The function of MF, adsorption, photo-oxidation, DOM, and PP particles on membrane fouling and decay effectiveness were inspected in an integrated water treatment system, of multichannel ceramic MF and PP particles, with UV radiation and air backwashing. Permeate flux (J) data were used to compute resistances of the membrane, boundary layer, and membrane fouling (R
m, R
b, R
f) by utilizing the resistance-in-series filtration model (J = ΔP / (R
m + R
b + R
f)), as the equivalent procedure to former research [
28], where ΔP means the transmembrane pressure. The model was shortened to J = ΔP / R
m, for the reason that the resistances of boundary layer and membrane fouling did not exist for a fresh membrane. The R
m could be computed by utilizing permeate flux data for a fresh membrane. The recovered membrane, the flux difference of which was below 5%, was applied in all of the investigations to diminish the membrane situation effect on the decay effectiveness. Actually, the membrane resistance (R
m) could be calculated from the flux of the recovered membrane, which was not a real fresh membrane, because of the membrane fouling included. At initial time of the synthetic feed, the model was reformed to J
0 = ΔP / (R
m + R
b), and R
b could be computed by using J
0 and R
m values, where J
0 is the initial permeate flux. After physical cleaning by a brush in the membrane channel inside, the resistances of irreversible and reversible membrane fouling (R
if, R
rf) could be determined from the permeate flux values (J).
3.1. The Function of Photo-Oxidation, Adsorption, and Membrane Filtration
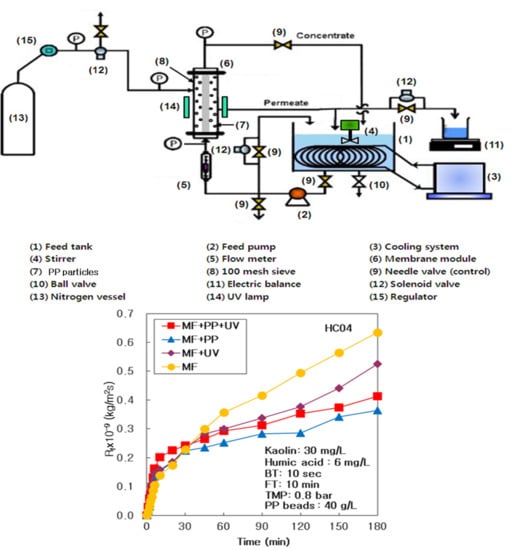
The (MF + PP) system using pure PP particles devoid of UV radiation, (MF + UV) system using UV radiation, and simply the MF system devoid of any PP particles and UV radiation were accomplished individually at HA 6 mg/L, and matched with the (MF + PP + UV) system of MF and PP particles with UV light. The membrane fouling resistance (R
f) of (MF + PP + UV), (MF + PP), (MF + UV), and only MF systems at 6 mg/L of HA were matched in
Figure 2a for the duration of 3 h. The R
f of the (MF + PP) system presented the lowest, and amplified intensely from the (MF + UV) to the MF system; however, that of the (MF + PP + UV) system was not the lowest value. It proved that the membrane fouling could be diminished powerfully by pure PP particles adsorption and the UV radiation photo-oxidation in this integrated water treatment system.
As arranged in
Table 2, the membrane resistance (R
m) was regulated at persistent value by incinerating the membrane in a furnace, and cleaning it in acidic and alkali solution. After 180 min procedure, a final R
f (R
f,180) was the maximum at 0.635 × 10
9 kg/m
2/s in the MF system, because of the highest R
b, that was created by a concentration polarization on the membrane surface; however, the R
f,180 was the lowest at 0.364 × 10
9 kg/m
2/s in the (MF + PP) system. The R
rf improved intensely when the system was made simpler, from the (MF + PP + UV) to the MF system in
Table 2; however, the R
if was the minimal at 0.225 × 10
9 kg/m
2/s in the (MF + PP) system and the maximal at 0.351 × 10
9 kg/m
2/s in the MF system.
As shown in
Figure 2b, to examine a comparative deterioration of treated flux, the dimensionless treated flux (J/J
0), where J
0 is the initial treated flux which was predicted by extrapolation using the first two J values at 1 and 2 min, could preserve the maximal in the (MF + PP) system and the minimal in the MF system. It proved that the J/J
0 in (MF + PP) could be greater than those of only the MF and the (MF + UV) systems, for the reason that pure PP particles adsorption could decrease the membrane fouling more powerfully than the UV radiation photo-oxidation. As summarized in
Table 2, the final value of J/J
0 (J
180/J
0) was the maximal at 0.711 in the (MF + PP) system after 180 min operation, which was 1.22 times greater than the minimal of 0.585 in the single MF system. The highest value of 224 L/m
2hr of permeate flux (J) could be attained in the (MF + PP) system, for the reason that the membrane fouling was repressed further powerfully via pure PP particles adsorption in the (MF + PP) system than in the single MF or (MF + UV) systems. Finally, the total permeate volume (V
T) was the maximal at 15.3 L in the (MF + PP) system and the minimal at 13.9 L in the MF system during 180 min operation.
