Acidic Gases Solubility in Bis(2-Ethylhexyl) Sulfosuccinate Based Ionic Liquids Using the Predictive Thermodynamic Model
Abstract
1. Introduction
2. Theory
2.1. Theory of Calculation Methods
2.2. Computational Details
2.3. Computational of Fractional Free Volume
3. Results and Discussions
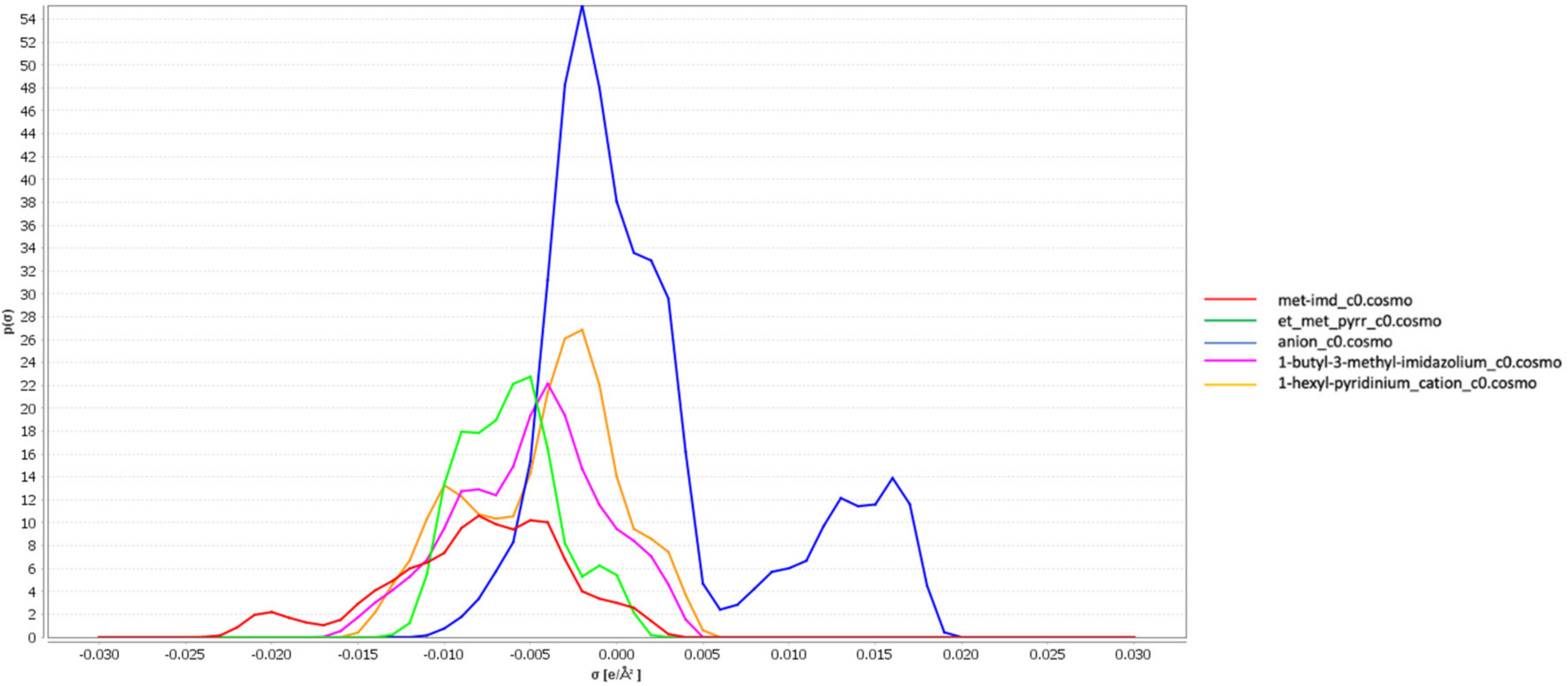
3.1. σ-Profiles of the Ionic Liquids
3.2. Screening Charges Densities
3.3. Densities and Volumetric Effects
3.4. Henry’s Law Predictions
3.5. Interpretation of Molecular Interactions with Sigma Profiles and Sigma Potentials
3.6. Free Volume Effects and Interactions Enthalpies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| [doc] | bis(2-Ethylhexyl) sulfosuccinate anion |
| [BF4] | tetrafluoroborate anion |
| [CF3SO3] | trifluoromethanesulfonate anion |
| [Tf2N] | bistriflamide anion |
| [bmim] | 1-butyl-3-methylimidazolium cation |
| [mim] | 1-methylimidazolium cation |
| [empyrr] | 1-ethyl-1-methylpyrrolidinium cation |
| [hpyr] | 1-hexylpyridinium cation |
| IL | ionic liquid |
| DFT | density functional theory |
| rmsd | root means square deviations |
References
- Mokhatab, S.; Poe, W.A.; Speight, J.G. Handbook of Natural Gas Transmission and Processing; Gulf Professional Publishing: Waltham, MA, USA, 2015; p. 261. [Google Scholar]
- Akhmetshina, A.I.; Yanbikov, N.R.; Atlaskin, A.A.; Trubyanov, M.M.; Mechergui, A.; Otvagina, K.V.; Razov, E.N.; Mochalova, A.E.; Vorotyntsev, I.V. Acidic gases separation from gas mixtures on the supported ionic liquid membranes providing the facilitated and solution-diffusion transport mechanisms. Membranes 2019, 9, 9. [Google Scholar] [CrossRef]
- Akhmetshina, A.I.; Petukhov, A.N.; Gumerova, O.R.; Vorotyntsev, A.V.; Nyuchev, A.V.; Vorotyntsev, I.V. Solubility of H2S and CO2 in imidazolium-based ionic liquids with bis(2-ethylhexyl) sulfosuccinate anion. J. Chem. Thermodyn. 2019, 130, 173–182. [Google Scholar] [CrossRef]
- Atlaskin, A.A.; Kryuchkov, S.S.; Yanbikov, N.R.; Smorodin, K.A.; Petukhov, A.N.; Trubyanov, M.M.; Vorotyntsev, V.M.; Vorotyntsev, I.V. Comprehensive experimental study of acid gases removal process by membrane-assisted gas absorption using imidazolium ionic liquids solutions absorbent. Sep. Purif. Technol. 2020, 239, 116578. [Google Scholar] [CrossRef]
