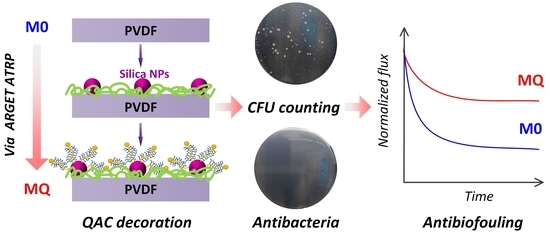
Membrane Biofouling Control by Surface Modification of Quaternary Ammonium Compound Using Atom-Transfer Radical-Polymerization Method with Silica Nanoparticle as Interlayer
Abstract
:1. Introduction
2. Materials and methods
2.1. Reagents
2.2. Membrane Fabrication
2.3. Membrane Characterization
2.4. Evaluation of Antibacterial Activity
2.5. Evaluation of Antibiofouling Performance
2.6. Stability Evaluation of the QAC-Modified Membrane
3. Results and Discussion
3.1. Membrane Characterization
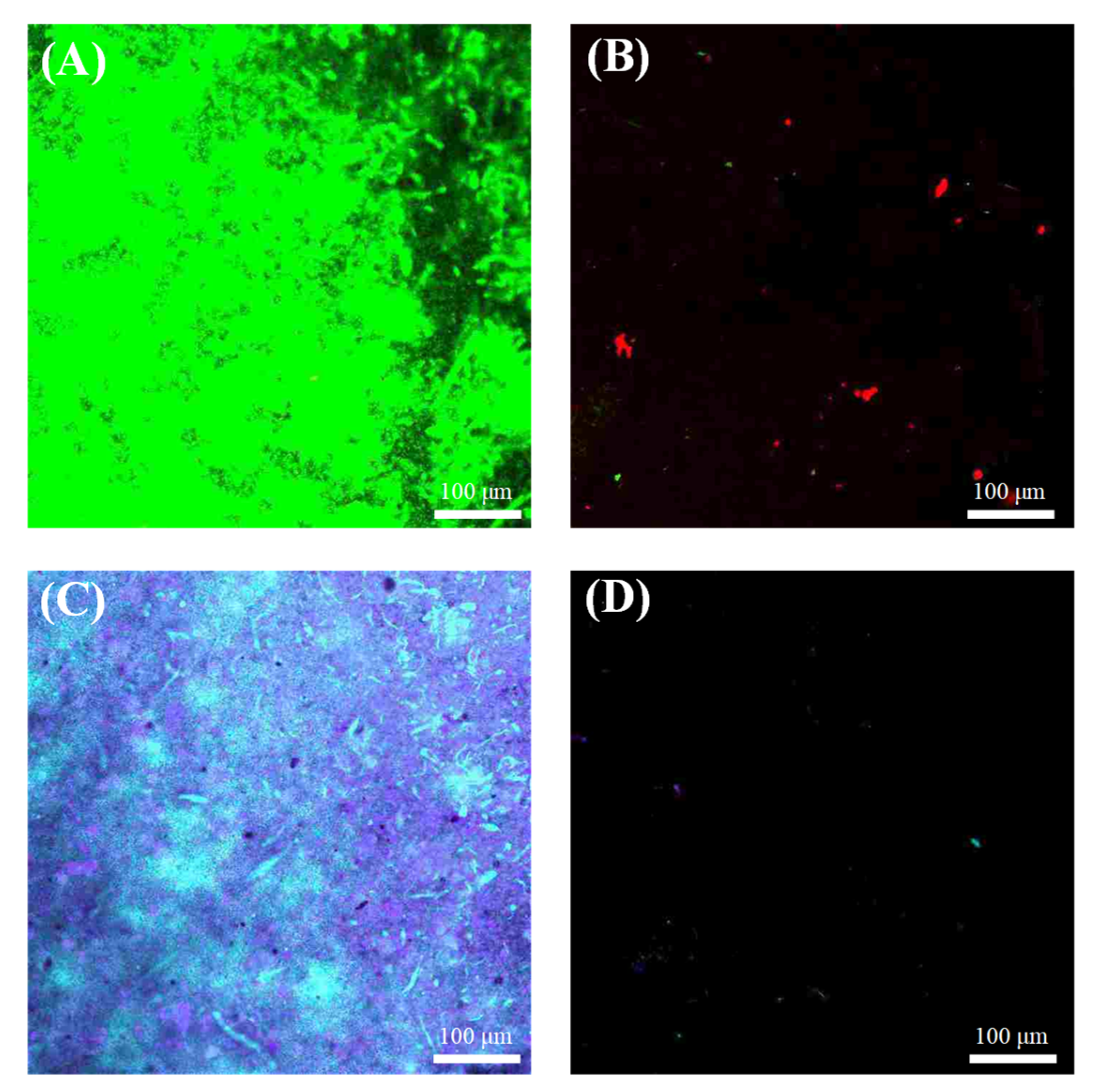
3.2. Evaluation of Antibacterial Activity
3.3. Antibiofouling Performance
3.4. Evaluation of QAC Stability by Repeated Fouling and Cleaning Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fane, A.G.; Wang, R.; Hu, M. Synthetic Membranes for Water Purification: Status and Future, Angew. Chem. Int. Edit. 2015, 54, 3368–3386. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Y.; Ladewig, B.P. A review of reverse osmosis membrane fouling and control strategies. Sci. Total Environ. 2017, 595, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xue, Z.; Saikaly, P.; Nunes, S.P.; Bluver, T.R.; Liu, W. Membrane biofouling in a wastewater nitrification reactor: Microbial succession from autotrophic colonization to heterotrophic domination. Water Res. 2016, 88, 337–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- She, Q.; Wang, R.; Fane, A.G.; Tang, C.Y. Membrane fouling in osmotically driven membrane processes: A review. J. Membr. Sci. 2016, 499, 201–233. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.Q.; Oh, Y.; Zhou, Z.; Shin, H.; Chae, S. Fouling in membrane bioreactors: An updated review. Water Res. 2017, 144, 151–180. [Google Scholar] [CrossRef]
- Lutchmiah, K.; Verliefde, A.R.; Roest, K.; Rietveld, L.C.; Cornelissen, E.R. Forward osmosis for application in wastewater treatment: A review. Water Res. 2014, 58, 179–197. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Y. Control and cleaning of membrane biofouling by energy uncoupling and cellular communication. Environ. Sci. Technol. 2011, 45, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, Z.; Wang, X.; Zheng, X.; Ma, J.; Wu, Z. Microbial responses to membrane cleaning using sodium hypochlorite in membrane bioreactors: Cell integrity, key enzymes and intracellular reactive oxygen species. Water Res. 2016, 88, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Faria, A.F.; Ma, J.; Elimelech, M. Mitigation of Biofilm Development on Thin-Film Composite Membranes Functionalized with Zwitterionic Polymers and Silver Nanoparticles. Environ. Sci. Technol. 2017, 51, 182–191. [Google Scholar] [CrossRef]
