Boron Nitride Nanotube (BNNT) Membranes for Energy and Environmental Applications
Abstract
:1. Introduction
2. Boron Nitride Nanotubes (BNNTs)
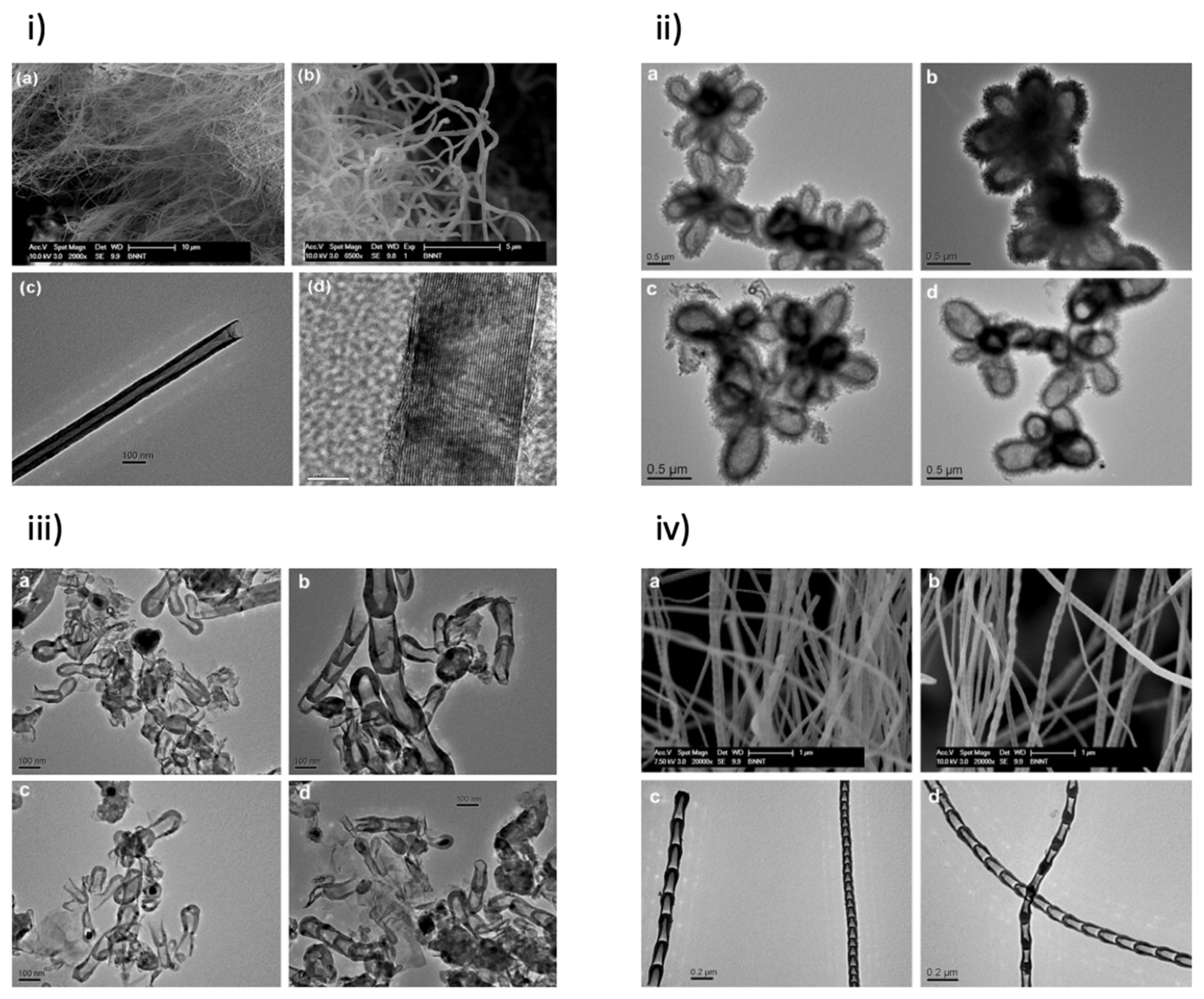
2.1. BNNT Synthesis Methods
2.2. Composites of BNNTs
2.3. BNNT Applications
3. BNNT Membranes
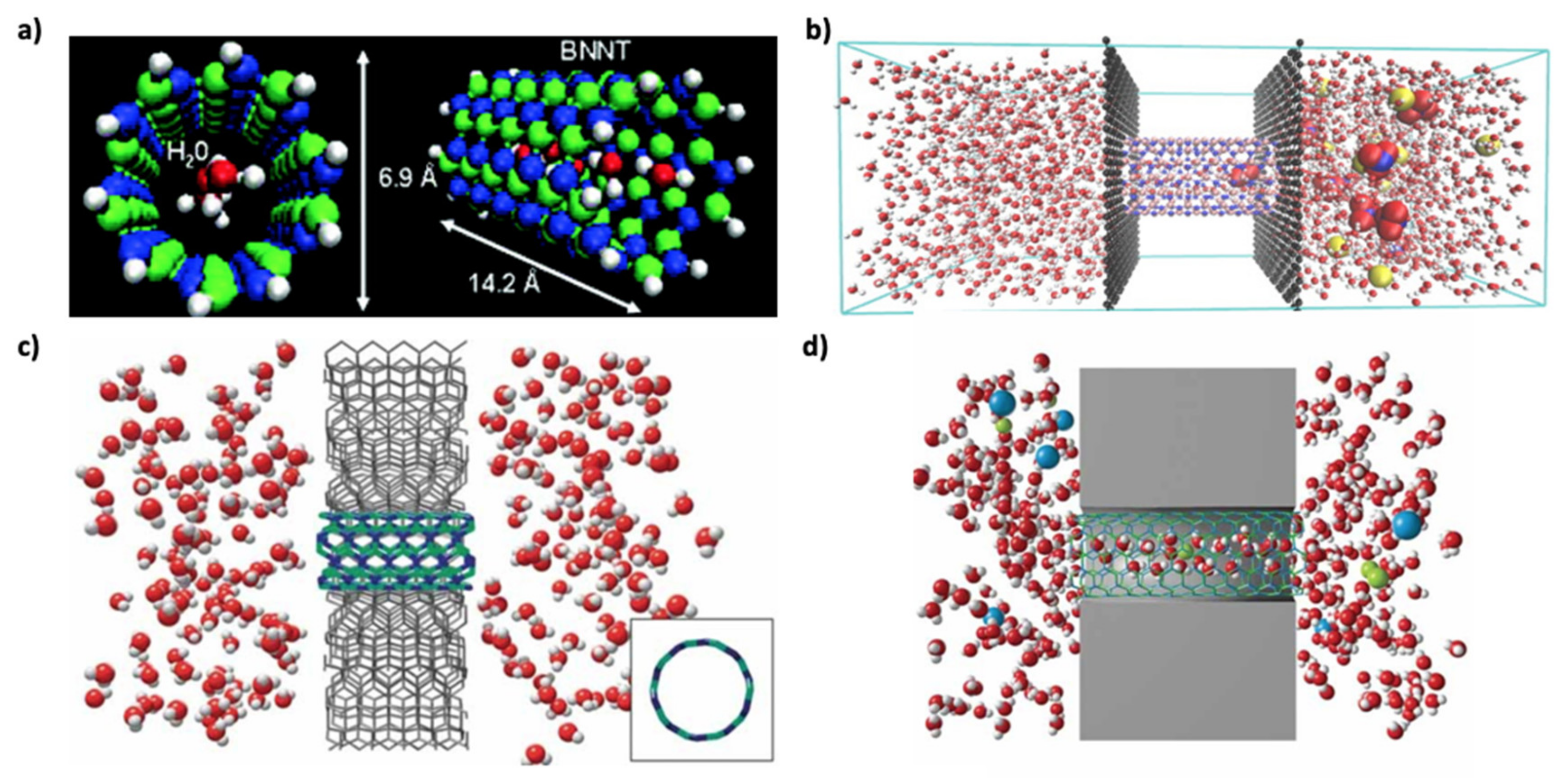
3.1. Wettability and Water Permeation
3.2. Thermal Conductivity and Resistance
3.3. Mechanical Strength