As paralleled in
Table 3 and
Table 4, the decay efficiencies of turbidity and DOM, that can be examined by UV
254 absorbance, reduced clearly when making the system simpler, step by step, from (MF + PP + UV), (MF + PP), and (MF + UV) to MF with air backwashing. The percentages of decay effectiveness by MF, adsorption, and UV photo-oxidation in this integrated system using air backwashing could be computed by deducting the turbid or organic materials decay effectiveness of MF, from those of (MF + PP) and (MF + UV) systems correspondingly, as prepared in
Table 5. The percentage of decay effectiveness by photo-oxidation with PP particles could be obtained by deducting the DOM or turbidity decay effectiveness of (MF + PP) from that of the (MF + PP + UV) system.
The percentages of MF, the PP particles adsorption, the UV radiation photo-oxidation, and the photo-oxidation with PP particles for turbidity decay were 87.6%, 3.8%, 3.5%, and 3.6%, respectively, as shown
Table 5. It verified that the function of MF and adsorption was important; however, those of UV photo-oxidation with/without the pure PP particles did not perform a dominant function to reduce the suspended inorganic matters in this integrated system, because inorganic materials, such as kaolin, could not be decayed by UV photo-oxidation.
However, the percentages of MF, PP particles adsorption, UV photo-oxidation, and photo-oxidation with PP particles for DOM (UV
254 absorbance) decay were 27.9%, 5.0%, 4.4%, and 0.2%, respectively, as shown in
Table 5. It proved that the function of MF was the most dominant, and those of adsorption by pure PP particles and UV photo-oxidation were more significant than that of photo-oxidation with PP particles for the DOM reduction in the integrated water treatment system. The adsorption on non-porous PP particles could not be effective, but pollutants could be coated and adsorbed on the PP particles surface. The pure PP particles adsorption and UV photo-oxidation could forcefully diminish membrane fouling, for the reason that the photo-oxidation and adsorption forced the main part of DOM decomposition in this integrated system using air backwashing.
3.2. The Influence of Humic Acid (HA) on Decay Effectiveness and Membrane Fouling
As compared in
Figure 3a, the resistances of membrane fouling (R
f) improved a small amount, as HA concentration was increased from 2 to 8 mg/L; however, R
f at HA of 10 mg/L was maintained at very high values during the 180 min operation. It proved that the dissolved organic materials, like as humic acid, could generate more rigorously the membrane fouling on the surface and inside of alumina membrane, beyond the specific HA concentration, such as 8 mg/L, in the integrated system of membrane and PP particles.
In the former research by [
26] about the integrated system of the similar multichannel MF (HC10, pore size 1.0 μm) and the equivalent pure PP particles using water backwashing, the R
f was highly influenced by HA and nearly persistent at 2–6 mg/L of HA; however, it improved intensely, as HA concentration was increased from 6 to 8 mg/L, and then diminished abruptly from 8 to 10 mg/L of HA. It proved that DOM, such as HA, could motivate the membrane fouling further strictly on the alumina membrane surface and inside; nevertheless, the dense fouling cake on the membrane might be detached via water backwashing at HA of 10 mg/L.
The feed flowrate (Q) was 1.0 L/min (1.667 × 10−5 m3/s) into the alumina membrane channel. The cross sectional area (A) of the membrane was 8.796 × 10−5 m2 (A = (π/4)D2 × 7, where D = 0.004 m). The mean linear velocity (v) inside the membrane channel was 0.1895 m/s (v = Q/A). The Reynolds number (Re) was 755.2 at 20 °C water, which had a laminar flow lower than 2100 (Re = ρvD/μ, where ρ = 998.23 kg/m3, μ = 1.002 × 10−3 kg/m/s at 20 °C water). The turn-over number (N) of the feed was 18 (N = t/T, where operation time t = 180 min, feed flow time T = V/Q = (10 L)/(1.0 L/min) = 10 min).