- Qi, L.; Hu, Y.; Liu, Z.; An, X.; Zeev, E.B. Improved anti-biofouling performance of thin-film composite forward-osmosis membranes containing passive and active moieties. Environ. Sci. Technol. 2018, 52, 9684–9693. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, J.; Tang, C.Y.; Wang, Z.; Ng, H.Y.; Wu, Z. Antibiofouling polyvinylidene fluoride membrane modified by quaternary ammonium compound: Direct contact-killing versus induced indirect contact-killing. Environ. Sci. Technol. 2016, 50, 5086–5093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, Y.; Wang, J.; Cao, B.; Tang, C.Y. In situ reduction of silver by polydopamine: A novel antimicrobial modification of a thin-film composite polyamide membrane. Environ. Sci. Technol. 2016, 50, 9543–9550. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yu, C.; Li, Q. Novel regenerable antimicrobial nanocomposite membranes: Effect of silver loading and valence state. J. Membr. Sci. 2017, 531, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Ping, M.; Wu, Z.; Tang, C.Y.; Wang, Z. Microfiltration membranes modified by silver-decorated biomimetic silica nanopollens for mitigating biofouling: Synergetic effects of nanopollens and silver nanoparticles. J. Membr. Sci. 2020, 597, 117773. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, Y.; Wu, Z.; Liu, M.; Wang, Z. Thin-film nanocomposite membranes incorporated with water stable metal-organic framework CuBTTri for mitigating biofouling. J. Membr. Sci. 2019, 582, 289–297. [Google Scholar] [CrossRef]
- Seyedpour, S.F.; Rahimpour, A.; Najafpour, G. Facile in-situ assembly of silver-based MOFs to surface functionalization of TFC membrane: A novel approach toward long-lasting biofouling mitigation. J. Membr. Sci. 2018, 573, 257–269. [Google Scholar] [CrossRef]
- Mozafari, M.; Seyedpour, S.F.; Salestan, S.K.; Rahimpour, A.; Shamsabadi, A.A.; Firouzjaei, M.D.; Esfahani, M.R.; Tiraferri, A.; Mohsenian, H.; Sangermano, M.; et al. Facile Cu-BTC surface modification of thin chitosan film coated polyethersulfone membranes with improved antifouling properties for sustainable removal of manganese. J. Membr. Sci. 2019, 588, 117200. [Google Scholar] [CrossRef]
- Zhu, J.; Hou, J.; Zhang, Y.; Tian, M.; He, T.; Liu, J.; Chen, V. Polymeric antimicrobial membranes enabled by nanomaterials for water treatment. J. Membr. Sci. 2018, 550, 173–197. [Google Scholar] [CrossRef]
- Ping, M.; Zhang, X.; Liu, M.; Wu, Z.; Wang, Z. Surface modification of polyvinylidene fluoride membrane by atom-transfer radical-polymerization of quaternary ammonium compound for mitigating biofouling. J. Membr. Sci. 2018, 570, 286–293. [Google Scholar] [CrossRef]
- Lee, S.; Cho, H.; Ha, Y.; Kim, S.; Chung, B.; Son, W.; Kang, K.; Jung, Y.; Park, K.; Lee, J. Enhancing the durability of filtration the ultrafine aerosol by electrospun polymer filter containing quaternary ammonium moiety. Polymer 2017, 121, 211–216. [Google Scholar] [CrossRef]
- Perreault, F.; Jaramillo, H.; Xie, M.; Ude, M.; Nghiem, L.D.; Elimelech, M. Biofouling mitigation in forward osmosis using graphene oxide functionalized thin-film composite membranes. Environ. Sci. Technol. 2016, 50, 5840–5848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.; Boo, C.; Shi, W.; Rolf, J.; Shaulsky, E.; Cheng, W.; Plata, D.L.; Qu, J.; Elimelech, M. Engineering Carbon Nanotube Forest Superstructure for Robust Thermal Desalination Membranes. Adv. Funct. Mater. 2019, 29, 1903125. [Google Scholar] [CrossRef]
