Treatment of Olive Mill Wastewater through Integrated Pressure-Driven Membrane Processes
Abstract
:1. Introduction
1.1. OMW Composition
1.2. OMW Membrane-Based Treatment Processes
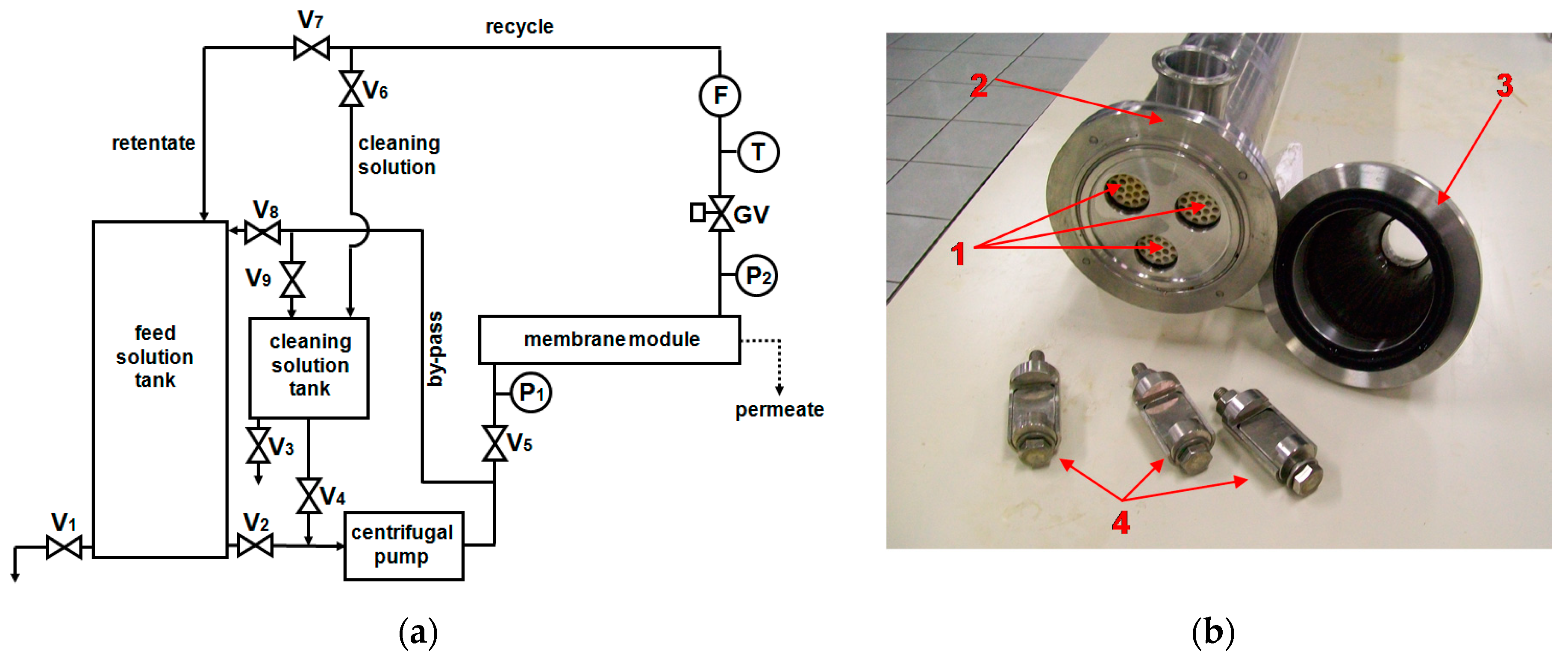
2. Materials and Methods
3. Results
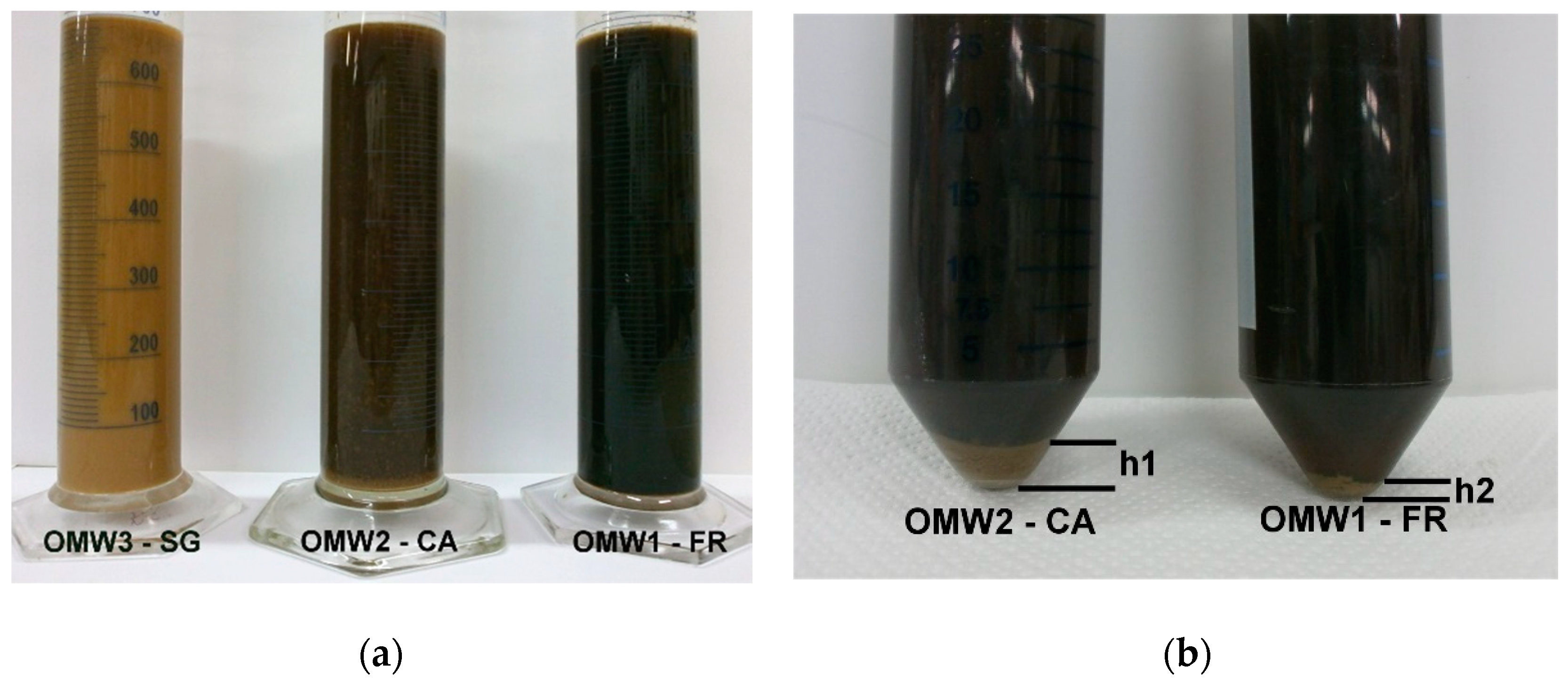
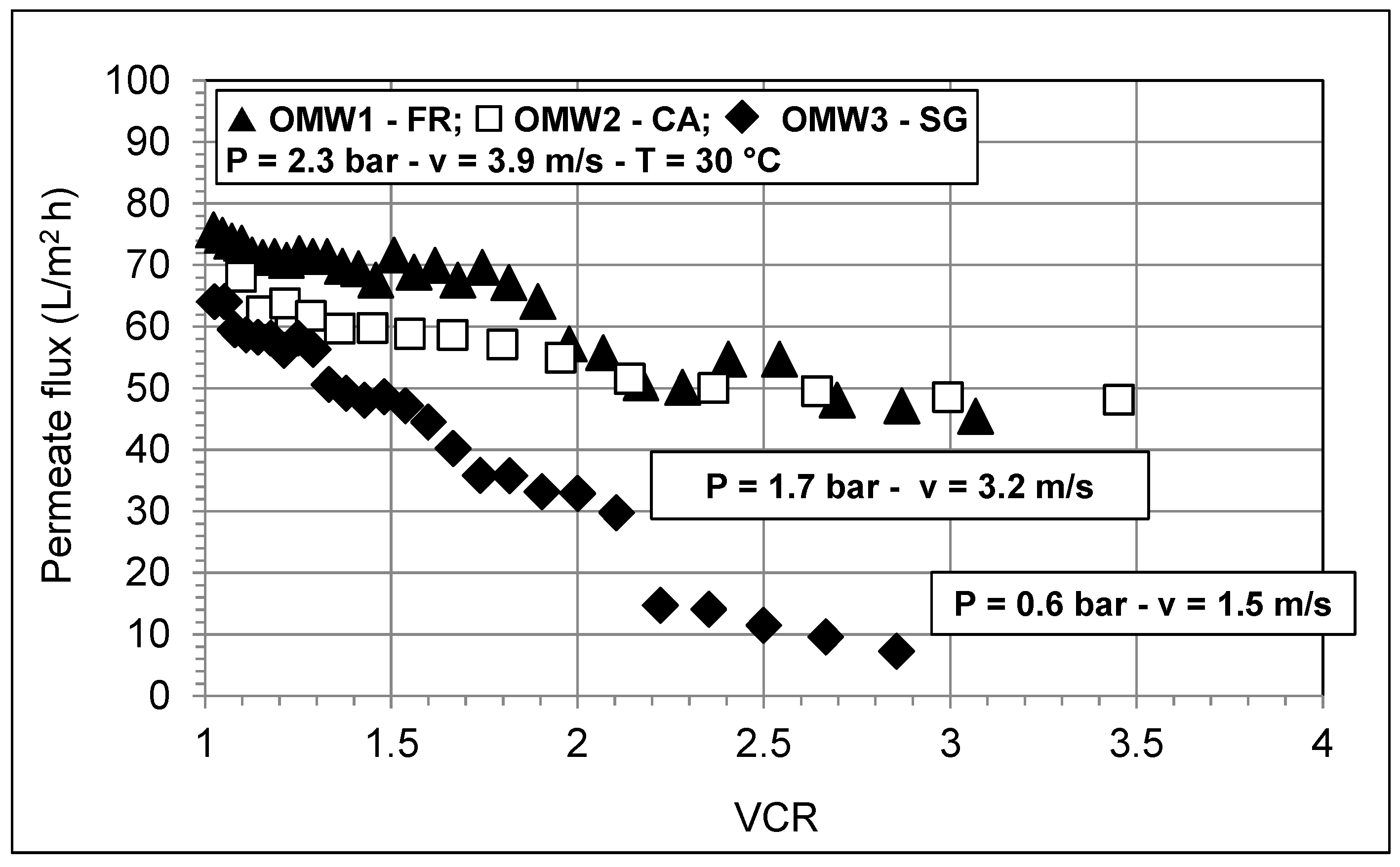
3.1. Feed Pretreatment and Microfiltration