- Atlaskin, A.A.; Kryuchkov, S.S.; Smorodin, K.A.; Markov, A.N.; Kazarina, O.V.; Zarubin, D.M.; Atlaskina, M.E.; Vorotyntsev, A.V.; Nyuchev, A.V.; Petukhov, A.N.; et al. Towards the potential of trihexyltetradecylphosphonium indazolide with aprotic heterocyclic ionic liquid as an efficient absorbent for membrane-assisted gas absorption technique for acid gas removal applications. Sep. Purif. Technol. 2020, 257, 117835. [Google Scholar] [CrossRef]
- Vorotyntsev, V.M.; Drozdov, P.N.; Vorotyntsev, I.V.; Murav’ev, D.V. Fine gas purification to remove slightly penetrating impurities using a membrane module with a feed reservoir. Dokl. Chem. 2006, 411, 243–245. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Sarwar, M.I.; Mecerreyes, D. Polymeric ionic liquids for CO2 capture and separation: Potential, progress and challenges. Polym. Chem. 2015, 6, 6435–6451. [Google Scholar] [CrossRef]
- Karkhanechi, H.; Salmani, S.; Asghari, M. A review on gas separation applications of supported ionic liquid membranes. ChemBioEng Rev. 2015, 2, 290–302. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Hancu, D.; Beckman, E.J.; Brennecke, J.F. Green processing using ionic liquids and CO2. Nature 1999, 399, 28–29. [Google Scholar] [CrossRef]
- Lei, Z.; Dai, C.; Chen, B. Gas solubility in ionic liquids. Chem. Rev. 2014, 114, 1289–1326. [Google Scholar] [CrossRef]
- Jin, F.; He, L.N.; Hu, Y.H. Advances in CO2 Capture, Sequestration, and Conversion; American Chemical Society: Washington, DC, USA, 2016. [Google Scholar]
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H. CO2 capture by a task-specific ionic liquid. J. Am. Chem. Soc. 2002, 124, 926–927. [Google Scholar] [CrossRef] [PubMed]
- Galán Sánchez, L.M.; Meindersma, G.W.; de Haan, A.B. Solvent properties of functionalized ionic liquids for CO2 absorption. Chem. Eng. Res. Design 2007, 85, 31–39. [Google Scholar] [CrossRef]
- Gutowski, K.E.; Maginn, E.J. Amine-functionalized task-specific ionic liquids: A mechanistic explanation for the dramatic increase in viscosity upon complexation with CO2 from molecular simulation. J. Am. Chem. Soc. 2008, 130, 14690–14704. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, B.F.; de la Fuente, J.C.; Gurkan, B.E.; Zadigian, D.J.; Price, E.A.; Huang, Y.; Brennecke, J.F. Experimental measurements of amine-functionalized anion-tethered ionic liquids with carbon dioxide. Ind. Eng. Chem. Res. 2011, 50, 111–118. [Google Scholar] [CrossRef]