- Yousefi, N.; Lu, X.; Elimelech, M.; Tufenkji, N. Environmental performance of graphene-based 3D macrostructures. Nat. Nanotechnol. 2019, 14, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Dreyer, D.; Bielawski, C.; Paul, D.; Freeman, B. Surface modification of water purification membranes: A review. Angew. Chem. Int. Ed. 2017, 56, 4662–4711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galiano, F.; André Schmidt, S.; Ye, X.; Kumar, R.; Mancuso, R.; Curcio, E.; Gabriele, B.; Hoinkis, J.; Figoli, A. UV-LED induced bicontinuous microemulsions polymerization for surface modification of commercial membranes-enhancing the antifouling properties. Sep. Purif. Technol. 2018, 194, 149–160. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, Z.; Huang, X.; Fan, W.; Yu, W.; Zhang, Z.; Li, L.; Mao, C. Hemocompatibility and oxygenation performance of polysulfone membranes grafted with polyethylene glycol and heparin by plasma-induced surface modification. J. Biomed. Mater. Res. B 2017, 105, 1737–1746. [Google Scholar] [CrossRef]
- Yue, W.; Li, H.; Xiang, T.; Qin, H.; Sun, S.; Zhao, C. Grafting of zwitterion from polysulfone membrane via surface-initiated ATRP with enhanced antifouling property and biocompatibility. J. Membr. Sci. 2013, 446, 79–91. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, X.; Ni, L.; Tang, Z.; Zhang, Y. Antibacterial cellulose membrane via one-step covalent immobilization of ammonium/amine groups. Desalination 2015, 359, 156–166. [Google Scholar] [CrossRef]
- Ye, G.; Lee, J.; Perreault, F.; Elimelech, M. Controlled architecture of dual-functional block copolymer brushes on thin-film composite membranes for integrated “Defending” and “Attacking” strategies against biofouling. ACS Appl. Mater. Interfaces 2015, 7, 23069–23079. [Google Scholar] [CrossRef]
- Carter, B.; Sengupta, A.; Qian, X.; Ulbricht, M.; Wichramasinghe, S. Controlling external versus internal pore modification of ultrafiltration membranes using surface-initiated AGET-ATRP. J. Membr. Sci. 2018, 554, 109–116. [Google Scholar] [CrossRef]
- Keating, J.; Sorci, M.; Kocsis, I.; Setaro, A.; Barboiu, M.; Underhill, P.; Belfort, G. Atmospheric pressure plasma—ARGET ATRP modification of poly(ether sulfone) membranes: A combination attack. J. Membr. Sci. 2018, 546, 151–157. [Google Scholar] [CrossRef]
- Xue, Q.; Cao, H.; Meng, F.; Quan, M.; Gong, Y.K. Cell membrane mimetic coating immobilized by mussel-inspired adhesion on commercial ultrafiltration membrane to enhance antifouling performance. J. Membr. Sci. 2017, 528, 1–11. [Google Scholar] [CrossRef]
- Yang, H.C.; Liao, K.J.; Huang, H.; Wu, Q.Y.; Wan, L.S. Mussel-inspired modification of a polymer membrane for ultra-high water permeability and oil-in-water emulsion separation. J. Mater. Chem. A 2014, 2, 10225–10230. [Google Scholar] [CrossRef]