3.4. Electrical Properties
4. Perspectives on Key Membrane Studies
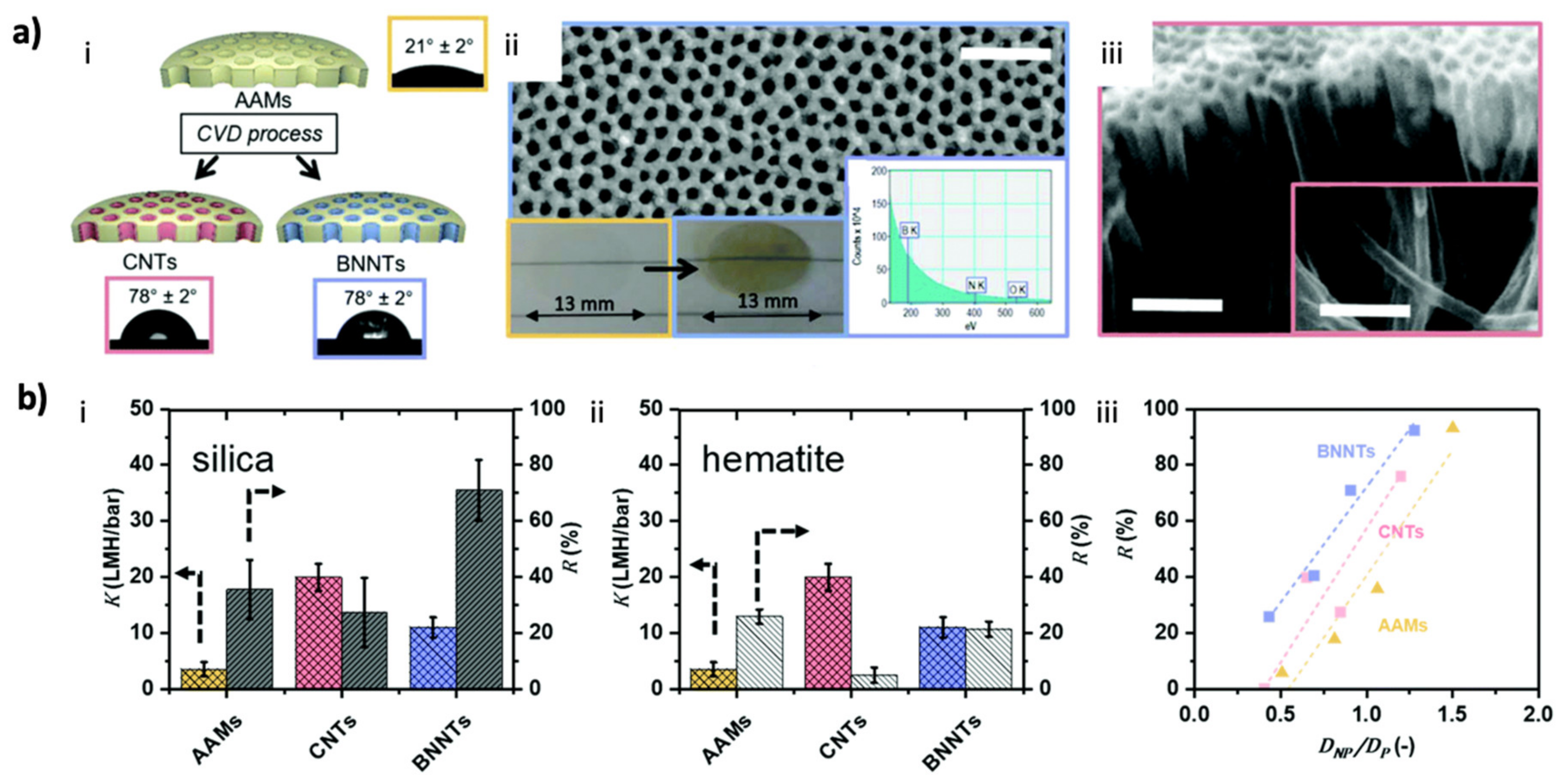
4.1. Water Treatment Membranes
4.2. Gas. Separation and Sensor Membranes
4.3. Battery Separator and Proton Exchange Membranes
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- AZoM. The applications of nanomaterials. AZO MATERIALS. 2001. Available online: https://www.azom.com/article.aspx?ArticleID=1066 (accessed on 10 November 2020).