- Gurkan, B.; Goodrich, B.F.; Mindrup, E.M.; Ficke, L.E.; Massel, M.; Seo, S.; Senftle, T.P.; Wu, H.; Glaser, M.F.; Shah, J.K.; et al. Molecular design of high capacity, low viscosity, chemically tunable ionic liquids for CO2 capture. J. Phys. Chem. Lett. 2010, 1, 3494–3499. [Google Scholar] [CrossRef]
- Wang, C.; Luo, H.; Jiang, D.-E.; Li, H.; Dai, S. Carbon dioxide capture by superbase-derived protic ionic liquids. Angew. Chem. Int. Ed. 2010, 49, 5978–5981. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Choi, W.Y.; Jang, J.H.; Yoo, K.P.; Lee, C.S. Solubility measurement and prediction of carbon dioxide in ionic liquids. Fluid Phase Equilibria 2005, 228–229, 439–445. [Google Scholar] [CrossRef]
- Scovazzo, P.; Camper, D.; Kieft, J.; Poshusta, J.; Koval, C.; Noble, R. Regular solution theory and CO2 gas solubility in room-temperature ionic liquids. Ind. Eng. Chem. Res. 2004, 43, 6855–6860. [Google Scholar] [CrossRef]
- Eike, D.M.; Brennecke, J.F.; Maginn, E.J. Predicting infinite-dilution activity coefficients of organic solutes in ionic liquids. Ind. Eng. Chem. Res. 2004, 43, 1039–1048. [Google Scholar] [CrossRef]
- Kroon, M.C.; Karakatsani, E.K.; Economou, I.G.; Witkamp, G.-J.; Peters, C.J. Modeling of the carbon dioxide solubility in imidazolium-based ionic liquids with the tpc-psaft equation of state. J. Phys. Chem. B 2006, 110, 9262–9269. [Google Scholar] [CrossRef]
- Kerlé, D.; Ludwig, R.; Geiger, A.; Paschek, D. Temperature dependence of the solubility of carbon dioxide in imidazolium-based ionic liquids. J. Phys. Chem. B 2009, 113, 12727–12735. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.N.; Boxall, J.A.; Lichtenthaler, R. Room temperature ionic liquids and their mixtures—A review. Fluid Phase Equilibria 2004, 219, 93–98. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Wang, W. Screening of ionic liquids to capture CO2 by COSMO-RS and experiments. AIChE J. 2008, 54, 2717–2728. [Google Scholar] [CrossRef]
- Maiti, A. Theoretical screening of ionic liquid solvents for carbon capture. ChemSusChem 2009, 2, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Palomar, J.; Gonzalez-Miquel, M.; Polo, A.; Rodriguez, F. Understanding the physical absorption of CO2 in ionic liquids using the cosmo-rs method. Ind. Eng. Chem. Res. 2011, 50, 3452–3463. [Google Scholar] [CrossRef]