- Li, M.; Xu, J.; Chang, C.Y.; Feng, C.; Zhang, L. Bioinspired fabrication of composite nanofiltration membrane based on the formation of DA/PEI layer followed by crosslinking. J. Membr. Sci. 2014, 459, 62–71. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Z.; Shan, M.; Zhou, B.; Li, Y.; Li, B.; Niu, J.; Qian, X. Synergetic effects of oxidized carbon nanotubes and graphene oxide on fouling control and anti-fouling mechanism of polyvinylidene fluoride ultrafiltration membranes. J. Membr. Sci. 2013, 448, 81–92. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Z.; Wu, Z. Effects of solvent compositions on physicochemical properties and anti-fouling ability of PVDF microfiltration membranes for wastewater treatment. Desalination 2012, 297, 79–86. [Google Scholar] [CrossRef]
- Liu, C.; Lee, J.; Ma, J.; Elimelech, M. Antifouling thin-film composite membranes by controlled architecture of zwitterionic polymer brush layer. Environ. Sci. Technol. 2017, 51, 2161–2169. [Google Scholar] [CrossRef]
- Perreault, F.; Tousley, M.E.; Elimelech, M. Thin-Film composite polyamide membranes functionalized with biocidal graphene oxide nanosheets. Environ. Sci. Technol. Lett. 2014, 1, 71–76. [Google Scholar] [CrossRef]
- Li, M.; Neoh, K.G.; Xu, L.Q.; Wang, R.; Kang, E.T.; Lau, T.; Olszyna, D.P.; Chiong, E. Surface modification of silicone for biomedical applications requiring long-term antibacterial, antifouling, and hemocompatible properties. Langmuir 2012, 28, 16408–16422. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Z.; Zhang, J.; Zhang, X.; Ma, J.; Wu, Z. A novel composite conductive microfiltration membrane and its anti-fouling performance with an external electric field in membrane bioreactors. Sci. Rep. 2015, 5, 9268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wang, Z.; Tang, C.Y.; Ma, J.; Liu, M.; Ping, M.; Chen, M.; Wu, Z. Modification of microfiltration membranes by alkoxysilane polycondensation induced quaternary ammonium compounds grafting for biofouling mitigation. J. Membr. Sci. 2018, 549, 165–172. [Google Scholar] [CrossRef]
- Zhang, F.; Srinivasan, M.P. Self-assembled molecular films of aminosilanes and their immobilization capacities. Langmuir 2004, 20, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhu, J.; Qiu, M.; He, C. Antifouling PVDF membrane grafted with zwitterionic poly(lysine methacrylamide) brushes. RSC Adv. 2016, 6, 61434–61442. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef]
- Liu, C.X.; Zhang, D.R.; He, Y.; Zhao, X.S.; Bai, R. Modification of membrane surface for anti-biofouling performance: Effect of anti-adhesion and anti-bacteria approaches. J. Membr. Sci. 2010, 346, 121–130. [Google Scholar] [CrossRef]
- Tiller, J.C. Antimicrobial surfaces. Bioact. Surf. 2010, 240, 193–217. [Google Scholar]
- Kugler, R.; Bouloussa, O.; Rondelez, F. Evidence of a charge-density threshold for optimum efficiency of biocidal cationic surfaces. Microbiology 2005, 151, 1341–1348. [Google Scholar] [CrossRef] [Green Version]