- Ghasemzadeh, G.; Momenpour, M.; Omidi, F.; Hosseini, M.R.; Ahani, M.; Barzegari, A. Applications of nanomaterials in water treatment and environmental remediation. Front. Environ. Sci. Eng. 2014, 8, 471–482. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A review of removal of pollutants from water/wastewater using different types of nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 825910. [Google Scholar] [CrossRef] [Green Version]
- Bottino, A.; Capannelli, G.; D’Asti, V.; Piaggio, P. Preparation and properties of novel organic-inorganic porous membranes. Sep. Purif. Technol. 2001, 22–23, 269–275. [Google Scholar] [CrossRef]
- Sharma, V.P.; Sharma, U.; Chattopadhyay, M.; Shukla, V. Advance applications of nanomaterials: A review. Mater. Today Proc. 2018, 5, 6376–6380. [Google Scholar] [CrossRef]
- Ray, S.S.; Bakshi, H.S.; Dangayach, R.; Singh, R.; Deb, C.K.; Ganesapillai, M.; Chen, S.-S.; Purkait, M.K. Recent developments in nanomaterials-modified membranes for improved membrane distillation performance. Membranes 2020, 10, 140. [Google Scholar]
- Kim, J.H.; Pham, T.V.; Hwang, J.H.; Kim, C.S.; Kim, M.J. Boron nitride nanotubes: Synthesis and applications. Nano Converg. 2018, 5, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chopra, N.G.; Luyken, R.J.; Cherrey, K.; Crespi, V.H.; Cohen, M.L.; Louie, S.G.; Zettl, A. Boron nitride nanotubes. Science 1995, 269, 966. [Google Scholar] [CrossRef]
- Golberg, D.V.; Bando, Y.; Huang, Y.; Terao, T.; Mitome, M.; Tang, C.; Zhi, C. Boron nitride nanotubes and nanosheets. ACS Nano 2010, 4, 2979–2993. [Google Scholar] [CrossRef]
- Arenal, R.; Ferrari, A.C.; Reich, S.; Wirtz, L.; Mevellec, J.-Y.; Lefrant, S.; Rubio, A.; Loiseau, A. Raman spectroscopy of single-wall boron nitride nanotubes. Nano Lett. 2006, 6, 1812–1816. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Tanur, A.E.; Walker, G. Synthesis and hydrogen storage properties of different types of boron nitride nanostructures. Int. J. Hydrogen Energy 2010, 35, 4138–4143. [Google Scholar] [CrossRef]
- Fakrach, B.; Rahmani, A.; Chadli, H.; Sbai, K.; Bentaleb, M.; Bantignies, J.-L.; Sauvajol, J.-L. Infrared spectrum of single-walled boron nitride nanotubes. Phys. Rev. B 2012, 85, 115437. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y. 5 boron nitride nanotubes: Synthesis and structure. In Nanotubes and Nanofibers; CRC Press: Boca Raton, FL, USA, 2006; Volume 157. [Google Scholar]
- Celik-Aktas, A.; Zuo, J.-M.; Stubbins, J.F.; Tang, C.; Bando, Y. Structure and chirality distribution of multiwalled boron nitride nanotubes. Appl. Phys. Lett. 2005, 86, 133110. [Google Scholar] [CrossRef]
- Zhi, C.; Bando, Y.; Tang, C.; Golberg, D. Boron nitride nanotubes. Mater. Sci. Eng. R Rep. 2010, 70, 92–111. [Google Scholar] [CrossRef]
- Kratschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C 60: A new form of carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- Amanda, L.T.; Cheol, P.; Joseph, W.L.; Hoa, H.L.; Luke, J.G.; Sang-Hyon, C.; Samantha, A.; Peter, G.; Sharon, L.; Jung, K.H.; et al. Boron nitride nanotube: Synthesis and applications. Proc. SPIE 2014. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Bhandari, S.; Tiwari, B.; Yapici, N.; Zhang, D.; Yap, Y.K. Boron nitride nanotubes: Recent advances in their synthesis, functionalization, and applications. Molecules 2016, 21, 922. [Google Scholar] [CrossRef]
- Smith, M.W.; Stevens, J.C.; Jordan, K.C. Induction-Coupled Plasma Synthesis of Boron Nitrade Nanotubes. U.S. Patent WO2015066428A3, 5 November 2015. [Google Scholar]
- Smith, M.W.; Jordan, K.C.; Stevens, J.C.; Whitney, R.R. Boron Nitride Nanotube SYNTHESIS via Direct Induction. U.S. Patent WO2016186721A1, 28 December 2017. [Google Scholar]
- Li, L.H.; Chen, Y.; Glushenkov, A.M. Boron nitride nanotube films grown from boron ink painting. J. Mater. Chem. 2010, 20, 9679–9683. [Google Scholar] [CrossRef] [Green Version]
- Li, L.H.; Chen, Y.; Glushenkov, A.M. Synthesis of boron nitride nanotubes by boron ink annealing. Nanotechnology 2010, 21, 105601. [Google Scholar] [CrossRef]
- Chen, Y.; Chadderton, L.T.; Fitz Gerald, J.; Williams, J.S. A solid-state process for formation of boron nitride nanotubes. Appl. Phys. Lett. 1999, 74, 2960–2962. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Chen, Y.; Liu, Y.; Fu, L.; Huang, C.; Llewellyn, D. Over 1.0 mm-long boron nitride nanotubes. Chem. Phys. Lett. 2008, 463, 130–133. [Google Scholar] [CrossRef]
- Han, W.; Bando, Y.; Kurashima, K.; Sato, T. Synthesis of boron nitride nanotubes from carbon nanotubes by a substitution reaction. Appl. Phys. Lett. 1998, 73, 3085–3087. [Google Scholar] [CrossRef]