- Gonzalez-Miquel, M.; Palomar, J.; Omar, S.; Rodriguez, F. CO2/N2 selectivity prediction in supported ionic liquid membranes (silms) by cosmo-rs. Ind. Eng. Chem. Res. 2011, 50, 5739–5748. [Google Scholar] [CrossRef]
- Lapkin, A.A.; Peters, M.; Greiner, L.; Chemat, S.; Leonhard, K.; Liauw, M.A.; Leitner, W. Screening of new solvents for artemisinin extraction process using ab initio methodology. Green Chem. 2010, 12, 241–251. [Google Scholar] [CrossRef]
- Kahlen, J.; Masuch, K.; Leonhard, K. Modelling cellulose solubilities in ionic liquids using COSMO-RS. Green Chem. 2010, 12, 2172–2181. [Google Scholar] [CrossRef]
- Klamt, A.; Eckert, F. Cosmo-rs: A novel and efficient method for the a priori prediction of thermophysical data of liquids. Fluid Phase Equilibria 2000, 172, 43–72. [Google Scholar] [CrossRef]
- Eckert, F.; Klamt, A. COSMOtherm, Version C2.1; COSMOlogic GmbH & Co. KG: Leverkusen, Germany, 2009. [Google Scholar]
- Ahlrichs, R.; Bär, M.; Häser, M.; Horn, H.; Kölmel, C. Electronic structure calculations on workstation computers: The program system turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted gaussian basis sets of triple zeta valence quality for atoms li to kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef] [PubMed]
- Sumon, K.Z.; Henni, A. Ionic liquids for CO2 capture using COSMO-RS: Effect of structure, properties and molecular interactions on solubility and selectivity. Fluid Phase Equilibria 2011, 310, 39–55. [Google Scholar] [CrossRef]
- Akhmetshina, A.I.; Petukhov, A.N.; Vorotyntsev, A.V.; Nyuchev, A.V.; Vorotyntsev, I.V. Absorption Behavior of Acid Gases in Protic Ionic Liquid/Alkanolamine Binary Mixtures. ACS Sustain. Chem. Eng. 2017, 5, 3429–3437. [Google Scholar] [CrossRef]
- Huang, K.; Cai, D.-N.; Chen, Y.-L.; Wu, Y.-T.; Hu, X.-B.; Zhang, Z.-B. Thermodynamic validation of 1-alkyl-3-methylimidazolium carboxylates as task-specific ionic liquids for H2S absorption. AIChE J. 2013, 59, 2227–2235. [Google Scholar] [CrossRef]
- Husson-Borg, P.; Majer, V.; Costa Gomes, M.F. Solubilities of oxygen and carbon dioxide in butyl methyl imidazolium tetrafluoroborate as a function of temperature and at pressures close to atmospheric pressure. J. Chem. Eng. Data 2003, 48, 480–485. [Google Scholar] [CrossRef]
