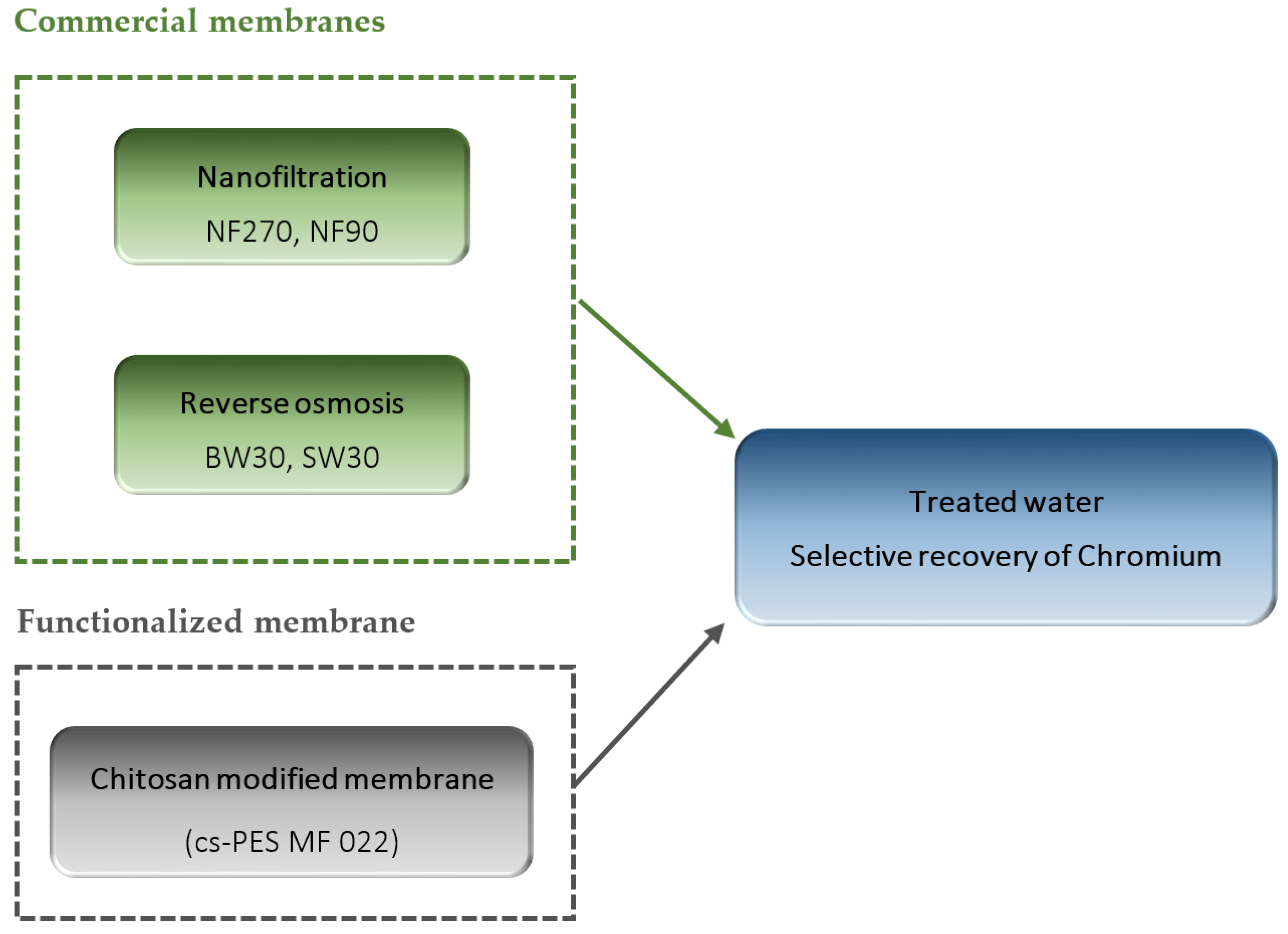
Tannery Effluent Treatment by Nanofiltration, Reverse Osmosis and Chitosan Modified Membranes
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.1.1. Chemicals
2.1.2. Solutions
Tannery Effluent of TAMEG-Rouiba
Synthetic Tannery Effluent
2.1.3. Membranes
Nanofiltration and Reverse Osmosis Membranes
Preparation of Chitosan Modified Membranes
Structural and Chemical Characterization of the Chitosan Modified Membrane
2.2. Methods
2.2.1. Experimental System
2.2.2. Data Analysis
2.2.3. Analytical Methods
3. Results and Discussion
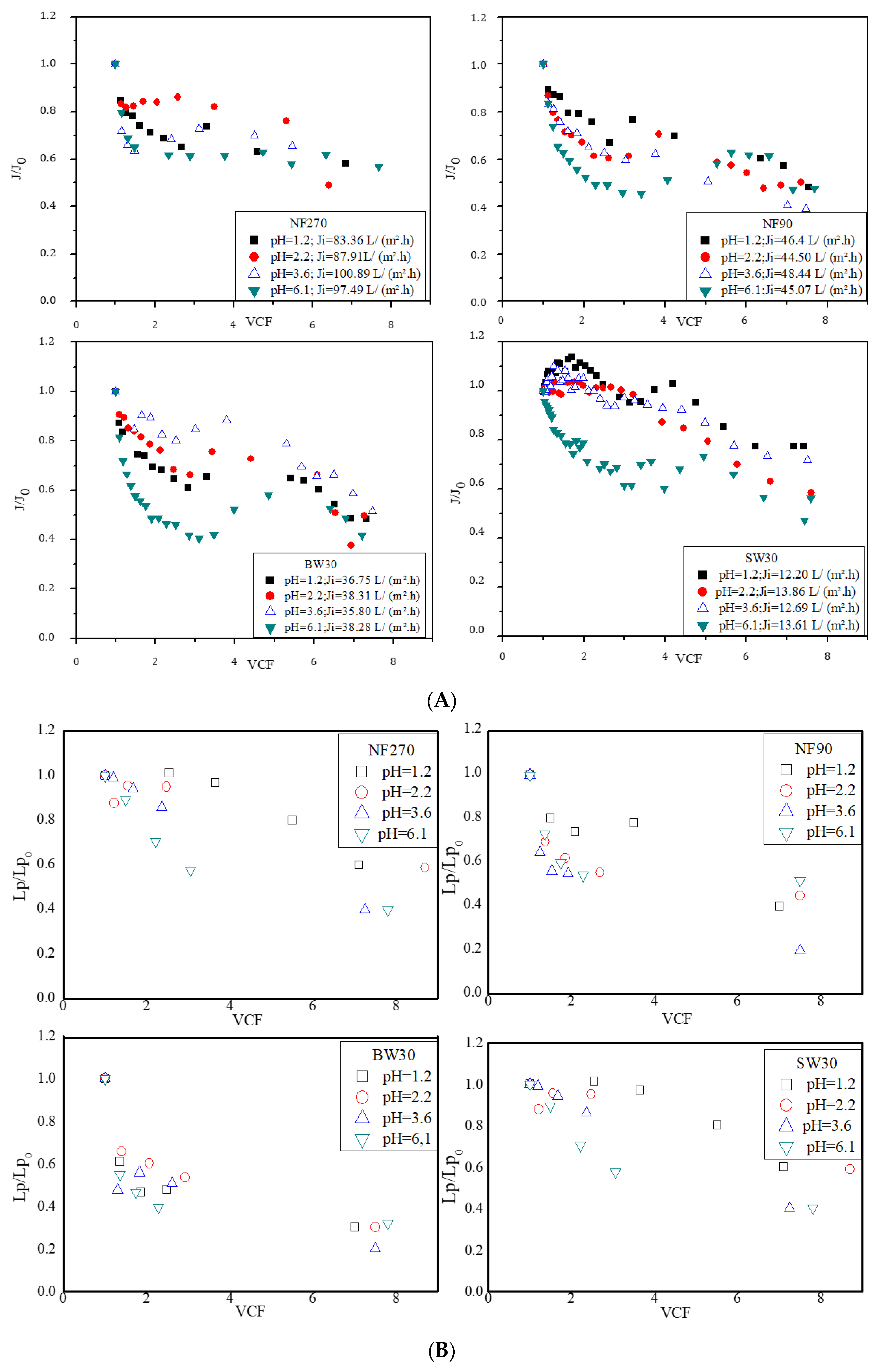
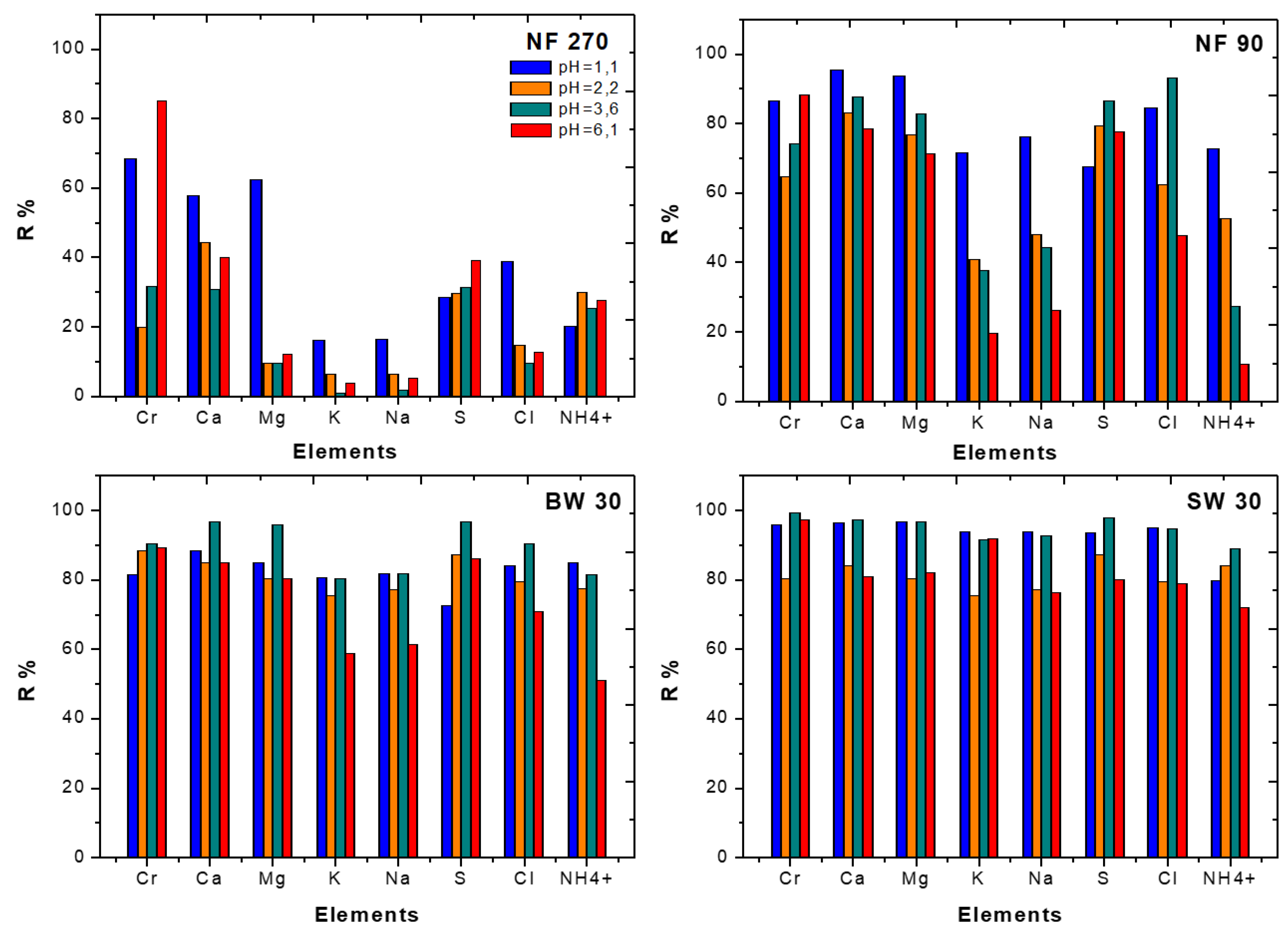
3.1. Treatment of Synthetic Tannery Effluent with Commercial NF and RO Membranes
3.1.1. Volumetric Permeate Fluxes and Membranes’ Hydraulic Permeability
3.1.2. Rejection Performance
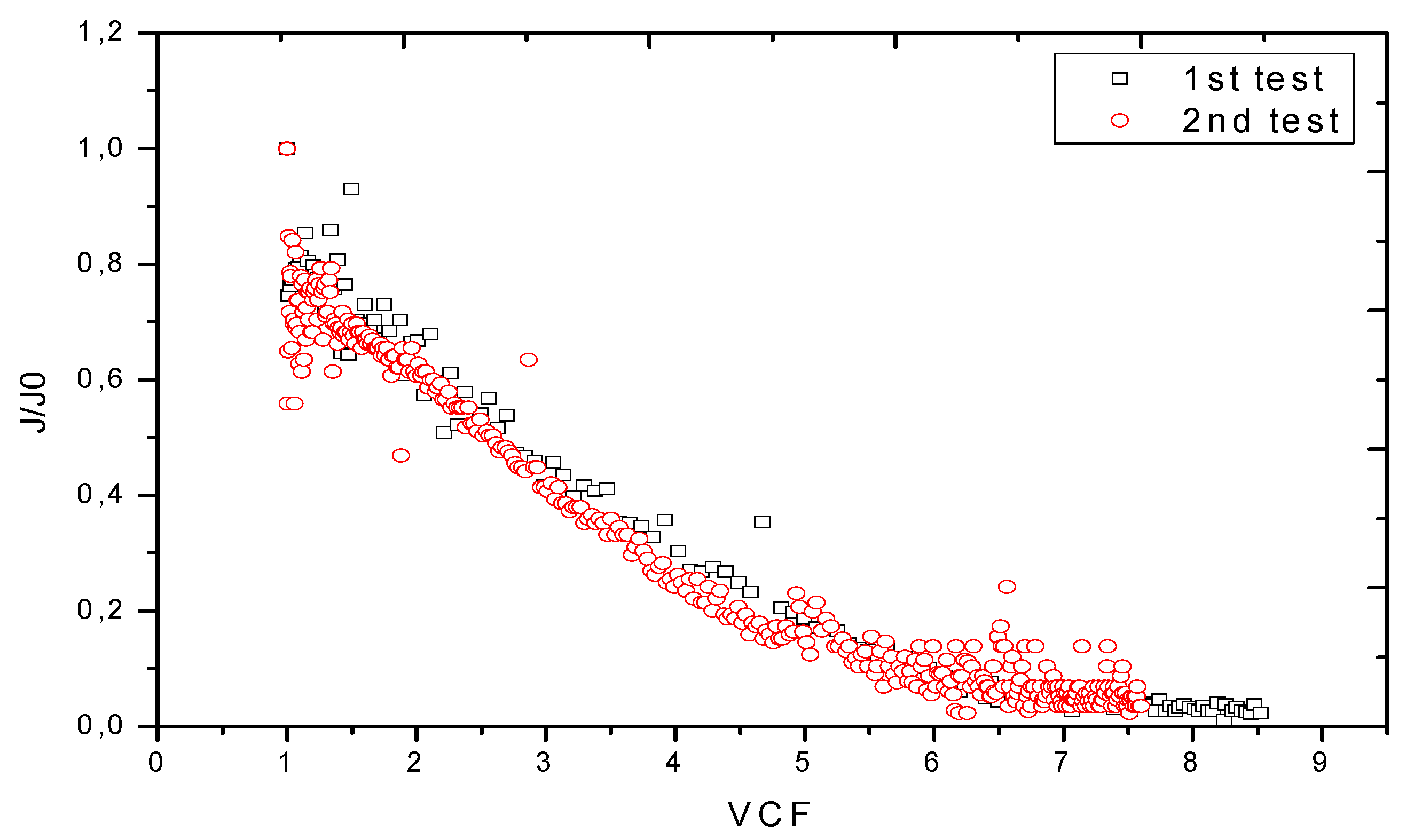
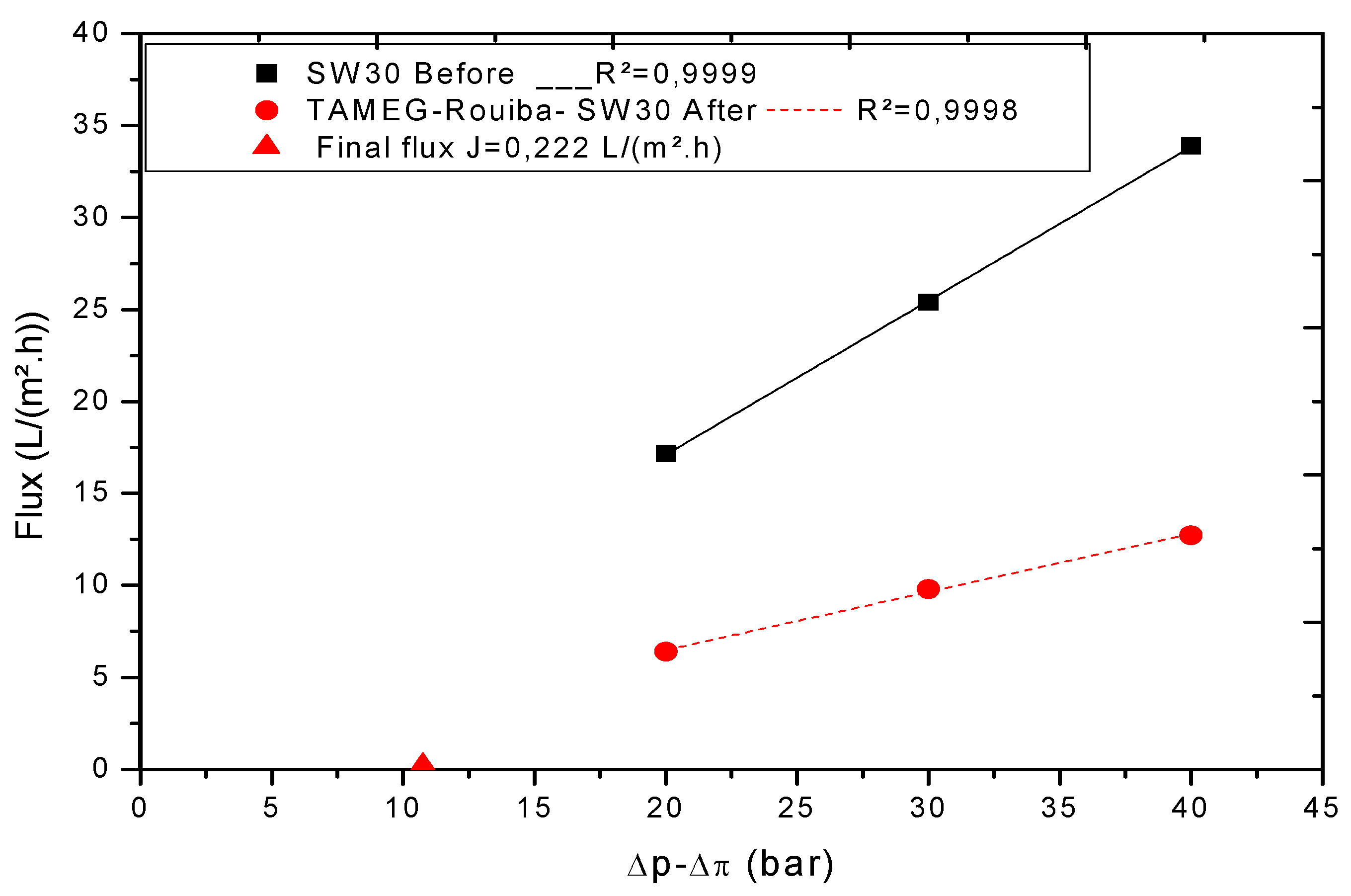
3.2. Treatment of Real TAMEG-Rouiba Tannery Effluent with SW30 Membrane
3.2.1. Volumetric Flux and Membrane Hydraulic Permeability
3.2.2. Rejection Performance
3.3. Treatment of the Synthetic Tannery Effluent with the Chitosan Modified Membranes
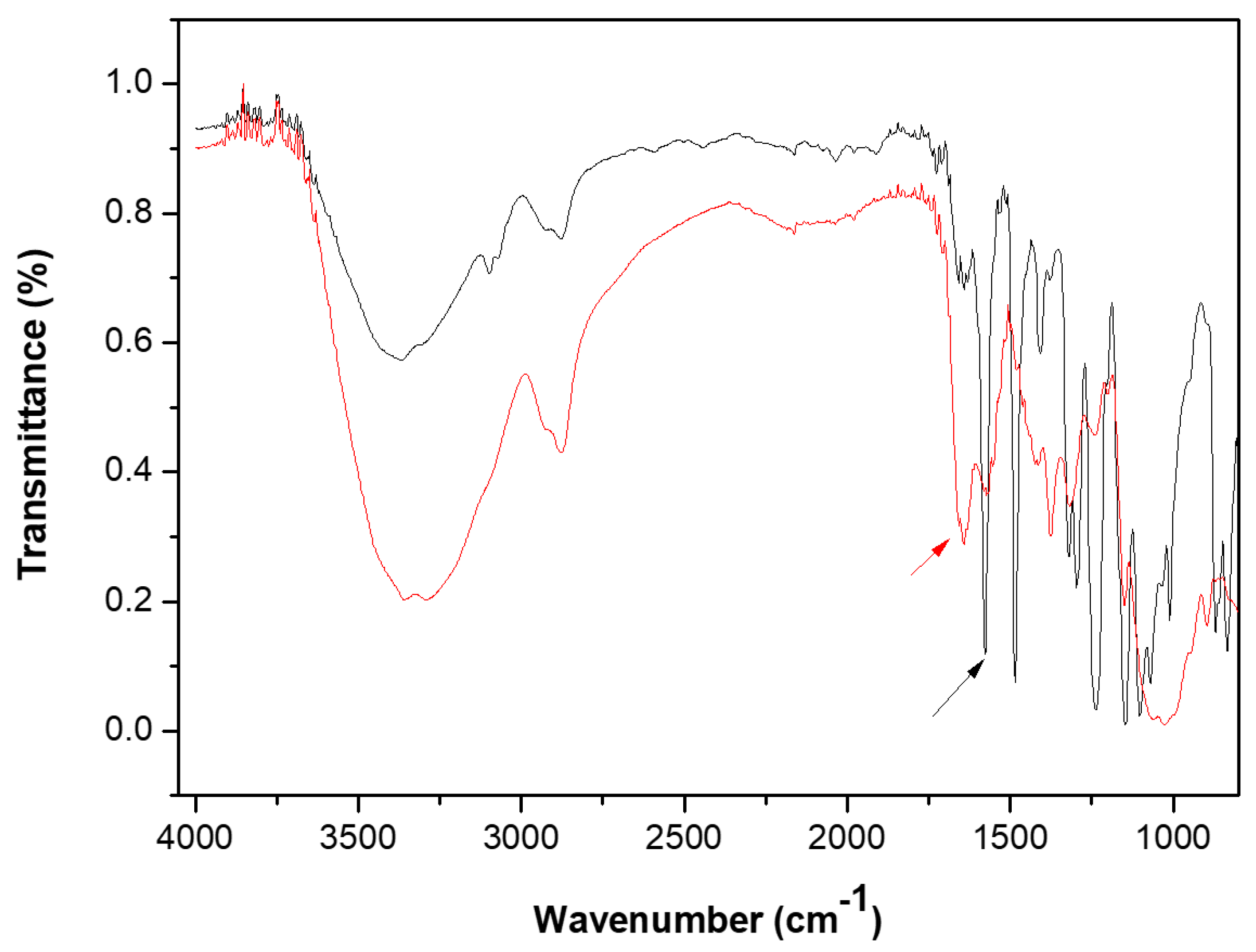
3.3.1. Structural and Chemical Characterization of the Chitosan Modified Membranes
Cross-Linking of the Chitosan Layer