- Bieser, A.M.; Tiller, J.C. Mechanistic considerations on contact-active antimicrobial surfaces with controlled functional group densities. Macromol. Biosci. 2011, 11, 526–534. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, X.; Wang, Z.; Liu, M.; Wang, L.; Wu, Z. Impacts of quaternary ammonium compounds on membrane bioreactor performance: Acute and chronic responses of microorganisms. Water Res. 2018, 134, 153–161. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, X.; Wang, Z.; Wang, L.; Wu, Z. QAC modified PVDF membranes: Antibiofouling performance, mechanisms, and effects on microbial communities in an MBR treating municipal wastewater. Water Res. 2017, 120, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Liu, S. Antibacterial surface design-Contact kill. Prog. Surf. Sci. 2016, 91, 136–153. [Google Scholar] [CrossRef]
- Murata, H.; Koepsel, R.R.; Matyjaszewski, K.; Russell, A.J. Permanent, non-leaching antibacterial surfaces-2: How high density cationic surfaces kill bacterial cells. Biomaterials 2007, 28, 4870–4879. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Pereira, A.M.; Pereira, M.C.; Melo, L.F.; Simoes, M. Physiological changes induced by the quaternary ammonium compound benzyldimethyldodecylammonium chloride on Pseudomonas fluorescens. J. Antimicrob. Chemoth. 2011, 66, 1036–1043. [Google Scholar] [CrossRef] [Green Version]
- Kolodkin-Gal, I.; Hazan, R.; Gaathon, A.; Carmeli, S.; Engelberg-Kulka, H. A linear pentapeptide is a quorum-sensing factor required for mazEF-mediated cell death in Escherichia coli. Science 2007, 318, 652–655. [Google Scholar] [CrossRef] [Green Version]








| No. | Compositions | Remarks |
|---|---|---|
| M0 | The base MF membrane | From Millipore |
| MP | M0 coated with a PDA/PEI layer | By crosslinking |
| MSi | MP decorated with silica NPs layer | Via silicification reaction |
| MBr | MSi grafted with BiBB | BiBB as an initiator |
| MQ | MBr grafted with QACs | Via ARGET-ATRP |
| No. | Surface element Composition (%) | |||||
|---|---|---|---|---|---|---|
| C | F | O | N | Si | Br | |
| M0 | 61.92 | 13.05 | 24.71 | 0.31 | ||
| MP | 63.57 | 11.03 | 22.98 | 2.42 | ||
| MSi | 54.21 | 22.92 | 18.51 | 1.55 | 2.81 | |
| MBr | 57.14 | 23.3 | 15.62 | 1.68 | 0.93 | 1.33 |
| MQ | 65.64 | 5.56 | 22.97 | 3.45 | 1.98 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, L.; Ping, M.; Zhang, X. Membrane Biofouling Control by Surface Modification of Quaternary Ammonium Compound Using Atom-Transfer Radical-Polymerization Method with Silica Nanoparticle as Interlayer. Membranes 2020, 10, 417. https://doi.org/10.3390/membranes10120417
Ren L, Ping M, Zhang X. Membrane Biofouling Control by Surface Modification of Quaternary Ammonium Compound Using Atom-Transfer Radical-Polymerization Method with Silica Nanoparticle as Interlayer. Membranes. 2020; 10(12):417. https://doi.org/10.3390/membranes10120417
Chicago/Turabian StyleRen, Lehui, Meng Ping, and Xingran Zhang. 2020. "Membrane Biofouling Control by Surface Modification of Quaternary Ammonium Compound Using Atom-Transfer Radical-Polymerization Method with Silica Nanoparticle as Interlayer" Membranes 10, no. 12: 417. https://doi.org/10.3390/membranes10120417
APA StyleRen, L., Ping, M., & Zhang, X. (2020). Membrane Biofouling Control by Surface Modification of Quaternary Ammonium Compound Using Atom-Transfer Radical-Polymerization Method with Silica Nanoparticle as Interlayer. Membranes, 10(12), 417. https://doi.org/10.3390/membranes10120417