di Venere, D.; Lavermicocca, P.; Rossi, M. Effect of Lactobacillus paracasei culture filtrates and artichoke polyphenols on cytokine production by dendritic cells. Nutrients 2016, 8, 635. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Chen, X.; Liu, M.; Guo, X. Effect of three lactobacilli with strain-specific activities on the growth performance, faecal microbiota and ileum mucosa proteomics of piglets. J. Anim. Sci. Biotechnol. 2017, 8, 52. [Google Scholar] [CrossRef]
- Bagnoli, F.; Bertholet, S.; Grandi, G. Inferring reasons for the failure of Staphylococcus aureus vaccines in clinical trials. Front. Cell Infect. Microbiol. 2012, 2, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proctor, R.A. Challenges for a universal Staphylococcus aureus vaccine. Clin. Infect. Dis. 2012, 54, 1179–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scully, I.L.; Liberator, P.A.; Jansen, K.U.; Anderson, A.S. Covering all the bases: Preclinical development of an effective Staphylococcus aureus vaccine. Front. Immunol. 2014, 5, 109. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.R.; Lee, K.S.; Park, S.J.; Min, K.H.; Lee, K.Y.; Choe, Y.H.; Lee, Y.R.; Kim, J.S.; Hong, S.J.; Lee, Y.C. PTEN down-regulates IL-17 expression in a murine model of toluene diisocyanate-induced airway disease. J. Immunol. 2007, 179, 6820–6829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miossec, P.; Kolls, J.K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov. 2012, 11, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; He, X.; Li, X.; Qian, Y. The roles and functional mechanisms of interleukin-17 family cytokines in mucosal immunity. Cell Mol. Immunol. 2016, 13, 418–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, P.; Liu, T.; Zhou, W.Y.; Zhuang, Y.; Peng, L.S.; Zhang, J.Y.; Yin, Z.N.; Mao, X.H.; Guo, G.; Shi, Y.; et al. Role of gamma-delta T cells in host response against Staphylococcus aureus-induced pneumonia. BMC Immunol. 2012, 13, 38. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, N.; Liu, B.; Bao, X.; Zhang, H.; Sheng, S.; Liang, Y.; Pan, H.; Wang, X. Oral Delivery of Novel Recombinant Lactobacillus Elicit High Protection against Staphylococcus aureus Pulmonary and Skin Infections. Vaccines 2021, 9, 984. https://doi.org/10.3390/vaccines9090984
Pan N, Liu B, Bao X, Zhang H, Sheng S, Liang Y, Pan H, Wang X. Oral Delivery of Novel Recombinant Lactobacillus Elicit High Protection against Staphylococcus aureus Pulmonary and Skin Infections. Vaccines. 2021; 9(9):984. https://doi.org/10.3390/vaccines9090984
Chicago/Turabian StylePan, Na, Bohui Liu, Xuemei Bao, Haochi Zhang, Shouxin Sheng, Yanchen Liang, Haiting Pan, and Xiao Wang. 2021. "Oral Delivery of Novel Recombinant Lactobacillus Elicit High Protection against Staphylococcus aureus Pulmonary and Skin Infections" Vaccines 9, no. 9: 984. https://doi.org/10.3390/vaccines9090984
APA StylePan, N., Liu, B., Bao, X., Zhang, H., Sheng, S., Liang, Y., Pan, H., & Wang, X. (2021). Oral Delivery of Novel Recombinant Lactobacillus Elicit High Protection against Staphylococcus aureus Pulmonary and Skin Infections. Vaccines, 9(9), 984. https://doi.org/10.3390/vaccines9090984