- Golberg, D.; Bando, Y.; Kurashima, K.; Sato, T. MoO3-promoted synthesis of multi-walled BN nanotubes from C nanotube templates. Chem. Phys. Lett. 2000, 323, 185–191. [Google Scholar] [CrossRef]
- Lourie, O.R.; Jones, C.R.; Bartlett, B.M.; Gibbons, P.C.; Ruoff, R.S.; Buhro, W.E. CVD Growth of Boron Nitride Nanotubes. Chem. Mater. 2000, 12, 1808–1810. [Google Scholar] [CrossRef]
- Tang, C.; Bando, Y.; Sato, T.; Kurashima, K. A novel precursor for synthesis of pure boron nitride nanotubes. Chem. Commun. 2002, 1290–1291. [Google Scholar] [CrossRef] [PubMed]
- Golberg, D.; Bando, Y.; Eremets, M.; Takemura, K.; Kurashima, K.; Yusa, H. Nanotubes in boron nitride laser heated at high pressure. Appl. Phys. Lett. 1996, 69, 2045–2047. [Google Scholar] [CrossRef]
- Laude, T.; Matsui, Y.; Marraud, A.; Jouffrey, B. Long ropes of boron nitride nanotubes grown by a continuous laser heating. Appl. Phys. Lett. 2000, 76, 3239–3241. [Google Scholar] [CrossRef]
- Yu, D.P.; Sun, X.S.; Lee, C.-S.; Bello, I.; Lee, S.T.; Gu, H.D.; Leung, K.M.; Zhou, G.W.; Dong, Z.F.; Zhang, Z. Synthesis of boron nitride nanotubes by means of excimer laser ablation at high temperature. Appl. Phys. Lett. 1998, 72, 1966–1968. [Google Scholar] [CrossRef] [Green Version]
- Arenal, R.; Stephan, O.; Cochon, J.-L.; Loiseau, A. Root-Growth mechanism for single-walled boron nitride nanotubes in laser vaporization technique. J. Am. Chem. Soc. 2007, 129, 16183–16189. [Google Scholar] [CrossRef]
- Yeh, Y.-W.; Raitses, Y.; Koel, B.E.; Yao, N. Stable synthesis of few-layered boron nitride nanotubes by anodic arc discharge. Sci. Rep. 2017, 7, 3075. [Google Scholar] [CrossRef]
- Loiseau, A.; Willaime, F.; Demoncy, N.; Hug, G.; Pascard, H. Boron nitride nanotubes with reduced numbers of layers synthesized by arc discharge. Phys. Rev. Lett. 1996, 76, 4737–4740. [Google Scholar] [CrossRef]
- Saito, Y.; Maida, M.; Matsumoto, T. Structures of boron nitride nanotubes with single-layer and multilayers produced by arc discharge. Jpn. J. Appl. Phys. 1999, 38, 159–163. [Google Scholar] [CrossRef]
- Smith, M.W.; Jordan, K.C.; Park, C.; Kim, J.-W.; Lillehei, P.T.; Crooks, R.; Harrison, J.S. Very long single- and few-walled boron nitride nanotubes via the pressurized vapor/condenser method. Nanotechnology 2009, 20, 505604. [Google Scholar] [CrossRef] [PubMed]
- Zhi, C.Y.; Bando, Y.; Tang, C.C.; Huang, Q.; Golberg, D. Boron nitride nanotubes: Functionalization and composites. J. Mater. Chem. 2008, 18, 3900–3908. [Google Scholar] [CrossRef]
- Wang, J.; Lee, C.H.; Yap, Y.K. Recent advancements in boron nitride nanotubes. Nanoscale 2010, 2, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Akdim, B.; Pachter, R.; Duan, X.; Adams, W.W. Comparative theoretical study of single-wall carbon and boron-nitride nanotubes. Phys. Rev. B 2003, 67, 245404. [Google Scholar] [CrossRef]
- Wang, B.-C.; Tsai, M.-H.; Chou, Y.-M. Comparative theoretical study of carbon nanotubes and boron-nitride nanotubes. Synth. Met. 1997, 86, 2379–2380. [Google Scholar] [CrossRef]
- Chen, C.-W.; Lee, M.-H.; Clark, S.J. Band gap modification of single-walled carbon nanotube and boron nitride nanotube under a transverse electric field. Nanotechnology 2004, 15, 1837–1843. [Google Scholar] [CrossRef]
- Lan, H.-P.; Ye, L.-H.; Zhang, S.; Peng, L.-M. Transverse dielectric properties of boron nitride nanotubes by ab initio electric field calculations. Appl. Phys. Lett. 2009, 94, 183110. [Google Scholar] [CrossRef]
- Dolati, S.; Fereidoon, A.; Kashyzadeh, K.R. A comparison study between boron nitride nanotubes and carbon nanotubes. Methods 2012, 49, 52. [Google Scholar]
- Jaffrennou, P.; Barjon, J.; Schmid, T.; Museur, L.; Kanaev, A.; Lauret, J.-S.; Zhi, C.Y.; Tang, C.; Bando, Y.; Golberg, D.; et al. Near-band-edge recombinations in multiwalled boron nitride nanotubes: Cathodoluminescence and photoluminescence spectroscopy measurements. Phys. Rev. B 2008, 77, 235422. [Google Scholar] [CrossRef] [Green Version]
- Li, L.H.; Chen, Y.; Lin, M.-Y.; Glushenkov, A.M.; Cheng, B.-M.; Yu, J. Single deep ultraviolet light emission from boron nitride nanotube film. Appl. Phys. Lett. 2010, 97, 141104. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Yague, M.A.; Larrañaga, A.; Gladkovskaya, O.; Stanley, A.; Tadayyon, G.; Guo, Y.; Sarasua, J.-R.; Tofail, S.A.M.; Zeugolis, D.I.; Pandit, A.; et al. Effects of polydopamine functionalization on boron nitride nanotube dispersion and cytocompatibility. Bioconjug. Chem. 2015, 26, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Tiano, A.L.; Park, C.; Lee, J.W.; Luong, H.H.; Gibbons, L.J.; Chu, S.-H.; Applin, S.; Gnoffo, P.; Lowther, S.; Kim, H.J. Boron Nitride Nanotubes: Synthesis and Applications; International Society for Optics and Photonics: Bellingham, WA, USA, 2018; p. 906006. [Google Scholar]