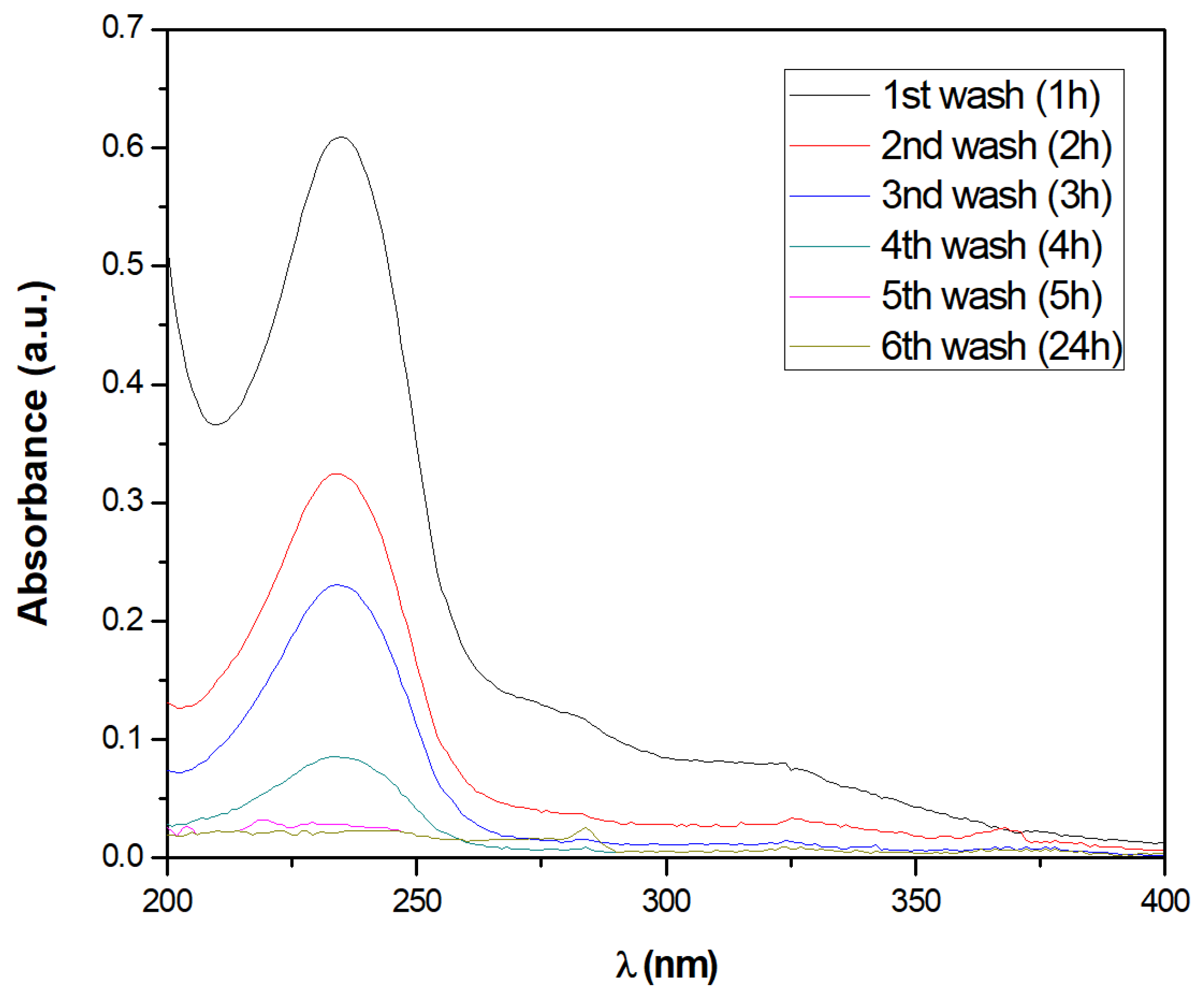
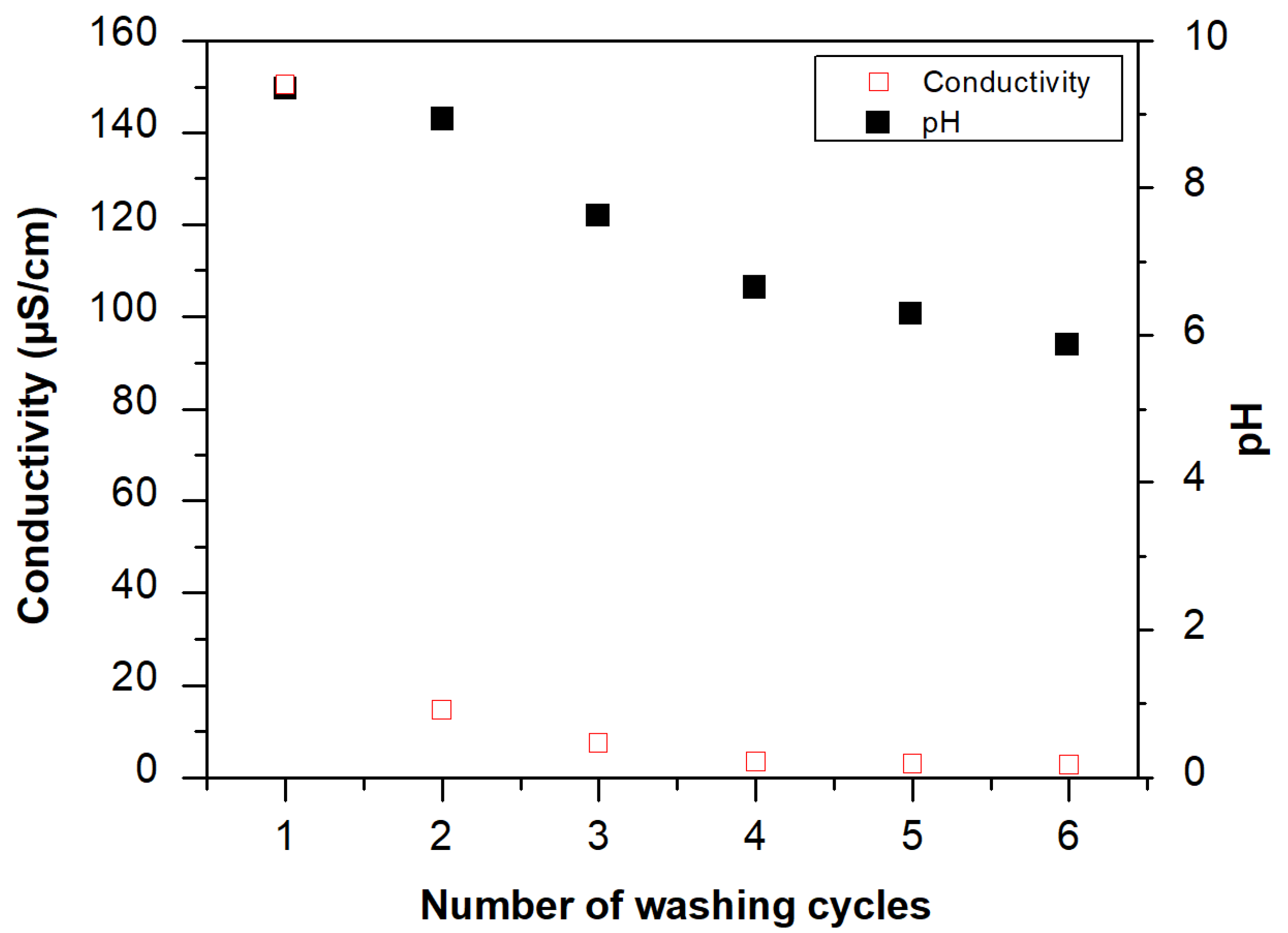
Stability of the Chitosan Modified Membranes
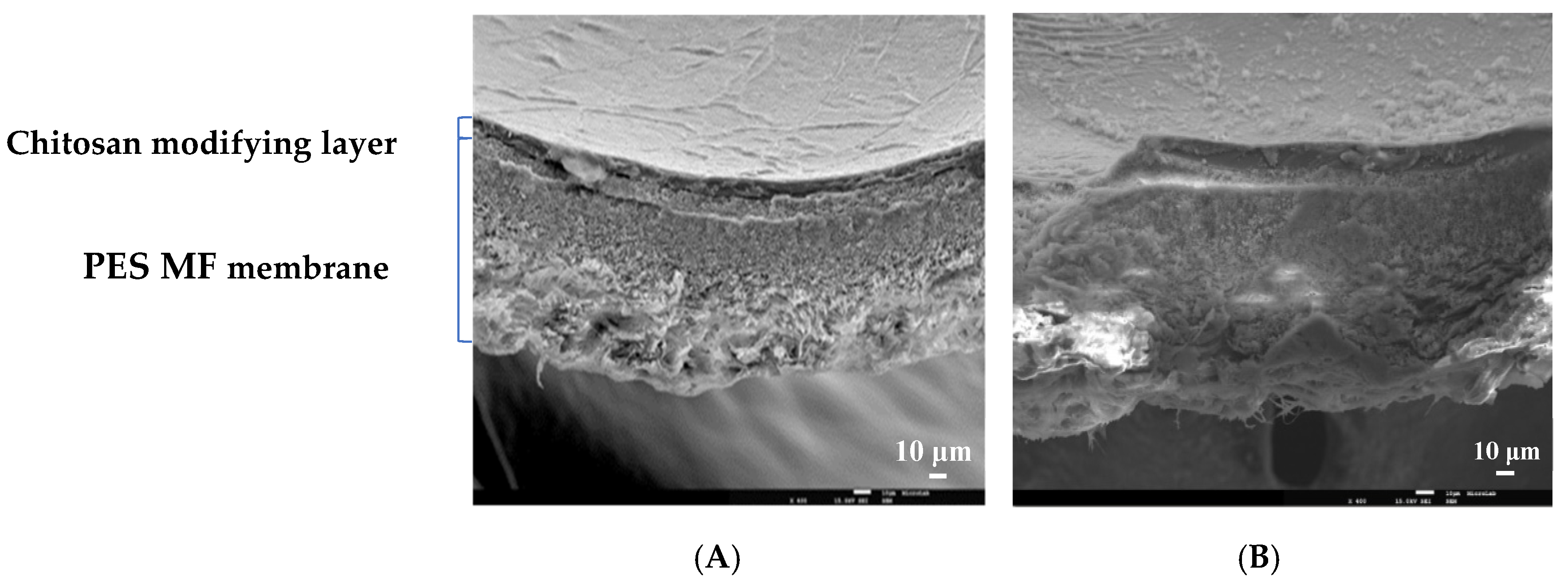
Structural Analysis of the Chitosan Modified Membranes
3.3.2. Treatment of the Synthetic Tannery Effluent with the Chitosan Modified Membrane
Permeate Flux and Membrane Hydraulic Permeability
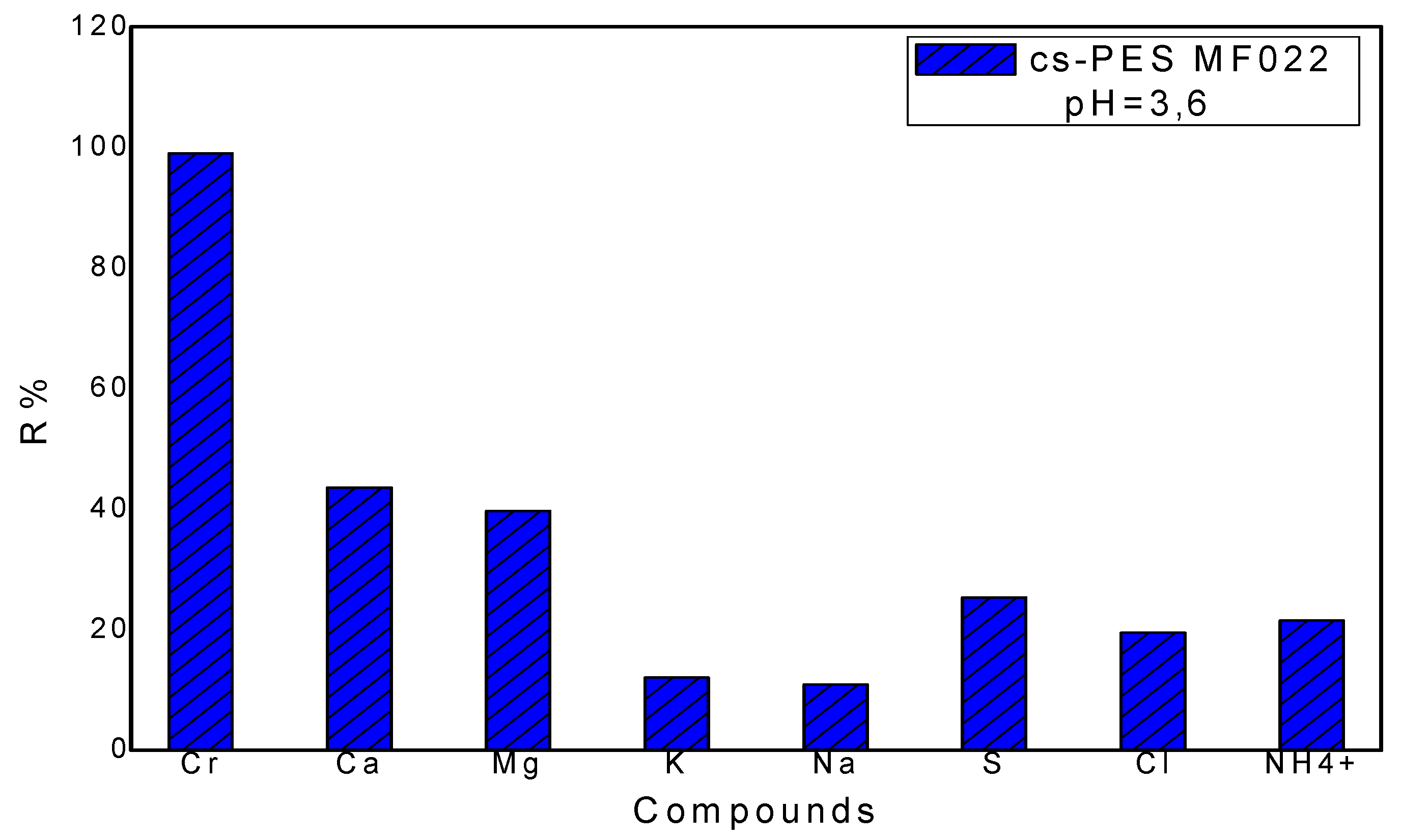
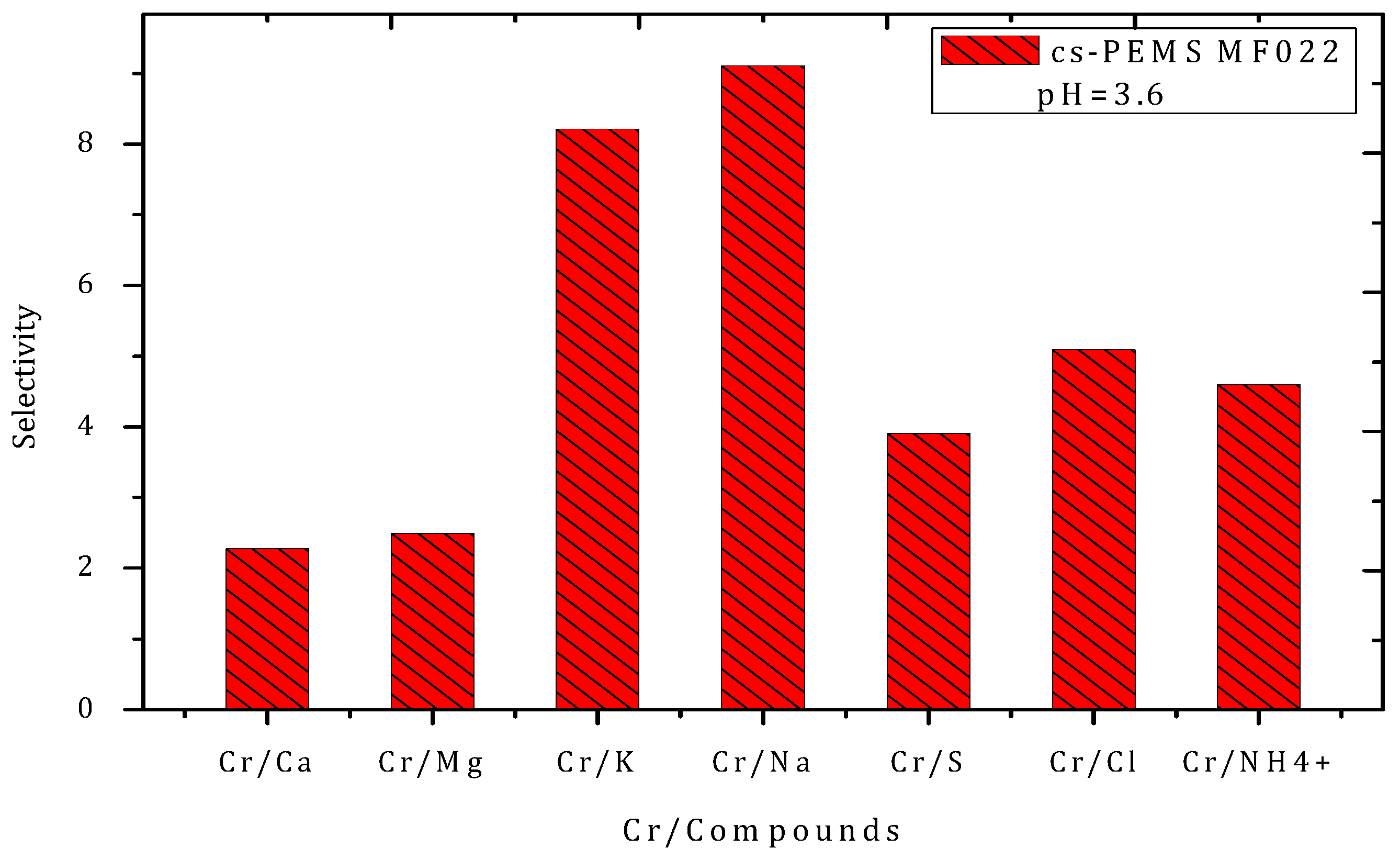
Rejection Performance and Membrane Selectivity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raper, E.; Stephenson, T.; Anderson, D.R.; Fisher, R.; Soares, A. Industrial Wastewater Treatment through Bioaugmentation. Process. Saf. Environ. Prot. 2018, 118, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Wondie, A.; Assefa, W. Bahir Dar tannery effluent characterization and its impact on the head of Blue Nile River. Afr. J. Environ. Sci. Technol. 2014, 8, 312–318. [Google Scholar] [CrossRef]
- Manjushree, C.; Mostafa, M.G.; Biswas, T.K.; Mandal, A.; Saha, A.K. Characterization of the Effluents from Leather Processing Industries. Environ. Process. 2015, 2, 173–187. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.; Lian, F.; Xia, R.; Wang, Z. Formulation and Durability of a Geopolymer Based on Metakaolin/Tannery Sludge. Waste Manag. 2018, 79, 717–728. [Google Scholar] [CrossRef]