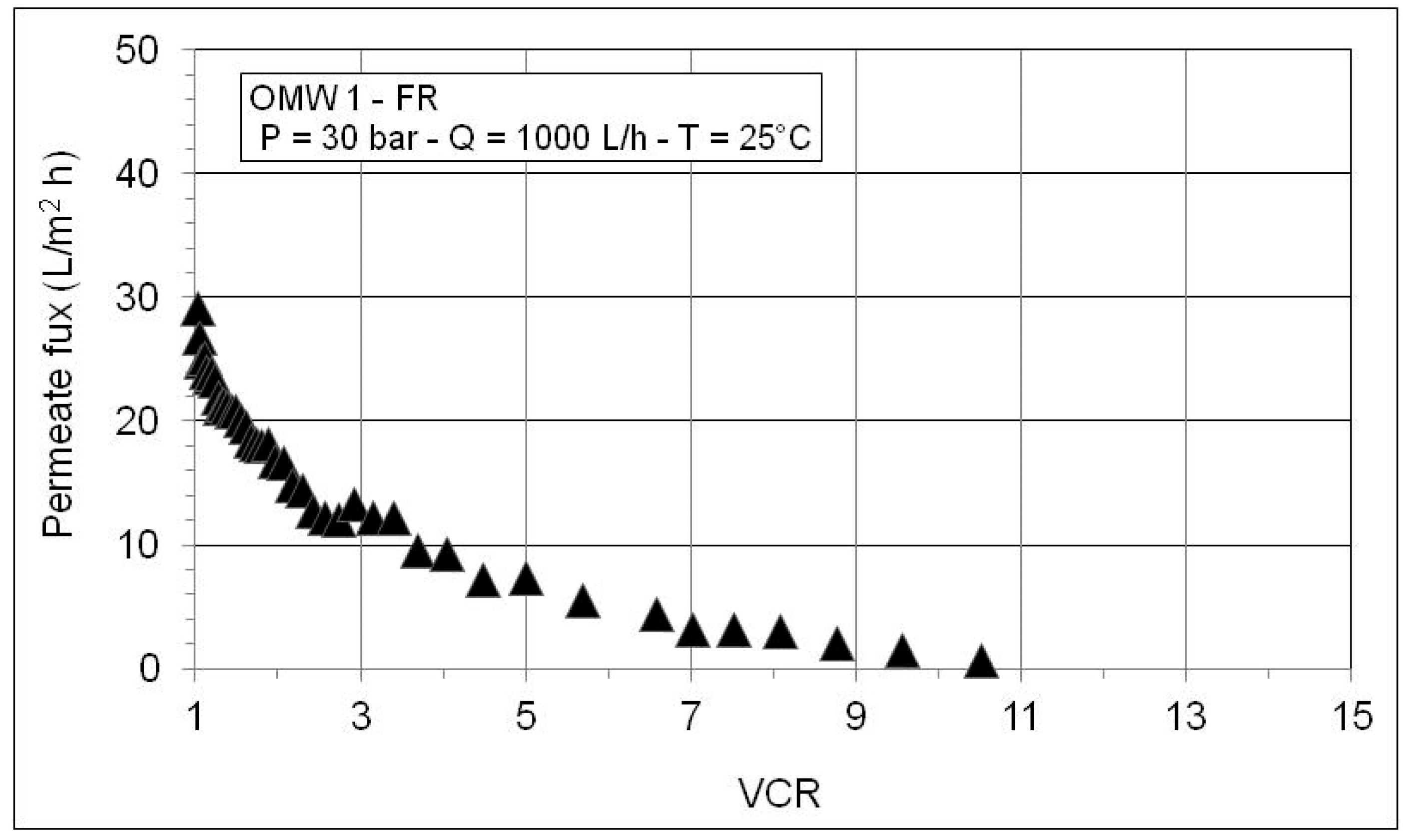
3.2. Nanofiltration and Reverse Osmosis
4. Discussion
4.1. Pretreatment and Microfiltration
4.2. Nanofiltration and Reverse Osmosis
4.3. Remarks
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Niaounakis, M.; Halvadakis, C.P. Olive Processing Waste Management: Literature Review and Patent Survey, 2nd ed.; Waste Management Series; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–498. [Google Scholar]
- Trends in World Olive Oil Consumption. Available online: https://www.oliveoilmarket.eu/trends-in-world-olive-oil-consumption (accessed on 10 August 2020).
- Consumption of Olive Oil Worldwide from 2012/13 to 2019/20. Available online: https://www.statista.com/statistics/940491/olive-oil-consumption-worldwide/ (accessed on 10 August 2020).