- Chen, X.; Zhang, L.; Park, C.; Fay, C.C.; Wang, X.; Ke, C. Mechanical strength of boron nitride nanotube-polymer interfaces. Appl. Phys. Lett. 2015, 107, 253105. [Google Scholar] [CrossRef]
- Li, T.; Tang, Z.; Huang, Z.; Yu, J. A comparison between the mechanical and thermal properties of single-walled carbon nanotubes and boron nitride nanotubes. Phys. E Low Dimens. Syst. Nanostruct. 2017, 85, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Alinezhad, H.; Ganji, M.D.; Soleymani, E.; Tajbakhsh, M. A comprehensive theoretical investigation about the bio-functionalization capability of single walled CNT, BNNT and SiCNT using DNA/RNA nucleobases. Appl. Surf. Sci. 2017, 422, 56–72. [Google Scholar] [CrossRef]
- Li, L.; Li, L.H.; Ramakrishnan, S.; Dai, X.J.; Nicholas, K.; Chen, Y.; Chen, Z.; Liu, X. Controlling wettability of boron nitride nanotube films and improved cell proliferation. J. Phys. Chem. C 2012, 116, 18334–18339. [Google Scholar] [CrossRef]
- Mintmire, J.W.; Dunlap, B.I.; White, C.T. Are fullerene tubules metallic? Phys. Rev. Lett. 1992, 68, 631–634. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science 2002, 297, 593. [Google Scholar] [CrossRef] [Green Version]
- De Marchi, L.; Neto, V.; Pretti, C.; Figueira, E.; Chiellini, F.; Morelli, A.; Soares, A.M.V.M.; Freitas, R. Toxic effects of multi-walled carbon nanotubes on bivalves: Comparison between functionalized and nonfunctionalized nanoparticles. Sci. Total Environ. 2018, 622, 1532–1542. [Google Scholar] [CrossRef]
- Meng, W.; Huang, Y.; Fu, Y.; Wang, Z.; Zhi, C. Polymer composites of boron nitride nanotubes and nanosheets. J. Mater. Chem. C 2014, 2, 10049–10061. [Google Scholar] [CrossRef]
- Xie, S.-Y.; Wang, W.; Fernando, K.A.S.; Wang, X.; Lin, Y.; Sun, Y.-P. Solubilization of boron nitride nanotubes. Chem. Commun. 2005, 3670–3672. [Google Scholar] [CrossRef] [PubMed]
- Zhi, C.; Bando, Y.; Tang, C.; Xie, R.; Sekiguchi, T.; Golberg, D. Perfectly dissolved boron nitride nanotubes due to polymer wrapping. J. Am. Chem. Soc. 2005, 127, 15996–15997. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Dmuchowski, C.M.; Park, C.; Fay, C.C.; Ke, C. Quantitative characterization of structural and mechanical properties of boron nitride nanotubes in high temperature environments. Sci. Rep. 2017, 7, 11388. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Fennimore, A.M.; Afanasiev, A.; Okawa, D.; Ikuno, T.; Garcia, H.; Li, D.; Majumdar, A.; Zettl, A. Isotope effect on the thermal conductivity of boron nitride nanotubes. Phys. Rev. Lett. 2006, 97, 085901. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Ajayi, T.D.; Morales, J.; Chowdhury, A.R.; Sauti, G.; Chu, S.; Park, C.; Xu, C. “Cheryl” Thermal properties of polymer-derived ceramic reinforced with boron nitride nanotubes. J. Am. Ceram. Soc. 2019, 102, 7584–7593. [Google Scholar] [CrossRef]
- Jakubinek, M.B.; Ashrafi, B.; Martinez-Rubi, Y.; Guan, J.; Rahmat, M.; Kim, K.S.; Dénommée, S.; Kingston, C.T.; Simard, B. Chapter 5—Boron nitride nanotube composites and applications. In Nanotube Superfiber Materials, 2nd ed.; Schulz, M.J., Shanov, V., Yin, Z., Cahay, M., Eds.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 91–111. [Google Scholar]
- Khatti, Z.; Hashemianzadeh, S.M. Boron nitride nanotube as a delivery system for platinum drugs: Drug encapsulation and diffusion coefficient prediction. Eur. J. Pharm. Sci. 2016, 88, 291–297. [Google Scholar] [CrossRef]
- Ciofani, G.; Mattoli, V. Boron Nitride Nanotubes in Nanomedicine; William Andrew Publishing: Norwich, NY, USA, 2016. [Google Scholar]
- Ciofani, G.; Raffa, V.; Menciassi, A.; Dario, P. Preparation of boron nitride nanotubes aqueous dispersions for biological applications. J. Nanosci. Nanotechnol. 2008, 8, 6223–6231. [Google Scholar] [CrossRef]
- Kang, J.H.; Park, C.; Sauti, G.; Smith, M.W.; Jordan, K.C.; Lowther, S.E.; Bryant, R.G. High Kinetic Energy Penetrator Shielding and High Wear Resistance Materials Fabricated with Boron Nitride Nanotubes (BNNTs) and BNNT Polymer Composites. U.S. Patent US9067385B2, 30 June 2015. [Google Scholar]
- Kang, J.H.; Park, C.; Harrison, J.S.; Smith, M.W.; Lowther, S.E.; Kim, J.-W.; Sauti, G. Energy Conversion Materials Fabricated with Boron Nitride Nanotubes (BNNTs) and BNNT Polymer Composites. KR Patent Application No. KR101810700B1, 19 December 2017. [Google Scholar]
- Son, M.; Park, H.; Liu, L.; Choi, H.; Kim, J.H.; Choi, H. Thin-film nanocomposite membrane with CNT positioning in support layer for energy harvesting from saline water. Chem. Eng. J. 2016, 284, 68–77. [Google Scholar] [CrossRef]