- Angelucci, D.M.; Stazi, V.; Andrew, J.; Daugulis, A.J.; Tomei, M.C. Treatment of Synthetic Tannery Wastewater in a Continuous Two-Phase Partitioning Bioreactor: Biodegradation of the Organic Fraction and Chromium Separation. J. Clean. Prod. 2017, 152, 321–329. [Google Scholar] [CrossRef]
- Kavouras, P.; Pantazopoulou, E.; Varitis, S.; Vourlias, G.; Chrissafis, K.; Dimitrakopulos, G.P.; Mitrakas, M.; Zouboulis, A.I.; Karakostas, T.; Xenidis, A. Incineration of Tannery Sludge under Oxic and Anoxic Conditions: Study of Chromium Speciation. J. Hazard. Mater. 2015, 283, 672–679. [Google Scholar] [CrossRef]
- Kiliç, E.; Font, J.; Puig, R.; Çolak, S.; Çelik, D. Chromium Recovery from Tannery Sludge with Saponin and Oxidative Remediation. J. Hazard. Mater. 2011, 185, 456–462. [Google Scholar] [CrossRef]
- Lofrano, G.; Meriç, S.; Zengin, G.E.; Orhon, D. Chemical and Biological Treatment Technologies for Leather Tannery Chemicals and Wastewaters: A Review. Sci. Total Environ. 2013, 461–462, 265–281. [Google Scholar] [CrossRef]
- Vignati, D.A.L.; Ferrari, B.J.D.; Roulier, J.-L.; Coquery, M.; Szalinska, E.; Bobrowski, A.; Czaplicka, A.; Kownacki, A.; Dominik, J. Chromium Bioavailability in Aquatic Systems Impacted by Tannery Wastewaters. Part 1: Understanding Chromium Accumulation by Indigenous Chironomids. Sci. Total Environ. 