- Kiritsakis, A.K. Olive Oil: From the Tree to the Table; Food & Nutrition Press Inc.: Trumbull, CT, USA, 1998. [Google Scholar]
- Tsagaraki, E.; Lazarides, H.N.; Petrotos, K.B. Olive Mill Wastewater Treatment. In Utilization of By-Products and Treatment of Waste in the Food Industry; Oreopoulou, V., Russ, W., Eds.; Springer: Boston, MA, USA, 2007; pp. 133–157. [Google Scholar]
- Obied, H.K.; Bedgood, D.R., Jr.; Prenzler, P.D.; Robards, K. Bioscreening of Australian olive mill waste extracts: Biophenol content, antioxidant, antimicrobial and molluscicidal activities. Food Chem. Toxicol. 2007, 45, 1238–1248. [Google Scholar] [CrossRef]
- Caputo, C.; Scacchia, F.; Pelagagge, P.M. Disposal of by-products in olive oil industry: Waste-to-energy solutions. Appl. Therm. Eng. 2003, 23, 197–214. [Google Scholar] [CrossRef]
- Paraskeva, P.; Diamadopoulos, E. Technologies for olive mill wastewater (OMW) treatment: A review. J. Chem. Technol. Biotechnol. 2006, 81, 1475–1485. [Google Scholar] [CrossRef]
- Yonar, T.; Mert, B.K.; Kestioglu, K.; Hung, Y.-T. Treatment of olive oil production wastewaters. In Handbook of Environment and Waste Management: Air and Water Pollution Control; Hung, Y.-T., Wang, L.K., Shammas, N.K., Eds.; World Scientific Pub: Singapore, 2012; Chapter 22; pp. 979–1014. [Google Scholar]
- Turano, E.; Curcio, S.; De Paola, M.G.; Calabrò, V.; Iorio, G. An integrated centrifugation–ultrafiltration system in the treatment of olive mill wastewater. J. Membr. Sci. 2002, 209, 519–531. [Google Scholar] [CrossRef]
- Garcia-Castello, E.; Cassano, A.; Criscuoli, A.; Conidi, C.; Drioli, E. Recovery and concentration of polyphenols from olive mill wastewaters by integrated membrane system. Water Res. 2010, 44, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Cassano, A.; Conidi, C.; Drioli, E. Comparison of the performance of UF membranes in olive mill wastewater treatment. Water Res. 2011, 45, 3197–3204. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Rodriguez-Vives, S.; Hodaifa, G.; Martinez-Ferez, A. Impacts of operating conditions on reverse osmosis performance of pretreated olive mill wastewater. Water Res. 2012, 46, 4621–4632. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Kiai, H.; Raiti, J.; Hafidi, A. Application of ultrafiltration for olive processing wastewaters treatment. J. Cleaner Prod. 2014, 65, 432–438. [Google Scholar] [CrossRef]
- Zirehpour, A.; Rahimpour, A.; Jahanshahi, M. The filtration performance and efficiency of olive mill wastewater treatment by integrated membrane process. Desalin. Water Treat. 2015, 53, 1254–1262. [Google Scholar] [CrossRef]
- Hamza, M.; Sayadi, S. Valorisation of olive mill wastewater by enhancement of natural hydroxytyrosol recovery. Int. J. Food Sci. Tech. 2015, 50, 826–833. [Google Scholar] [CrossRef]
- Gebreyohannes, A.Y.; Mazzei, R.; Giorno, L. Trends and current practices of olive mill wastewater treatment: Application of integrated membrane process and its future perspective. Sep. Purif. Technol. 2016, 162, 45–60. [Google Scholar] [CrossRef]
