Amine-Containing Membranes with Functionalized Multi-Walled Carbon Nanotubes for CO2/H2 Separation
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Dispersion and Modification of MWNTs
2.3. Membrane Synthesis
2.4. Characterization
3. Results and Discussion
3.1. Characterization of MWNTs
3.2. Effect of AF-MWNT Loading on the Amine Content of the Membrane
3.3. Effect of AF-MWNT Loading on Membrane Performance at a Low Feed Pressure
3.4. Effect of AF-MWNT Loading on Membrane Performance at a High Feed Pressure
3.5. Comparison with Literature Data
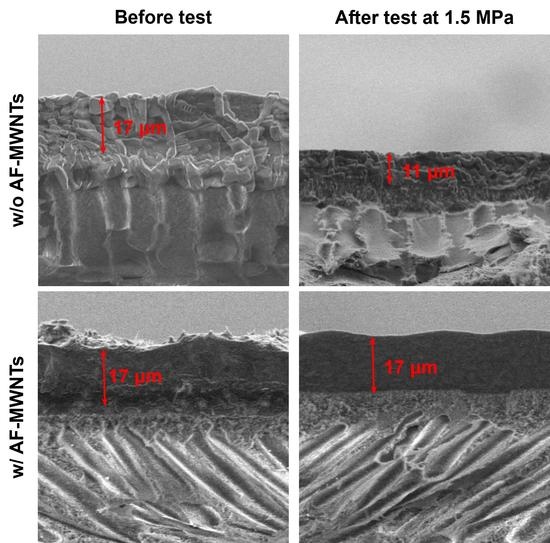
3.6. Effect of AF-MWNT Loading on Membrane Stability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roh, H.-S.; Jun, K.-W.; Dong, W.-S.; Chang, J.-S.; Park, S.-E.; Joe, Y.-I. Highly active and stable Ni/Ce-ZrO2 catalyst for H2 production from methane. J. Mol. Catal. A Chem. 2002, 181, 137–142. [Google Scholar] [CrossRef]
- Merkel, T.C.; Zhou, M.; Baker, R.W. Carbon dioxide capture with membranes at an IGCC power plant. J. Membr. Sci. 2012, 389, 441–450. [Google Scholar] [CrossRef]
- Momirlan, M.; Veziroglu, T.N. The properties of hydrogen as fuel tomorrow in sustainable energy system for a cleaner planet. Int. J. Hydrogen Energy 2005, 30, 795–802. [Google Scholar] [CrossRef]
- Ramasubramanian, K.; Zhao, Y.; Ho, W.S.W. CO2 capture and H2 purification: Prospects for CO2-selective membrane processes. AlChE J. 2013, 59, 1033–1045. [Google Scholar] [CrossRef]
- Dijkstra, J.; Jansen, D. Novel concepts for CO2 capture. Energy 2004, 29, 1249–1257. [Google Scholar] [CrossRef]
- Ho, W.S.W.; Sirkar, K. Membrane Handbook; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Ho, W.S.W.; Dalrymple, D. Facilitated transport of olefins in Ag+-containing polymer membranes. J. Membr. Sci. 1994, 91, 13–25. [Google Scholar] [CrossRef]
- Zhao, Y.; Ho, W.S.W. CO2-selective membranes containing sterically hindered amines for CO2/H2 separation. Ind. Eng. Chem. Res. 2012, 52, 8774–8782. [Google Scholar] [CrossRef]
- Quinn, R.; Laciak, D.; Pez, G. Polyelectrolyte-salt blend membranes for acid gas separations. J. Membr. Sci. 1997, 131, 61–69. [Google Scholar] [CrossRef]
- Okada, O.; Teramoto, M.; Yegani, R.; Matsuyama, H.; Shimada, K.; Morimoto, K. CO2-Facilitated Transport Membrane and Method for Producing the Same. U.S. Patent 8,197,576, 12 June 2012. [Google Scholar]
- Zou, J.; Ho, W.S.W. CO2-selective polymeric membranes containing amines in crosslinked poly(vinyl alcohol). J. Membr. Sci. 2006, 286, 310–321. [Google Scholar] [CrossRef]
- Deng, L.; Kim, T.-J.; Hägg, M.-B. Facilitated transport of CO2 in novel PVAm/PVA blend membrane. J. Membr. Sci. 2009, 340, 154–163. [Google Scholar] [CrossRef]
- Dimilia, R.A.; Reed, J.S. Dependence of compaction on the glass transition temperature of the binder plase. Am. Ceram. Soc. Bull. 1983, 62, 484. [Google Scholar]
- Zhao, Y.; Jung, B.T.; Ansaloni, L.; Ho, W.S.W. Multiwalled carbon nanotube mixed matrix membranes containing amines for high pressure CO2/H2 separation. J. Membr. Sci. 2014, 459, 233–243. [Google Scholar] [CrossRef]
- Han, Y.; Wu, D.; Ho, W.S.W. Nanotube-reinforced facilitated transport membrane for CO2/N2 separation with vacuum operation. J. Membr. Sci. 2018, 567, 261–271. [Google Scholar] [CrossRef]
- Deng, L.; Hägg, M.-B. Carbon nanotube reinforced PVAm/PVA blend FSC nanocomposite membrane for CO2/CH4 separation. Int. J. Greenh. Gas Con. 2014, 26, 127–134. [Google Scholar] [CrossRef]