2019, 653, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Vidal, G.; Nieto, J.; Cooman, K.; Gajardo, M.; Bornhardt, C. Unhairing Effluents Treated by an Activated Sludge System. J. Hazard. Mater. 2004, 112, 143–149. [Google Scholar] [CrossRef]
- Agustini, C.; Costa, M.; Gutterres, M. Biogas Production from Tannery Solid Wastes—Scale-up and Cost Saving Analysis. J. Clean. Prod. 2018, 187, 158–164. [Google Scholar] [CrossRef]
- Mendoza-Roca, J.A.; Galiana-Aleixandre, M.V.; Lora-García, J.; Bes-Piá, A. Purification of Tannery Effluents by Ultrafiltration in View of Permeate Reuse. Sep. Purif. Technol. 2010, 70, 296–301. [Google Scholar] [CrossRef]
- Religa, P.; Kowalik, A.; Gierycz, P. A new approach to chromium concentration from salt mixture solution using nanofiltration. Sep. Purif. Technol. 2011, 82, 114–120. [Google Scholar] [CrossRef]
- Dasgupta, J.; Mondal, D.; Chakraborty, S.; Sikder, J.; Curcio, S.; Arafat, H.A. Nanofiltration Based Water Reclamation from Tannery Effluent Following Coagulation Pretreatment. Ecotoxicol. Environ. Saf. 2015, 121, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Colla, V.; Branca, T.A.; Rosito, F.; Lucca, C.; Vivas, B.P.; Delmiro, V.M. Sustainable Reverse Osmosis Application for Wastewater Treatment in the Steel Industry. J. Clean. Prod. 2016, 130, 103–115. [Google Scholar] [CrossRef]