- Pulido, J.M.O. A review on the use of membrane technology and fouling control for olive mill wastewater treatment. Sci. Total Environ. 2016, 563–564, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Pizzichini, M.; Russo, C. Process for Recovering the Component of Olive Mill Wastewater with Membrane Technologies. WIPO Patent WO2005123603 A1, 29 December 2005. [Google Scholar]
- Galanakis, C.M.; Tornberg, E.; Gekas, V. Clarification of high-added value products from olive mill wastewater. J. Food Eng. 2010, 99, 190–197. [Google Scholar] [CrossRef]
- Ochando-Pulido, M.; Martinez-Ferez, A. Chapter 7: On the Purification of Agro-Industrial Wastewater by Membrane Technologies: The Case of Olive Mill Effluents. In Desalination; Taner, Y., Ed.; IntechOpen: London, UK, 2017; pp. 127–142. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, M.S.; Martins, R.C.; Quinta-Ferreira, R.M.; Gando-Ferreira, L.M. Optimization of operating conditions for the valorization of olive mill wastewater using membrane processes. Environ. Sci. Pollut. Res. 2018, 25, 21968–21981. [Google Scholar] [CrossRef] [PubMed]
- Ochando-Pulido, M.; Corpas-Martínez, J.R.; Vellido-Perez, J.A.; Martinez-Ferez, A. Optimization of polymeric nanofiltration performance for olive-oil-washing wastewater phenols recovery and reclamation. Sep. Purif. Technol. 2020, 236, 116261. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Stoller, M.; Martinez-Fereza, A. Boundary flux modelling for purification optimization of differently-pretreated agro-industrial wastewater with nanofiltration. Sep. Purif. Technol. 2018, 93, 147–154. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Stoller, M.; Víctor-Ortega, M.D.; Martínez-Férez, A. Analysis of the Fouling Build-up of a Spiral Wound Reverse Osmosis Membrane in the Treatment of Two-phase Olive Mill Wastewater. Chem. Eng. Trans. 2016, 47, 403–408. [Google Scholar]
- Zagklis, D.P.; Vavouraki, A.I.; Kornaros, M.E.; Paraskeva, C.A. Purification of olive mill wastewater phenols through membrane filtration and resin adsorption/desorption. J. Hazard. Mater. 2015, 285, 69–76. [Google Scholar] [CrossRef]
- Paraskeva, C.A.; Papadakis, V.G.; Tsarouchi, E.; Kanellopoulou, D.G.; Koutsoukos, P.G. Membrane processing for olive mill wastewater fractionation. Desalination 2007, 213, 218–229. [Google Scholar] [CrossRef]
- Bazzarelli, F.; Piacentini, E.; Poerio, T.; Mazzei, R.; Cassano, A.; Giorno, L. Advances in membrane operations for water purification and biophenols recovery/valorization from OMWWs. J. Membr. Sci. 2016, 497, 402–409. [Google Scholar] [CrossRef]
- Bazzarelli, F.; Poerio, T.; Mazzei, R.; D’Agostino, N.; Giorno, L. Study of OMWWs suspended solids destabilization to improve membrane processes performance. Sep. Purif. Technol. 2015, 149, 183–189. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Martinez-Ferez, A. Operation setup of a nanofiltration membrane unit for purification of two-phase olives and olive oil washing wastewaters. Sci. Total Environ. 2018, 612, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Ochando-Pulido, J.M.; Víctor-Ortega, M.D.; Martínez-Ferez, A. Membrane fouling insight during reverse osmosis purification of pretreated olive mill wastewater. Sep. Purif. Technol. 2016, 168, 177–187. [Google Scholar] [CrossRef]