- Park, S.; Yang, E.; Park, H.; Choi, H. Fabrication of functionalized halloysite nanotube blended ultrafiltration membranes for high flux and fouling resistance. Environ. Eng. Res. 2019, 25, 771–778. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.A.; Cho, Y.H.; Choi, H.-G.; Nam, S.-E.; Kim, J.F.; Kim, Y.; Park, Y.; Park, H. Facile integration of halloysite nanotubes with bioadhesive as highly permeable interlayer in forward osmosis membranes. J. Ind. Eng. Chem. 2019, 73, 276–285. [Google Scholar] [CrossRef]
- Roh, J.S.; Lee, H.; Lee, T.H.; Yoon, H.W.; Choi, T.H.; Do, S.-H.; Yoo, S.Y.; Freeman, B.D.; Song, T.; Paik, U.; et al. Unprecedentedly Low CO2 Transport through Vertically Aligned, Conical Silicon Nanotube Membranes. Nano Lett. 2020, 20, 4754–4760. [Google Scholar] [CrossRef] [PubMed]
- Albu, S.P.; Ghicov, A.; Macak, J.M.; Hahn, R.; Schmuki, P. Self-Organized, Free-Standing TiO2Nanotube Membrane for Flow-through Photocatalytic Applications. Nano Lett. 2007, 7, 1286–1289. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, K.; Hoivik, N.; Jakobsen, H. Progress on free-standing and flow-through TiO2 nanotube membranes. Sol. Energy Mater. Sol. Cells 2012, 98, 24–38. [Google Scholar] [CrossRef]
- Casanova, S.; Mistry, S.; Mazinani, S.; Borg, M.K.; Chew, Y.M.J.; Mattia, D. Enhanced nanoparticle rejection in aligned boron nitride nanotube membranes. Nanoscale 2020, 12, 21138–21145. [Google Scholar] [CrossRef]
- Celik, E.; Choi, H. Carbon nanotube/polyethersulfone composite membranes for water filtration. In Modern Applications in Membrane Science and Technology; American Chemical Society (ACS): Washington, DC, USA, 2011; pp. 257–269. [Google Scholar]
- Das, R.; Ali, E.; Hamid, S.B.A.; Ramakrishna, S.; Chowdhury, Z.Z. Carbon nanotube membranes for water purification: A bright future in water desalination. Desalination 2014, 336, 97–109. [Google Scholar] [CrossRef]
- Choi, H.-G.; Shah, A.A.; Nam, S.-E.; Park, Y.; Park, H. Thin-film composite membranes comprising ultrathin hydrophilic polydopamine interlayer with graphene oxide for forward osmosis. Desalination 2019, 449, 41–49. [Google Scholar] [CrossRef]
- Abdelkarim, A.A.; Leaper, S.; Alberto, M.; Vijayaraghavan, A.; Fan, X.; Holmes, S.M.; Souaya, E.R.; Badawy, M.I.; Gorgojo, P. High flux and fouling resistant flat sheet polyethersulfone membranes incorporated with graphene oxide for ultrafiltration applications. Chem. Eng. J. 2018, 334, 789–799. [Google Scholar] [CrossRef] [Green Version]
- Bi, R.; Zhang, Q.; Zhang, R.; Su, Y.; Jiang, Z. Thin film nanocomposite membranes incorporated with graphene quantum dots for high flux and antifouling property. J. Membr. Sci. 2018, 553, 17–24. [Google Scholar] [CrossRef]
- Alayande, A.B.; Chae, S.; Kim, I.S. Surface morphology-dependent spontaneous bacterial behaviors on graphene oxide membranes. Sep. Purif. Technol. 2019, 226, 68–74. [Google Scholar] [CrossRef]
- Alayande, A.B.; Park, H.; Vrouwenvelder, J.S.; Kim, I.S. Implications of Chemical Reduction Using Hydriodic Acid on the Antimicrobial Properties of Graphene Oxide and Reduced Graphene Oxide Membranes. Small 2019, 15, e1901023. [Google Scholar] [CrossRef]
- Arsuaga, J.M.; Sotto, A.; del Rosario, G.; Martínez, A.; Molina, S.; Teli, S.B.; de Abajo, J. Influence of the type, size, and distribution of metal oxide particles on the properties of nanocomposite ultrafiltration membranes. J. Membr. Sci. 2013, 428, 131–141. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Aber, S.; Vatanpour, V.; Mahmoodi, N.M. Development of hydrophilic microporous PES ultrafiltration membrane containing CuO nanoparticles with improved antifouling and separation performance. Mater. Chem. Phys. 2019, 222, 338–350. [Google Scholar] [CrossRef]
- Chu, B.; Hsiao, B.S.; Ma, H. High Flux Fluid Separation Membranes Comprising a Cellulose or Cellulose Derivative Layer. International Patent Application No. EP2150356A4, 30 May 2012. [Google Scholar]
- Li, X.-L.; Zhu, L.-P.; Zhu, B.-K.; Xu, Y.-Y. High-flux and anti-fouling cellulose nanofiltration membranes prepared via phase inversion with ionic liquid as solvent. Sep. Purif. Technol. 2011, 83, 66–73. [Google Scholar] [CrossRef]
- Qadir, D.; Mukhtar, H.; Keong, L.K. Mixed Matrix Membranes for Water Purification Applications. Sep. Purif. Rev. 2017, 46, 62–80. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Leo, C.P.; Ahmad, A.L.; Ramli, W.K.W. Membranes with Great Hydrophobicity: A Review on Preparation and Characterization. Sep. Purif. Rev. 2015, 44, 109–134. [Google Scholar] [CrossRef]
- Li, L.H.; Chen, Y. Superhydrophobic Properties of Nonaligned Boron Nitride Nanotube Films. Langmuir 2010, 26, 5135–5140. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, X.; Dai, X.J.; Li, L.H.; Chen, Y. Surface wetting processing on BNNT films by selective plasma modes. Chin. Sci. Bull. 2013, 58, 3403–3408. [Google Scholar] [CrossRef] [Green Version]