- Wenten, I.G.; Khoiruddin. Reverse Osmosis Applications: Prospect and Challenges. Desalination 2016, 391, 112–125. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Tamer, T.M.; Hassan, M.A.; Omer, A.M.; Valachová, K.; Eldin, M.S.M.; Collins, M.N.; Šoltés, L. Antibacterial and Antioxidative Activity of O-Amine Functionalized Chitosan. Carbohydr. Polym. 2017, 169, 441–450. [Google Scholar] [CrossRef]
- Rokhati, N.; Istirokhatun, T.; Samsudin, A.M. Layer by Layer Composite Membranes of Alginate-Chitosan Crosslinked by Glutaraldehyde in Pervaporation Dehydration of Ethanol. Int. J. Renew. Energy Dev. 2016, 5, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, B.; Seitz, D.; Mencke, A.; Kokott, A.; Ziegler, G. Glutaraldehyde and Oxidised Dextran as Crosslinker Reagents for Chitosan-Based Scaffolds for Cartilage Tissue Engineering. J. Mater. Sci. Mater. Med. 2009, 20, 1495–1503. [Google Scholar] [CrossRef]
- Beppu, M.M.; Vieira, R.S.; Aimoli, C.G.; Santana, C.C. Crosslinking of Chitosan Membranes Using Glutaraldehyde: Effect on Ion Permeability and Water Absorption. J. Membr. Sci. 2007, 301, 126–130. [Google Scholar] [CrossRef]