- Ansaloni, L.; Zhao, Y.; Jung, B.T.; Ramasubramanian, K.; Baschetti, M.G.; Ho, W.S.W. Facilitated transport membranes containing amino-functionalized multi-walled carbon nanotubes for high-pressure CO2 separations. J. Membr. Sci. 2015, 490, 18–28. [Google Scholar] [CrossRef]
- Van Damme, M.; Sap, W.; Van Aert, H. Processless Lithographic Printing Plate. U.S. Patent 6,790,595, 14 September 2004. [Google Scholar]
- Morgan, M.; Fielding, L.; Armes, S. Synthesis and characterisation of sterically stabilised polypyrrole particles using a chemically reactive poly(vinyl amine)-based stabiliser. Colloid. Polym. Sci. 2013, 291, 77–86. [Google Scholar] [CrossRef][Green Version]
- De Lannoy, C.-F.; Soyer, E.; Wiesner, M.R. Optimizing carbon nanotube-reinforced polysulfone ultrafiltration membranes through carboxylic acid functionalization. J. Membr. Sci. 2013, 447, 395–402. [Google Scholar] [CrossRef]
- Shanmugharaj, A.; Bae, J.; Lee, K.Y.; Noh, W.H.; Lee, S.H.; Ryu, S.H. Physical and chemical characteristics of multiwalled carbon nanotubes functionalized with aminosilane and its influence on the properties of natural rubber composites. Compos. Sci. Technol. 2007, 67, 1813–1822. [Google Scholar] [CrossRef]
- Xing, R.; Ho, W.S.W. Crosslinked polyvinylalcohol–polysiloxane/fumed silica mixed matrix membranes containing amines for CO2/H2 separation. J. Membr. Sci. 2011, 367, 91–102. [Google Scholar] [CrossRef]
- Chen, Y.; Ho, W.S.W. High-molecular-weight polyvinylamine/piperazine glycinate membranes for CO2 capture from flue gas. J. Membr. Sci. 2016, 514, 376–384. [Google Scholar] [CrossRef]
- Mawhinney, D.B.; Naumenko, V.; Kuznetsova, A.; Yates, J.T.; Liu, J.; Smalley, R. Infrared spectral evidence for the etching of carbon nanotubes: Ozone oxidation at 298 K. J. Am. Chem. Soc. 2000, 122, 2383–2384. [Google Scholar] [CrossRef]
- Shaffer, M.S.; Fan, X.; Windle, A. Dispersion and packing of carbon nanotubes. Carbon 1998, 36, 1603–1612. [Google Scholar] [CrossRef]
- Lee, G.-W.; Kim, J.; Yoon, J.; Bae, J.-S.; Shin, B.C.; Kim, I.S.; Oh, W.; Ree, M. Structural characterization of carboxylated multi-walled carbon nanotubes. Thin Solid Films 2008, 516, 5781–5784. [Google Scholar] [CrossRef]
- Bai, H.; Ho, W.S.W. Carbon dioxide-selective membranes for high-pressure synthesis gas purification. Ind. Eng. Chem. Res. 2011, 50, 12152–12161. [Google Scholar] [CrossRef]
- Kathi, J.; Rhee, K.-Y.; Lee, J.H. Effect of chemical functionalization of multi-walled carbon nanotubes with 3-aminopropyltriethoxysilane on mechanical and morphological properties of epoxy nanocomposites. Compos. Part A Appl. Sci. Manuf. 2009, 40, 800–809. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Du, J.; Wang, J.; Su, S.; Wilkie, C.A. Additional XPS studies on the degradation of poly (methyl methacrylate) and polystyrene nanocomposites. Polym. Degrad. Stab. 2004, 83, 29–34. [Google Scholar] [CrossRef]
- Graf, N.; Yegen, E.; Gross, T.; Lippitz, A.; Weigel, W.; Krakert, S.; Terfort, A.; Unger, W.E. XPS and NEXAFS studies of aliphatic and aromatic amine species on functionalized surfaces. Surf. Sci. 2009, 603, 2849–2860. [Google Scholar] [CrossRef]
- Beard, B.C. Cellulose nitrate as a binding energy reference in N (1s) XPS studies of nitrogen-containing organic molecules. Appl. Surf. Sci. 1990, 45, 221–227. [Google Scholar] [CrossRef]
- Dietrich, P.M.; Streeck, C.; Glamsch, S.; Ehlert, C.; Lippitz, A.; Nutsch, A.; Kulak, N.; Beckhoff, B.; Unger, W. Quantification of silane molecules on oxidized silicon: Are there options for a traceable and absolute determination? Anal. Chem. 2015, 87, 10117–10124. [Google Scholar] [CrossRef]