- Won, C.Y.; Aluru, N.R. Water Permeation through a Subnanometer Boron Nitride Nanotube. J. Am. Chem. Soc. 2007, 129, 2748–2749. [Google Scholar] [CrossRef]
- Zhi, C.; Bando, Y.; Terao, T.; Tang, C.; Kuwahara, H.; Golberg, D. Towards thermoconductive, electrically insulating polymeric composites with boron nitride nanotubes as fillers. Adv. Funct. Mater. 2009, 19, 1857–1862. [Google Scholar] [CrossRef]
- Kallem, P.; Yanar, N.; Choi, H. Nanofiber-Based Proton Exchange Membranes: Development of Aligned Electrospun Nanofibers for Polymer Electrolyte Fuel Cell Applications. ACS Sustain. Chem. Eng. 2018, 7, 1808–1825. [Google Scholar] [CrossRef]
- Hajian, A.; Lindström, S.B.; Pettersson, T.; Hamedi, M.M.; Wågberg, L. Understanding the Dispersive Action of Nanocellulose for Carbon Nanomaterials. Nano Lett. 2017, 17, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.P.; Hurst, J.B.; Choi, S.R. Boron Nitride Nanotubes-Reinforced Glass Composites. J. Am. Ceram. Soc. 2006, 89, 388–390. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.-L.; Bi, J.-Q.; Sun, K.; Du, M.; Long, N.-N.; Bai, Y.-J. Fabrication of Alumina Ceramic Reinforced with Boron Nitride Nanotubes with Improved Mechanical Properties. J. Am. Ceram. Soc. 2011, 94, 3636–3640. [Google Scholar] [CrossRef]
- Cong, Z.; Lee, S. Study of mechanical behavior of BNNT-reinforced aluminum composites using molecular dynamics simulations. Compos. Struct. 2018, 194, 80–86. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Chang, K.J.; Louie, S.G. Electronic structure of radially deformed BN andBC3nanotubes. Phys. Rev. B 2001, 63, 205408. [Google Scholar] [CrossRef]
- Zhi, C.; Bando, Y.; Tang, C.; Golberg, D. Engineering of electronic structure of boron-nitride nanotubes by covalent functionalization. Phys. Rev. B 2006, 74, 153413. [Google Scholar] [CrossRef] [Green Version]
- Nakhmanson, S.M.; Calzolari, A.; Meunier, V.; Bernholc, J.; Nardelli, M.B. Spontaneous polarization and piezoelectricity in boron nitride nanotubes. Phys. Rev. B 2003, 67, 235406. [Google Scholar] [CrossRef] [Green Version]
- Suk, M.E.; Raghunathan, A.V.; Aluru, N.R. Fast reverse osmosis using boron nitride and carbon nanotubes. Appl. Phys. Lett. 2008, 92, 133120. [Google Scholar] [CrossRef]
- Azamat, J.; Khataee, A. Removal of nitrate ion from water using boron nitride nanotubes: Insights from molecular dynamics simulations. Comput. Theor. Chem. 2016, 1098, 56–62. [Google Scholar] [CrossRef]
- Hilder, T.A.; Gordon, D.; Chung, S.-H. Salt rejection and water transport through boron nitride nanotubes. Small 2009, 5, 2183–2190. [Google Scholar] [CrossRef]
- Hilder, T.A.; Gordon, D.; Chung, S.-H. Boron Nitride Nanotubes Selectively Permeable to Cations or Anions. Small 2009, 5, 2870–2875. [Google Scholar] [CrossRef] [PubMed]
- Azamat, J.; Khataee, A.; Joo, S.W. Separation of a heavy metal from water through a membrane containing boron nitride nanotubes: Molecular dynamics simulations. J. Mol. Model. 2014, 20, 2468. [Google Scholar] [CrossRef]
- Azamat, J.; Hazizadeh, B. Removal of Cd(II) from water using carbon, boron nitride and silicon carbide nanotubes. Membr. Water Treat. 2018, 9, 63–68. [Google Scholar]
- Liang, L.; Li, J.-C.; Zhang, L.; Zhang, Z.; Shen, J.-W.; Li, L.; Wu, J. Computer simulation of water desalination through boron nitride nanotubes. Phys. Chem. Chem. Phys. 2017, 19, 30031–30038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jia, L.; Zhang, J.; Li, J.; Liang, L.; Kong, Z.; Shen, J.-W.; Wang, X.; Zhang, W.; Wang, H. Understanding the effect of chemical modification on water desalination in boron nitride nanotubes via molecular dynamics simulation. Desalination 2019, 464, 84–93. [Google Scholar] [CrossRef]
- Lim, H.; Suh, B.L.; Kim, M.J.; Yun, H.; Kim, J.; Kim, B.J.; Jang, S.G. High-performance, recyclable ultrafiltration membranes from P4VP-assisted dispersion of flame-resistive boron nitride nanotubes. J. Membr. Sci. 2018, 551, 172–179. [Google Scholar] [CrossRef]
- Casanovaab, S.; Liuc, T.-Y.; Chewab, Y.-M.J.; Livingstoncd, A.; Mattiaab, D. High flux thin-film nanocomposites with embedded boron nitride nanotubes for nanofiltration. J. Membr. Sci. 2020, 597, 117749. [Google Scholar] [CrossRef]
- Madenli, E.C.; Yanar, N.; Choi, H. Enhanced antibacterial properties and suppressed biofilm growth on multi-walled carbon nanotube (MWCNT) blended polyethersulfone (PES) membranes. J. Environ. Chem. Eng. 2020, 104755. [Google Scholar] [CrossRef]
- Siria, A.; Poncharal, P.; Biance, A.-L.; Fulcrand, R.; Blase, X.; Purcell, S.T.; Bocquet, L. Giant osmotic energy conversion measured in a single transmembrane boron nitride nanotube. Nature 2013, 494, 455–458. [Google Scholar] [CrossRef]