- Poon, L.; Wilson, L.D.; Headley, J.V. Chitosan-Glutaraldehyde Copolymers and Their Sorption Properties. Carbohydr. Polym. 2014, 109, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Baroni, P.; Vieira, R.S.; Meneghetti, E.; da Silva, M.G.C.; Beppu, M.M. Evaluation of batch adsorption of chromium ions on natural and crosslinked chitosan membranes. J. Hazard. Mater. 2008, 152, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U.; Siddique, T.A.; Talebian, S.; Lee, J.J.L.; Salleh, A.; Ang, B.C.; Afifi, A.M. Effect of Deacetylation on Property of Electrospun Chitosan/PVA Nanofibrous Membrane and Removal of Methyl Orange, Fe(III) and Cr(VI) Ions. Carbohydr. Polym. 2017, 177, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Abou El-Reash, Y.G. Magnetic Chitosan Modified with Cysteine-Glutaraldehyde as Adsorbent for Removal of Heavy Metals from Water. J. Environ. Chem. Eng. 2016, 4, 3835–3847. [Google Scholar] [CrossRef]
- Askari, M.; Rezaei, B.; Shoushtari, M.A.; Majid, A. Performance improvements in structural characteristics of chitosan-based nanofibrous composite membrane for using in liquid filtration. J. Taiwan Inst. Chem. Eng. 2015, 56, 77–83. [Google Scholar] [CrossRef]
- Horzum, N.; Boyacı, E.; Eroğlu, A.E.; Shahwan, T.; Demir, M.M. Sorption Efficiency of Chitosan Nanofibers toward Metal Ions at Low Concentrations. Biomacromolecules 2010, 11, 3301–3308. [Google Scholar] [CrossRef] [Green Version]