- Jakša, G.; Štefane, B.; Kovač, J. XPS and AFM characterization of aminosilanes with different numbers of bonding sites on a silicon wafer. Surf. Interface Anal. 2013, 45, 1709–1713. [Google Scholar] [CrossRef]
- Martin, H.J.; Schulz, K.H.; Bumgardner, J.D.; Walters, K.B. XPS study on the use of 3-aminopropyltriethoxysilane to bond chitosan to a titanium surface. Langmuir 2007, 23, 6645–6651. [Google Scholar] [CrossRef] [PubMed]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef]
- Ge, L.; Zhu, Z.; Rudolph, V. Enhanced gas permeability by fabricating functionalized multi-walled carbon nanotubes and polyethersulfone nanocomposite membrane. Sep. Purif. Technol. 2011, 78, 76–82. [Google Scholar] [CrossRef]
- Skoulidas, A.I.; Sholl, D.S.; Johnson, J.K. Adsorption and diffusion of carbon dioxide and nitrogen through single-walled carbon nanotube membranes. J. Chem. Phys. 2006, 124, 054708. [Google Scholar] [CrossRef]
- Kumar, P.P.; Rao, V.S.; Chandra, S.S. Deflection behavior of carbon nanotube reinforced polymer composite beams using first order shear deformation theory. Mater. Today 2018, 5, 26836–26842. [Google Scholar] [CrossRef]
- MacDonald, R.A.; Laurenzi, B.F.; Viswanathan, G.; Ajayan, P.M.; Stegemann, J.P. Collagen–carbon nanotube composite materials as scaffolds in tissue engineering. J. Biomed. Mater. Res. 2005, 74, 489–496. [Google Scholar] [CrossRef]
- Han, Y.; Ho, W.S.W. Recent advances in polymeric membranes for CO2 capture. Chin. J. Chem. Eng. 2018, 26, 2238–2254. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of permeability/selectivity tradeoff relations in polymeric gas separation membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Lin, H.; Van Wagner, E.; Freeman, B.D.; Toy, L.G.; Gupta, R.P. Plasticization-enhanced hydrogen purification using polymeric membranes. Science 2006, 311, 639–642. [Google Scholar] [CrossRef]
- Rowe, B.W.; Robeson, L.M.; Freeman, B.D.; Paul, D.R. Influence of temperature on the upper bound: Theoretical considerations and comparison with experimental results. J. Membr. Sci. 2010, 360, 58–69. [Google Scholar] [CrossRef]
- Härtel, G.; Püschel, T. Permselectivity of a PA6 membrane for the separation of a compressed CO2/H2 gas mixture at elevated pressures. J. Membr. Sci. 1999, 162, 1–8. [Google Scholar] [CrossRef]












| T (°C) | Pressure (MPa) | Dry Gas Flow Rate (cm3/min) | Relative Humidity (%) | |||
|---|---|---|---|---|---|---|
| Feed | Sweep | Feed | Sweep | Feed | Sweep | |
| 107 | 0.2 | 0.1 | 100 | 30 | 59.2 | 47.4 |
| 107 | 1.5 | 0.1 | 100 | 30 | 100 | 72.5 |
| Carriers | AC Value (mmol/g) |
|---|---|
| PVAm | 23.3 |
| AIBA-K | 14.4 |
| APTES | 4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Han, Y.; Pang, R.; Ho, W.S.W. Amine-Containing Membranes with Functionalized Multi-Walled Carbon Nanotubes for CO2/H2 Separation. Membranes 2020, 10, 333. https://doi.org/10.3390/membranes10110333
Yang Y, Han Y, Pang R, Ho WSW. Amine-Containing Membranes with Functionalized Multi-Walled Carbon Nanotubes for CO2/H2 Separation. Membranes. 2020; 10(11):333. https://doi.org/10.3390/membranes10110333
Chicago/Turabian StyleYang, Yutong, Yang Han, Ruizhi Pang, and W.S. Winston Ho. 2020. "Amine-Containing Membranes with Functionalized Multi-Walled Carbon Nanotubes for CO2/H2 Separation" Membranes 10, no. 11: 333. https://doi.org/10.3390/membranes10110333
APA StyleYang, Y., Han, Y., Pang, R., & Ho, W. S. W. (2020). Amine-Containing Membranes with Functionalized Multi-Walled Carbon Nanotubes for CO2/H2 Separation. Membranes, 10(11), 333. https://doi.org/10.3390/membranes10110333