- Chae, S.H.; Kim, Y.M.; Park, H.; Seo, J.; Lim, S.J.; Kim, J.H. Modeling and simulation studies analyzing the Pressure-Retarded Osmosis (PRO) and PRO-hybridized processes. Energies 2019, 12, 243. [Google Scholar] [CrossRef] [Green Version]
- Cetindag, S.; Pendse, A.; Praino, R.F.; Kim, S.; Shan, J.W. Enhanced electrokinetic energy conversion & ion-selective transport in macroscopic vertically aligned BNNT membranes. In Proceedings of the 2019 MRS Fall Meeting and Exhibit, Boston, MA, USA, 1–6 December 2019; p. B36.006. [Google Scholar]
- Yanar, N.; Kallem, P.; Son, M.; Park, H.; Kang, S.; Choi, H. A New era of water treatment technologies: 3D printing for membranes. J. Ind. Eng. Chem. 2020, 91, 1–14. [Google Scholar] [CrossRef]
- Yanar, N.; Son, M.; Park, H.; Choi, H. Bio-mimetically inspired 3D-printed honeycombed support (spacer) for the reduction of reverse solute flux and fouling of osmotic energy driven membranes. J. Ind. Eng. Chem. 2020, 83, 343–350. [Google Scholar] [CrossRef]
- Yanar, N.; Son, M.; Park, H.; Choi, H. Toward greener membranes with 3D printing technology. Environ. Eng. Res. 2020, 26, 200027. [Google Scholar] [CrossRef] [Green Version]
- Yanar, N.; Son, M.; Yang, E.; Kim, Y.; Park, H.; Nam, S.-E.; Choi, H. Investigation of the performance behavior of a forward osmosis membrane system using various feed spacer materials fabricated by 3D printing technique. Chemosphere 2018, 202, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jagirdar, P.; Naserifar, S.; Sahimi, M. Molecular simulation study of gas solubility and diffusion in a polymer-boron nitride nanotube composite. J. Phys. Chem. B 2016, 120, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
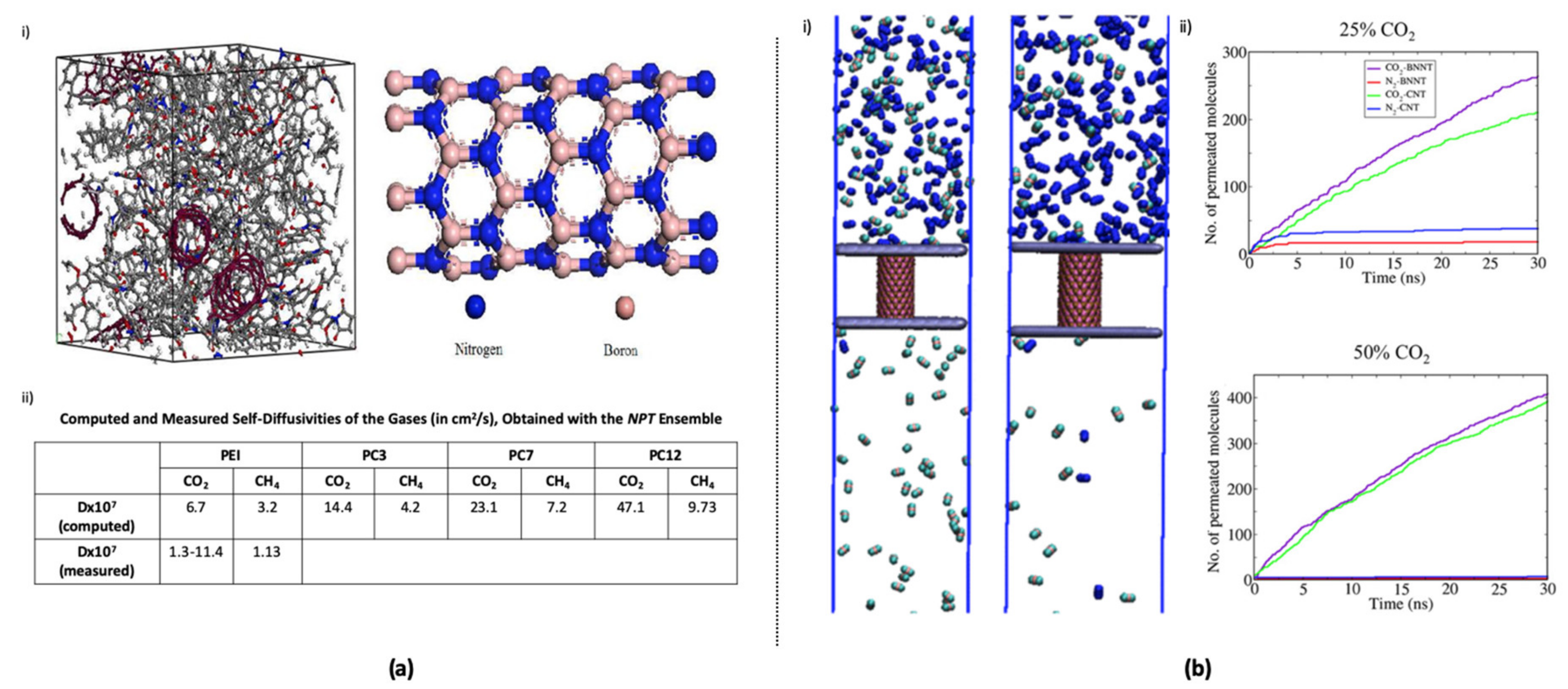
- Maurya, M.; Sappidi, P.K.; Singh, J.K. Selective separation of CO2 from flue gas using carbon and boron nitride nanotubes as a membrane. Energy Fuels 2020, 34, 7223–7231. [Google Scholar] [CrossRef]
- Yoo, S.-M.; Sharma, B.; Kim, J.-S. Boron nitride nanotubes (BNNTs) decorated Pd-ternary alloy (Pd63·2Ni34·3Co2.5) for H2 sensing. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Rahman, M.; Mateti, S.; Cai, Q.; Sultana, I.; Fan, Y.; Wang, X.; Hou, C.; Chen, Y. High temperature and high rate lithium-ion batteries with boron nitride nanotubes coated polypropylene separators. Energy Storage Mater. 2019, 19, 352–359. [Google Scholar] [CrossRef]
- Kaneko, K.; Hori, K.; Noda, S. Nanotubes make battery lighter and safer. Carbon 2020, 167, 596–600. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, Z.; Hua, W.; Liu, D.; Tao, T.; Rahman, M.; Lei, W.; Huang, S.; Chen, Y. Functionalized boron nitride nanosheets/graphene interlayer for fast and long-life lithium-sulfur batteries. Adv. Energy Mater. 2017, 7, 1602380. [Google Scholar] [CrossRef]
- Scheers, J.; Fantini, S.; Johansson, P. A review of electrolytes for lithium-sulphur batteries. J. Power Sources 2014, 255, 204–218. [Google Scholar] [CrossRef]
- Assink, R. Fouling mechanism of separator membranes for the iron/chromium redox battery. J. Membr. Sci. 1984, 17, 205–217. [Google Scholar] [CrossRef]



| Fabrication Method | Advantage | Disadvantage |
|---|---|---|
| Annealing of ball-milled BN powders in nitrogen (Ball milling) [24] | ||
| BN substitution method from CNT templates [26] |
|
|
| Borazine as precursor CVD [17] |
| Impurity [17] |
| Boron and magnesium oxide as precursors for CVD (BOCVD) [28] |
|
|
| Heating of Hexagonal BN [29,30] |
| • High-temperature requirement [29,30] |
| Laser Ablation/Vaporization [31] |
|
|
| Plasma Arc discharge [8] | • Simple and inexpensive setup [33] |
|
| Pressure Vapor/Condenser Method (PVC) [36] |
| • A significant fraction within the yarns of BN phases [9] |
 |  | ||
|---|---|---|---|
| BNNTs | BNNTs and CNTs | CNTs | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanar, N.; Yang, E.; Park, H.; Son, M.; Choi, H. Boron Nitride Nanotube (BNNT) Membranes for Energy and Environmental Applications. Membranes 2020, 10, 430. https://doi.org/10.3390/membranes10120430
Yanar N, Yang E, Park H, Son M, Choi H. Boron Nitride Nanotube (BNNT) Membranes for Energy and Environmental Applications. Membranes. 2020; 10(12):430. https://doi.org/10.3390/membranes10120430
Chicago/Turabian StyleYanar, Numan, Eunmok Yang, Hosik Park, Moon Son, and Heechul Choi. 2020. "Boron Nitride Nanotube (BNNT) Membranes for Energy and Environmental Applications" Membranes 10, no. 12: 430. https://doi.org/10.3390/membranes10120430
APA StyleYanar, N., Yang, E., Park, H., Son, M., & Choi, H. (2020). Boron Nitride Nanotube (BNNT) Membranes for Energy and Environmental Applications. Membranes, 10(12), 430. https://doi.org/10.3390/membranes10120430