- Camper, D.; Bara, J.; Koval, C.; Noble, R. Bulk-fluid solubility and membrane feasibility of rmim-based room-temperature ionic liquids. Ind. Eng. Chem. Res. 2006, 45, 6279–6283. [Google Scholar] [CrossRef]
- Shannon, M.S.; Tedstone, J.M.; Danielsen, S.P.O.; Hindman, M.S.; Irvin, A.C.; Bara, J.E. Free volume as the basis of gas solubility and selectivity in imidazolium-based ionic liquids. Ind. Eng. Chem. Res. 2012, 51, 5565–5576. [Google Scholar] [CrossRef]
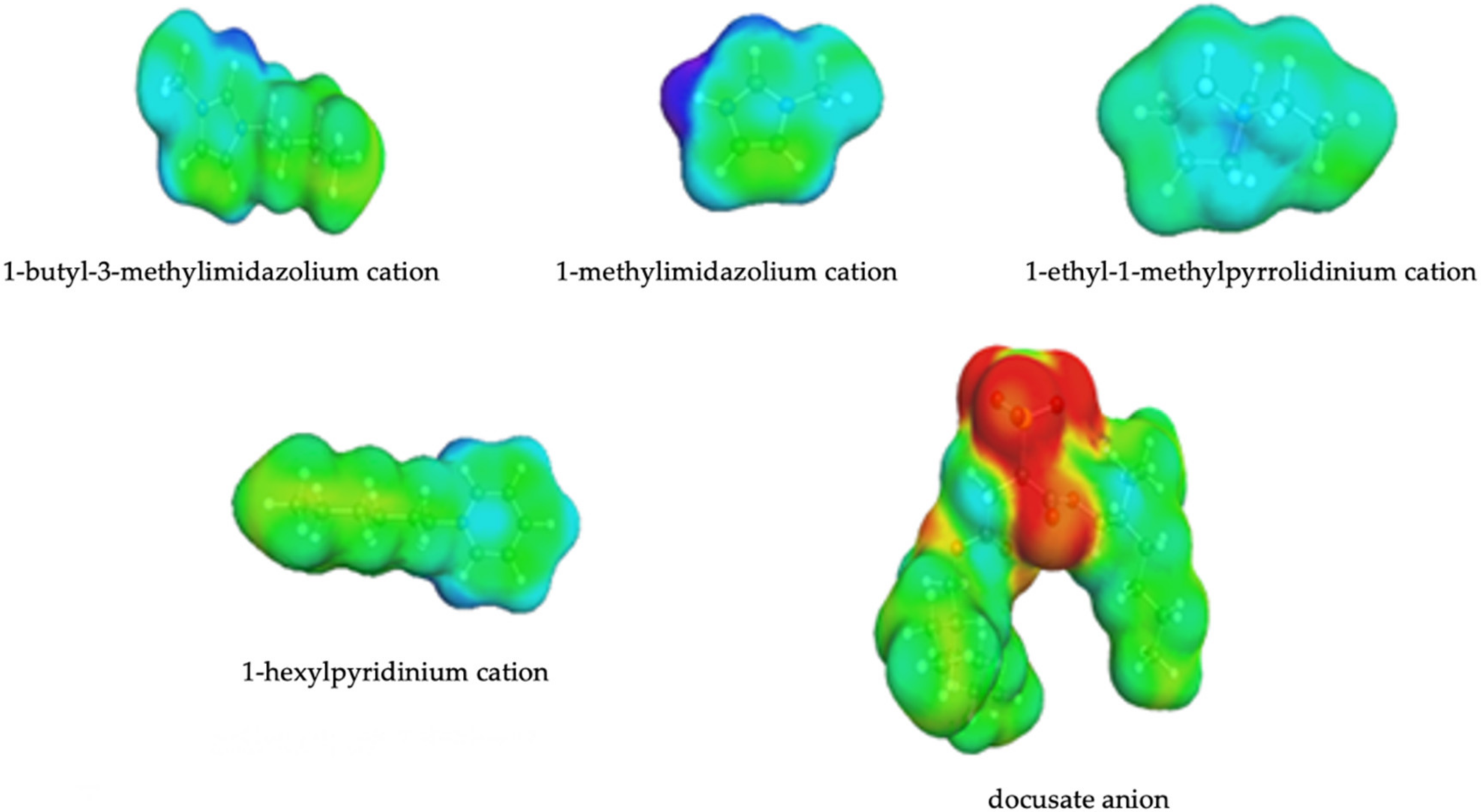

| Abbreviation | Name | Structure | Molar mass/g/mol |
|---|---|---|---|
| [bmim] | 1-butyl-3-methylimicazolium cation |  | 139.17 |
| [mim] | 1-methylimidazolium cation | 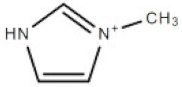 | 83.10 |
| [empyrr] | 1-ethyl-1-methylpyrrolidinium cation | 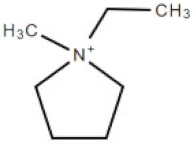 | 114.21 |
| [hpyr] | 1-hexylpyridinium cation | 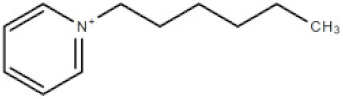 | 164.21 |
| [doc] | docusate anion (bis(2-ethylhexyl) sulfosuccinate, dioctyl sulfosuccinate (DOSS)) | 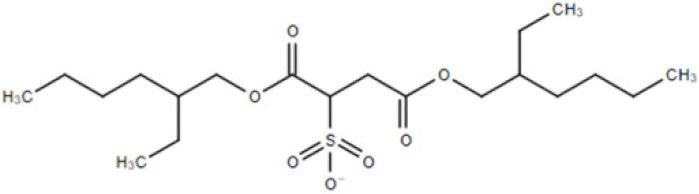 | 421.57 |
| ILs | Linear Regression Fit | Correlation Coefficient, R |
|---|---|---|
| [mim][doc] | ρexperimental= 0.743ρpredicted + 0.3393 | 1 |
| [bmim][doc] | ρexperimental= 0.7418ρpredicted + 0.3338 | 1 |
| ILs | ρ (g/cm3) | Dev., % | Vm (cm3/mol) | Dev., % | Reference | ||
|---|---|---|---|---|---|---|---|
| Experim. | Predict. | Experim. | Predict. | ||||
| [mim][doc] | 1.084 | 1.017 | 6.59 | 456.587 | 471.762 | 3.32 | [31] |
| [bmim][doc] | 1.084 | 1.026 | 5.63 | 506.637 | 546.376 | 7.84 | [31] |
| [hpyrr][doc] | - | 1.013 | - | - | 575.931 | - | This work |
| [empyrr][doc] | - | 1.069 | - | - | 528.870 | - | This work |
| [bmim][BF4] | 1.205 | 1.194 | 0.90 | 187.824 | 189.028 | 0.64 | [32] |
| [bmim][CF3SO3] | 1.301 | 1.319 | 1.40 | 220.934 | 218.526 | 1.09 | [32] |
| [hxmim][Tf2N] | 1.370 | 1.395 | 1.80 | 326.886 | 320.866 | 1.84 | [32] |
| [emim][Tf2N] | 1.521 | 1.531 | 0.65 | 291.970 | 288.358 | 1.24 | [32] |
| ILs | H(bar) | ||||
|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | 343.15 K | |
| CO2 | |||||
| mim[doc] | 62.694 | 75.498 | 89.712 | 105.323 | 122.303 |
| bmim[doc] | 45.864 | 55.511 | 66.304 | 78.252 | 91.351 |
| hpyrr[doc] | 45.202 | 54.566 | 65.028 | 76.598 | 89.276 |
| empyrr[doc] | 44.107 | 53.610 | 64.301 | 76.199 | 89.309 |