- Nagireddi, S.; Katiyar, V.; Uppaluri, R. Pd(II) adsorption characteristics of glutaraldehyde cross-linked chitosan copolymer resin. Int. J. Biol. Macromol. 2017, 94 Pt A, 72–84. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Kuang, Y.; Yang, R.; Ma, J.; Zhao, S.; Liao, Y.; Mao, H. Adsorptive Removal of Cr3+ from aqueous solutions using chitosan microfibers immobilized with plant polyphenols as biosorbents with high capacity and selectivity. Appl. Surf. Sci. 2017, 404, 418–425. [Google Scholar] [CrossRef]
- Bassi, R.; Prasher, S.O.; Simpson, B.K. Removal of selected metal ions from aqueous solutions using chitosan flakes. Sep. Sci. Technol. 2000, 35, 547–560. [Google Scholar] [CrossRef]
- Pietrelli, L.; Francolini, I.; Piozzi, A.; Sighicelli, M.; Silvestro, I.; Vocciante, M. Chromium(III) Removal from Wastewater by Chitosan Flakes. Appl. Sci. 2010, 10, 1925–1936. [Google Scholar] [CrossRef] [Green Version]
- Benhadji, A.; Ahmed, M.T.; Maachi, R. Electrocoagulation and Effect of Cathode Materials on the Removal of Pollutants from Tannery Wastewater of Rouïba. Desalination 2011, 277, 128–134. [Google Scholar] [CrossRef]
- TAMEG. Available online: https://dz.kompass.com/c/tannerie-megisserie-spa/dz128962/ (accessed on 15 March 2020).