- Sygouni, V.; Pantziaros, A.G.; Iakovides, I.C.; Sfetsa, E.; Bogdou, P.I.; Christoforou, E.A.; Paraskeva, C.A. Treatment of two-phase olive mill wastewater and recovery of phenolic compounds using membrane technology. Membranes 2019, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tundis, R.; Conidi, C.; Loizzo, M.R.; Sicari, V.; Cassano, A. Olive Mill Wastewater Polyphenol-Enriched Fractions by Integrated Membrane Process: A Promising Source of Antioxidant, Hypolipidemic and Hypoglycaemic Compounds. Antioxidants 2020, 9, 602. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Paraskeva, C.A. Preliminary design of a phenols purification plant. J. Chem. Technol. Biotechnol. 2020, 95, 373–383. [Google Scholar] [CrossRef]
- Coskun, T.; Debik, E.; Demir, N.M. Treatment of olive mill wastewaters by nanofiltration and reverse osmosis membranes. Desalination 2010, 259, 65–70. [Google Scholar] [CrossRef]
- Petrotos, K.B.; Kokkora, M.I.; Papaioannou, C.; Gkoutsidis, P.E. Olive mill wastewater concentration by two-stage reverse osmosis in tubular configuration, in a scheme combining open and tight membranes. Desalin. Water Treat. 2016, 57, 20621–20630. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 15th ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 1981. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Rodriguez, S.G.S. Particulate and Organic Matter Fouling of Seawater Reverse Osmosis Systems, Characterization, Modeling and Applications; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Han, Y.; Giorno, L.; Gugliuzza, A. Photoactive Gel for Assisted Cleaning during Olive Mill Wastewater Membrane Microfiltration. Membranes 2017, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Fraga, M.C.; Sanches, S.; Crespo, J.G.; Pereira, V.J. Assessment of a New Silicon Carbide Tubular Honeycomb Membrane for Treatment of Olive Mill Wastewaters. Membranes 2017, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Ochando-Pulido, J.M.; Martinez-Ferez, A. On the Recent Use of Membrane Technology for Olive Mill Wastewater Purification. Membranes 2015, 5, 513–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Process | pH | EC | TSS | COD | Ph | Ch | Proposed Process | References |
|---|---|---|---|---|---|---|---|---|
| (mS/cm) | (g/L) | (g/L) | (mg/L) | (g/L) | ||||
| BP | 4.5–5 | 0.1–2.7 | 65.7–130 | 1.2–2.4% | 2.2–4.5 | [5] | ||
| 4.5 | 9.0 | 12% | −180.0 | - | - | [21] | ||
| 4.5 | - | 8.0 | 47.8 | 3740 | - | UF-NF | [22] | |
| 4.7 | - | 7.8 | 59.1 | 4560 | - | |||
| 2P | 5.1 | 1.8 | - | 13.4 | 749 | - | NF | [23] |
| 5.25 | 2.1 | - | 14.0 | 776 | - | |||
| 5.5 | 2.2 | - | 4.2 | - | - | NF | [24] | |
| 4.9 | 1.7 | 5.6 | 16.4 | 181 | - | RO | [25] | |
| 4.9 | 1.3 | 0.6% | 7.8 | - | [21] | |||
| 3P | 4.7–5.2 | - | 0.9–27.6 | 40–103.4 | 0.37–0.5% | 1.5–4.7 | [5] | |
| 5.0 | - | 17.6 | 212 | - | MF-NF-OD | [11] | ||
| - | - | 44 | 107.2 | 2640 | 12.3 | UF-NF-RO | [26] | |
| 5.4 | 7.9 | 6.6% | 151.4 | - | - | [21] | ||
| 5.13 | 5.08 | 11.7 | 16.5 | 850 | 13.1 | UF-NF-RO | [27] |
| Channel Diameter | 4 mm |
| Number of Channels | 19 |
| Filtration Surface Area | 0.24 m2 |
| Length | 1020 mm |
| Material | Ultrapure α-alumina (>99.7%) |