| H2S | |||||
| mim[doc] | 0.721 | 1.0254 | 1.416 | 1.908 | 2.513 |
| bmim[doc] | 0.523 | 0.7545 | 1.058 | 1.445 | 1.926 |
| hpyrr[doc] | 0.528 | 0.760 | 1.061 | 1.445 | 1.923 |
| empyrr[doc] | 0.461 | 0.673 | 0.953 | 1.315 | 1.771 |
| ILs | Vf (cm3/mol) | FFV |
|---|---|---|
| mim[doc] | 252.1296811 | 0.536972372 |
| bmim[doc] | 320.5894064 | 0.589633588 |
| hpyrr[doc] | 338.0120416 | 0.589782822 |
| empyrr[doc] | 311.7823921 | 0.592451172 |
| ILs | Misfit Interactions Energy Kcal/mol | H-Bonding Interactions Energy Kcal/mol | VdW Interactions Energy Kcal/mol | Total Kcal/mol | ||||
|---|---|---|---|---|---|---|---|---|
| CO2 | H2S | CO2 | H2S | CO2 | H2S | CO2 | H2S | |
| mim[doc] | 1.02556 | 1.11703 | 0 | −0.6868 | −2.89609 | −3.65569 | −1.87053 | −2.08448 |
| bmim[doc] | 1.03132 | 1.12246 | 0 | −0.72946 | −2.88958 | −3.64518 | −1.85827 | −2.1119 |
| hpyrr[doc] | 1.02511 | 1.12872 | 0 | −0.71923 | −2.89069 | −3.65656 | −1.8658 | −2.10608 |
| empyrr[doc] | 1.06730 | 1.12549 | 0 | −0.76074 | −2.86842 | −3.60433 | −1.80113 | −2.09859 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechergui, A.; Akhmetshina, A.I.; Kazarina, O.V.; Atlaskina, M.E.; Petukhov, A.N.; Vorotyntsev, I.V. Acidic Gases Solubility in Bis(2-Ethylhexyl) Sulfosuccinate Based Ionic Liquids Using the Predictive Thermodynamic Model. Membranes 2020, 10, 429. https://doi.org/10.3390/membranes10120429
Mechergui A, Akhmetshina AI, Kazarina OV, Atlaskina ME, Petukhov AN, Vorotyntsev IV. Acidic Gases Solubility in Bis(2-Ethylhexyl) Sulfosuccinate Based Ionic Liquids Using the Predictive Thermodynamic Model. Membranes. 2020; 10(12):429. https://doi.org/10.3390/membranes10120429
Chicago/Turabian StyleMechergui, Amal, Alsu I. Akhmetshina, Olga V. Kazarina, Maria E. Atlaskina, Anton N. Petukhov, and Ilya V. Vorotyntsev. 2020. "Acidic Gases Solubility in Bis(2-Ethylhexyl) Sulfosuccinate Based Ionic Liquids Using the Predictive Thermodynamic Model" Membranes 10, no. 12: 429. https://doi.org/10.3390/membranes10120429
APA StyleMechergui, A., Akhmetshina, A. I., Kazarina, O. V., Atlaskina, M. E., Petukhov, A. N., & Vorotyntsev, I. V. (2020). Acidic Gases Solubility in Bis(2-Ethylhexyl) Sulfosuccinate Based Ionic Liquids Using the Predictive Thermodynamic Model. Membranes, 10(12), 429. https://doi.org/10.3390/membranes10120429