- Executive Decree No. 06-141 of 20 Rabie El Aouel 1427 Corresponding to 19 April 2006, Official Journal of the Algerian Republic N° 26. Available online: https://and.dz/site/wp-content/uploads/D%C3%A9cret-executif-n%C2%B0-06-141.pdf (accessed on 10 September 2020).
- Tu, K.L.; Nghiem, L.D.; Chivas, A.R. Coupling Effects of Feed Solution pH and Ionic Strength on the Rejection of Boron by NF/RO Membranes. Chem. Eng. J. 2011, 168, 700–706. [Google Scholar] [CrossRef] [Green Version]
- Madsen, H.T.; Søgaard, E.G. Applicability and Modelling of Nanofiltration and Reverse Osmosis for Remediation of Groundwater Polluted with Pesticides and Pesticide Transformation Products. Sep. Purif. Technol. 2014, 125, 111–119. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 2nd ed.; John Wiley & Sons: Chichester, West Sussex, UK, 2004; pp. 206–207. [Google Scholar]
- Bouroche, A.; Le Bars, M. Techniques de Separation Par Membranes; INRA: Paris, France, 1994. [Google Scholar]
- Chemical Equilibrium Diagrams. Available online: https://sites.google.com/site/chemdiagr/ (accessed on 15 March 2020).
- Ji, Y.-L.; Qian, W.-J.; An, Q.-F.; Huang, S.-H.; Lee, K.-R.; Gao, C.-J. Mussel-Inspired Zwitterionic Dopamine Nanoparticles as Building Blocks for Constructing Salt Selective Nanocomposite Membranes. J. Membr. Sci. 2019, 572, 140–151. [Google Scholar] [CrossRef]
- Blakemore, L.C.; Searle, P.L.; Daley, B.K. Methods for Chemical Analysis of Soils; Department of Scientific and Industrial Research: Wellington, New Zealand, 1987. [Google Scholar]
- Rodier, J.; Legube, B.; Merlet, N. L’analyse de l’eau; 9th ed.; Dunod: Paris, France, 2009; pp. 47, 50, 407, 413, 974. [Google Scholar]
- Hilal, N.; Kochkodan, V.; Al Abdulgader, H.; Johnson, D. A combined ion exchange–nanofiltration process for water desalination: II. Membrane selection. Desalination 2015, 363, 51–57. [Google Scholar] [CrossRef]
- Childress, A.E.; Elimelech, M. Relating nanofiltration membrane performance to membrane charge (electrokinetic) characteristics. Environ. Sci. Technol. 2000, 34, 3710–3716. [Google Scholar] [CrossRef]



| Parameter | Temperature | Total Suspended Solids (TSS) | pH | Conductivity | Turbidity | 2 COD | 3 BOD |
|---|---|---|---|---|---|---|---|
| Unit | °C | g/L | Sorensen scale | mS/cm | 1 NTU | mg O2/L | mg O2/L |
| Value | 20 | 1.79 | 7.1 | 9.6 | 322 | 942 | 92 |
| Max. levels allowed | 30 | 0.04 | 6.5–8.5 | - | - | 130 | 40 |
| Parameter | 1 COD | 2 BOD | Cr | Fe | Ni | Cu | B | Ca | K | Mg | Na | P | S | Si | Sr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | mg O2/L | mg O2/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L |
| Value | 92 | - | 50 | 4.64 | 0.27 | 1.54 | 0.12 | 81 | 79.8 | 67.2 | 259 | 0.36 | 58.3 | 9.7 | 0.97 |
| Parameter | T | pH | Conductivity | Cl− | NH4+ | Cr3+ | Ca2+ | K+ | Mg2+ | Na+ | SO42− |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | °C | - | mS/cm | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L |
| Value | 20 | 3.6 | 2.8 | 746.1 | 48.4 | 50 | 81 | 79.8 | 67.2 | 259 | 312.3 |
| Membrane | Max. Temperature (°C) | Max. Pressure (bar) | Salt Rejection (%) |
|---|---|---|---|
| NF270 | 45 | 35 | 80 (NaCl), 50 (CaCl2), 99.3 (MgSO4) |
| NF90 | 35–45 | 41 | 90–96 (NaCl) |
| BW30 | 45 | 41 | 99.4 (NaCl), 99.4 (CaCl2), 99.7 (MgSO4) |
| SW30 | 45 | 69 | 99.4 (NaCl) |
| Membrane | Pure Water Permeability L/(h m2 bar) | Degree of Recovery (%) | |
|---|---|---|---|
| Before | After | ||
| NF270 (pH 1.2) | 7.729 | 7.353 | 95.135 |
| NF 270 (pH 2.2) | 8.594 | 7.263 | 84.512 |
| NF270 (pH 3.6) | 8.325 | 8.241 | 98.991 |
| NF270 (pH 6.1) | 8.858 | 7.218 | 81.486 |
| NF90 (pH 1.2) | 5.906 | 1.524 | 25.804 |
| NF90 (pH 2.2) | 7.172 | 0.840 | 11.712 |
| NF90 (pH 3.6) | 6.751 | 2.600 | 38.513 |
| NF90 (pH 6.1) | 7.320 | 3.971 | 54.249 |
| BW30 (pH 1.2) | 3.128 | 2.707 | 86.541 |
| BW30 (pH 2.2) | 3.280 | 2.884 | 87.927 |
| BW30 (pH 3.6) | 3.788 | 2.911 | 76.848 |
| BW30(pH 6.1) | 3.395 | 2.618 | 77.113 |
| SW30 (pH 1.2) | 0.893 | 0.33 | 36.954 |
| SW30 (pH 2.2) | 0.804 | 0.293 | 36.443 |
| SW30 (pH 3.6) | 0.809 | 0.485 | 59.951 |
| SW30 (pH 6.1) | 0.803 | 0.503 | 62.640 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakmout, A.; Sadi, F.; Portugal, C.A.M.; Crespo, J.G.; Velizarov, S. Tannery Effluent Treatment by Nanofiltration, Reverse Osmosis and Chitosan Modified Membranes. Membranes 2020, 10, 378. https://doi.org/10.3390/membranes10120378
Zakmout A, Sadi F, Portugal CAM, Crespo JG, Velizarov S. Tannery Effluent Treatment by Nanofiltration, Reverse Osmosis and Chitosan Modified Membranes. Membranes. 2020; 10(12):378. https://doi.org/10.3390/membranes10120378
Chicago/Turabian StyleZakmout, Asmaa, Fatma Sadi, Carla A. M. Portugal, João G. Crespo, and Svetlozar Velizarov. 2020. "Tannery Effluent Treatment by Nanofiltration, Reverse Osmosis and Chitosan Modified Membranes" Membranes 10, no. 12: 378. https://doi.org/10.3390/membranes10120378
APA StyleZakmout, A., Sadi, F., Portugal, C. A. M., Crespo, J. G., & Velizarov, S. (2020). Tannery Effluent Treatment by Nanofiltration, Reverse Osmosis and Chitosan Modified Membranes. Membranes, 10(12), 378. https://doi.org/10.3390/membranes10120378