| Pore size of the inner layer | 0.2 µm |
| Membrane | Manufacturer | Minimum Rejection | Application |
|---|---|---|---|
| Desal AG | GE Power&Water | 99.3% (NaCl) | Brackish water RO |
| Desal SC | GE Power&Water | 98.5% (NaCl) | Brackish water RO |
| Desal DK | GE Power&Water, | 98% (MgSO4) | NF |
| SW30 HR | DOW | 99.6% (NaCl) | Seawater RO |
| SW30 ULE | DOW | 99.55% (NaCl) | Seawater RO |
| BW30 | DOW | 99.0% (NaCl) | Brackish water RO |
| NF 90 | DOW | 97.0% (MgSO4), 85.0% (NaCl) | NF |
| pH (—) | TSS (mg/L) | COD (mg/L) | Conductivity (µS/cm) | Phenols (mg/L) | |
|---|---|---|---|---|---|
| OMW1-FR | 4.43 | 870 | 30,000 | 5310 | 2000 |
| OMW2-CA | 5.80 | 1700 | 66,500 | 13,940 | 1500 |
| OMW3-SG | 5.17 | 15,000 | 159,500 | 12,780 | 3300 |
| VCR | pH | Conductivity (µs/cm) | COD (mg/L) | Phenols (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FD | PR | FD | PR | R (%) | FD | PR | R (%) | FD | PR | R (%) | |
| OMW1-FR | |||||||||||
| 1.00 | 4.43 | 4.39 | 5310 | 5050 | 4.90 | 30,000 | 19,780 | 34.07 | 2000 | 1430 | 28.50 |
| 1.29 | 4.44 | 4.39 | 5450 | 5330 | 2.20 | ||||||
| 1.82 | 4.44 | 4.4 | 5420 | 5290 | 2.40 | 33,425 | 20,450 | 38.82 | 3900 | 2820 | 27.69 |
| 3.07 | 4.5 | 4.41 | 5450 | 5260 | 3.49 | 38,700 | 23,100 | 40.31 | 4500 | 3200 | 28.89 |
| OMW2-CA | |||||||||||
| 1.00 | 5.8 | 5.9 | 13,940 | 13,690 | 1.79 | 66,500 | 43,370 | 34.78 | 1500 | 996 | 33.60 |
| 1.22 | 5.79 | 5.88 | 13,600 | 13,350 | 1.84 | 70,889 | 40,990 | 42.18 | |||
| 1.55 | 5.77 | 5.91 | 13,560 | 13,410 | 1.11 | 75,500 | 42,440 | 43.79 | 1590 | 1050 | 33.96 |
| 2.14 | 5.79 | 5.87 | 13,920 | 13,650 | 1.94 | 85,000 | 40,740 | 52.07 | |||
| 3.45 | 5.81 | 5.9 | 13,990 | 13,500 | 3.50 | 95,000 | 42,850 | 54.89 | 1910 | 1200 | 37.17 |
| OMW3-SG | |||||||||||
| 1.00 | 5.17 | 5.23 | 12,780 | 12,240 | 4.23 | 128,800 | 49,490 | 61.58 | 3300 | 1950 | 40.91 |
| 1.67 | 5.37 | 5.38 | 12,470 | 12,060 | 3.29 | 164,350 | 55,350 | 66.32 | |||
| 2.86 | 5.37 | 5.39 | 12,470 | 12,050 | 3.37 | 209,000 | 64,590 | 69.10 | 4800 | 2820 | 41.25 |
| Membrane | Permeate Flux (L/m2h) | Conductivity | COD | Phenols | |||
|---|---|---|---|---|---|---|---|
| PR (µS/cm) | R (%) | PR (mg/L) | R (%) | PR (mg/L) | R (%) | ||
| Desal AG | 7.00 | 138.6 | 98.95 | 2608 | 93.51 | 6.0 | 99.44 |
| Desal SC | 14.35 | 170.3 | 98.71 | 2326 | 94.21 | 9.1 | 99.15 |
| Desal DK | 60.61 | 1473 | 88.84 | 8249 | 79.47 | 112.8 | 89.46 |
| SW30 HR | 12.66 | 109.6 | 99.17 | 1736 | 95.68 | 2.9 | 99.73 |
| SW30 ULE | 12.04 | 151.8 | 98.85 | 2359 | 94.13 | 4.4 | 99.59 |
| BW30 | 33.26 | 159.7 | 98.79 | 2387 | 94.06 | 9.0 | 99.16 |
| NF 90 | 27.27 | 150.5 | 98.86 | 2668 | 93.36 | 7.5 | 99.30 |
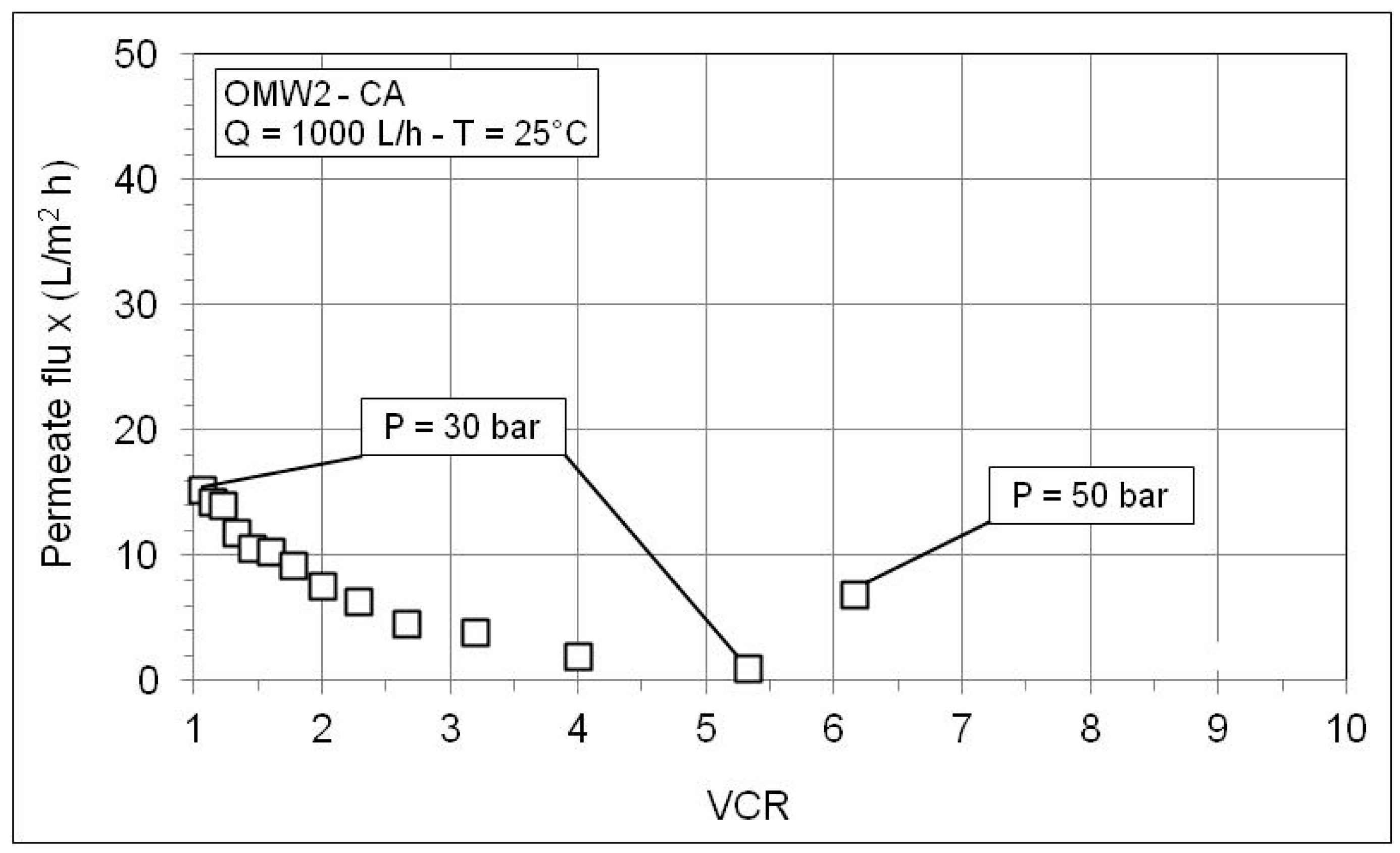
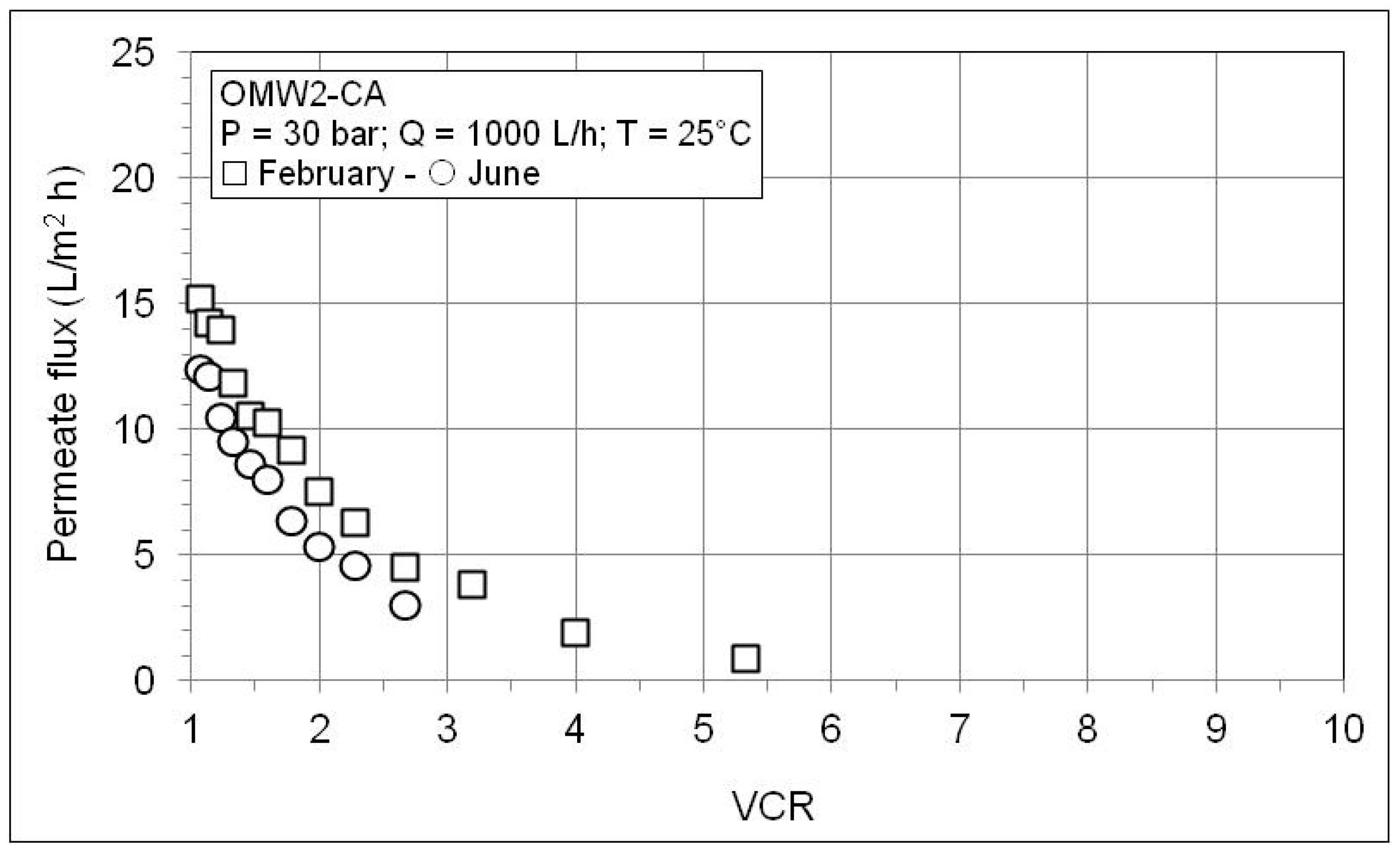
| VCR | P (bar) | pH | Conductivity (µS/cm) | COD (mg/L) | Phenols (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FD | PR | FD | PR | R (%) | FD | PR | R (%) | FD | PR | R (%) | ||
| OMW1-FR | ||||||||||||
| 1.00 | 30 | 4.48 | 3.79 | 5100 | 89.7 | 98.24 | 19,650 | 628 | 96.80 | 2900 | 6.0 | 99.79 |
| 1.96 | 30 | 4.58 | 3.61 | 8880 | 109.0 | 98.77 | 36,170 | 926 | 97.44 | |||
| 6.56 | 30 | 4.68 | 3.83 | 20,900 | 253.0 | 98.79 | ||||||
| 10.50 | 30 | 26,700 | 1382 | 94.82 | 140,120 | 2558 | 98.17 | 24300 | 28.0 | 99.88 | ||
| OMW2-CA | ||||||||||||
| 1.00 | 30 | 5.88 | 4.81 | 13,200 | 116.3 | 99.12 | 40,180 | 2010 | 95.00 | 1070 | 5.0 | 99.53 |
| 1.23 | 30 | 5.87 | 4.84 | 15,200 | 133.4 | 99.12 | 53,990 | 2231 | 95.87 | |||
| 1.60 | 30 | 5.86 | 4.86 | 16,370 | 199.2 | 98.78 | 65,860 | 2495 | 96.21 | |||
| 2.29 | 30 | 5.9 | 4.9 | 18,910 | 239.7 | 98.73 | 86,960 | 3840 | 95.58 | |||
| 4.00 | 30 | 5.98 | 4.92 | 20,490 | 318.9 | 98.44 | 109,220 | 8855 | 91.89 | |||
| 5.33 | 30 | 6.02 | 4.96 | 27,400 | 904.0 | 96.70 | 151,700 | 15,325 | 89.90 | 5600 | 57.3 | 98.98 |
| 6.15 | 50 | 6.12 | 4.89 | 30,200 | 702.0 | 97.68 | 191,350 | 4075 | 97.87 | 6420 | 24.2 | 99.62 |
| OMW3-SG | ||||||||||||
| 1.00 | 30 | 5.45 | 5.27 | 12,100 | 115.2 | 99.05 | 58,560 | 2933 | 94.99 | 2250 | 2.2 | 99.90 |
| 1.24 | 30 | 17,130 | 128.4 | 99.25 | 78,280 | 3189 | 95.93 | |||||
| 1.62 | 30 | 20,500 | 192.7 | 99.06 | 104,320 | 4543 | 95.65 | |||||
| 2.33 | 30 | 5.5 | 5.21 | 25,800 | 738.0 | 97.14 | 120,800 | 10,000 | 91.72 | |||
| 2.74 | 30 | 29,000 | 1592 | 94.51 | 159,220 | 13,855 | 91.30 | 7500 | 54.0 | 99.28 | ||
| 3.62 | 40 | 33,800 | 1734 | 94.87 | 215,850 | 16,620 | 92.30 | |||||
| 4.57 | 50 | 5.59 | 5.16 | 36,100 | 3320 | 90.80 | 261,900 | 8720 | 96.67 | 9850 | 26.0 | 99.74 |
| VCR | P (bar) | pH | Conductivity (µs/cm) | COD (mg/L) | Phenols (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FD | PR | FD | PR | R (%) | FD | PR | R (%) | FD | PR | R (%) | ||
| 1.00 | 30 | 5.91 | 4.82 | 13,500 | 117.4 | 99.13 | 40,200 | 2050 | 94.90 | 1025 | 5.2 | 99.49 |
| 1.14 | 30 | 5.87 | 4.84 | 14,200 | 135.2 | 99.05 | 44,500 | 2120 | 95.24 | |||
| 1.33 | 30 | 5.93 | 4.85 | 15,300 | 198.7 | 98.70 | 55,450 | 2290 | 95.87 | |||
| 1.60 | 30 | 5.94 | 4.87 | 16,450 | 240.1 | 98.54 | 67,800 | 2370 | 96.50 | |||
| 2.00 | 30 | 5.89 | 4.83 | 17,930 | 303.8 | 98.31 | 84,300 | 3540 | 95.80 | 2320 | 11.0 | 99.53 |
| 2.67 | 30 | 5.93 | 4.90 | 19,800 | 318.4 | 98.39 | 98,650 | 5900 | 94.02 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bottino, A.; Capannelli, G.; Comite, A.; Costa, C.; Firpo, R.; Jezowska, A.; Pagliero, M. Treatment of Olive Mill Wastewater through Integrated Pressure-Driven Membrane Processes. Membranes 2020, 10, 334. https://doi.org/10.3390/membranes10110334
Bottino A, Capannelli G, Comite A, Costa C, Firpo R, Jezowska A, Pagliero M. Treatment of Olive Mill Wastewater through Integrated Pressure-Driven Membrane Processes. Membranes. 2020; 10(11):334. https://doi.org/10.3390/membranes10110334
Chicago/Turabian StyleBottino, Aldo, Gustavo Capannelli, Antonio Comite, Camilla Costa, Raffaella Firpo, Anna Jezowska, and Marcello Pagliero. 2020. "Treatment of Olive Mill Wastewater through Integrated Pressure-Driven Membrane Processes" Membranes 10, no. 11: 334. https://doi.org/10.3390/membranes10110334
APA StyleBottino, A., Capannelli, G., Comite, A., Costa, C., Firpo, R., Jezowska, A., & Pagliero, M. (2020). Treatment of Olive Mill Wastewater through Integrated Pressure-Driven Membrane Processes. Membranes, 10(11), 334. https://doi.org/10.3390/membranes10110334