As arranged in
Table 6, the R
f,180 after 3 h procedure at 10 mg/L of humic acid was the highest 2.087 × 10
9 kg/m
2/s, which was 6.17 times greater than the lowest R
f,180 of 0.338 × 10
9 kg/m
2/s at 2 mg/L; however, the values increased slowly from 0.338 × 10
9 to 0.421 × 10
9 kg/m
2/s when HA was amplified from 2 to 8 mg/L. It proved that the sever membrane fouling could occur beyond the specific HA concentration in this integrated system of ceramic membrane and PP particles using UV radiation. In addition, the R
rf,180 increased slowly from 0.078 × 10
9 to 0.152 × 10
9 kg/m
2/s as HA was amplified from 2 to 8 mg/L, and increased dramatically to 0.864 × 10
9 kg/m
2/s at HA of 10 mg/L. Additionally, the R
if,180 increased suddenly from 0.269 × 10
9 to 1.223 × 10
9 kg/m
2/s as HA was increased from 8 to 10 mg/L.
In the former research by [
26] about the integrated system using water backwashing, as compared in
Table 6, the membrane resistance (R
m) was regulated persistently by the furnace incineration and acid/alkali cleaning. After 180 min procedure, the final R
f (R
f,180) at HA of 8 mg/L was 1.963 × 10
9 kg/m
2/s, which was 1.76 times higher than 1.113 × 10
9 kg/m
2/s of the R
f,180 value at HA of 6 mg/L. The R
f value (1.246 × 10
9 kg/m
2/s) with water backwashing was much lower than 2.087 × 10
9 kg/m
2/s of air backwashing at HA of 10 mg/L. It proved that water backwashing might be more efficient to inhibit the membrane fouling at high HA condition, than air backwashing, in this integrated system of ceramic membrane and PP particles.
As compared in
Figure 3b, the J/J
0 showed a little reduction trend HA concentration was increased from 2 to 8 mg/L; however, it decreased abruptly at HA of 10 mg/L, for the reason that the membrane fouling might be produced via additional organic macromolecules beyond the specific HA concentration. As presented in
Table 6, after 180 min operation the final J
180/J
0 dropped a little from 0.728 to 0.695 as HA was increased from to 8 mg/L; however, it diminished dramatically to 0.329 at 10 mg/L, due to severe membrane fouling beyond HA of 8 mg/L.
In the former research by [
26] about the integrated system using water backwashing, as compared in
Table 6, the final J/J
0 (J
180/J
0) after 180 min procedure was 0.437 at HA of 6 mg/L, which was 1.33 times greater than 0.307 at HA of 8 mg/L. It proved that the permeated flux could showed a different trend, depending on backwashing media of air or water. The J
180/J
0 value using air backwashing was 0.329, which was much lower than 0.406 of water backwashing at HA of 10 mg/L. It verified that water backwashing was able to maintain the higher flux more effectively than air backwashing in this integrated system of ceramic membrane and PP particles.
Furthermore, the V
T decreased slowly from 15.21 L at HA 2 mg/L to 13.93 L at 8 mg/L; however, it suddenly dropped to the lowest, 8.18 L, at HA of 10 mg/L, as displayed in
Table 6. It proved that NOM concentration, like HA, could be a major cause of membrane fouling in this integrated water treatment system of multichannel alumina membrane and pure PP particles using air backwashing.
In the former research by [
26], about the integrated system using water backwashing, as compared in
Table 6, the V
T of 12.48 L in HA of 4 mg/L presented 1.38 times more than 9.05 L of V
T in HA of 8 mg/L. It proved that the HA effect on the total treated volume might be more severe for air backwashing than water backwashing.
As paralleled in
Table 7, the decay effectiveness of turbidity improved a small amount, from 94.7% to 95.4%, as HA concentration was increased. It proved that the suspended particles, like kaolin, might be rejected more successfully at higher HA concentrations, due to the secondary membrane, which was the gel deposit on the membrane surface, via the robust membrane fouling in the integrated system of ceramic membrane and PP particles.
In the former study by [
26], about the integrated system using water backwashing, as arranged in
Table 7, the turbidity decay effectiveness displayed a growing trend in relation to increasing HA concentration. This phenomenon for water backwashing showed a similar trend to the results of this study with air backwashing. It proved that the backwashing media could not disturb the decay effectiveness of turbidity in the integrated system of PP particles and multichannel alumina membrane.
In the former research by [
26], for the integrated system using water backwashing, as shown in paralleled data in
Table 8, the DOM decay effectiveness improved as HA concentration was increased, and in conclusion presented the extreme result of 49.7% in HA of 10 mg/L. It proved that DOM might be treated more excellently in an extreme DOM situation in this integrated system of multichannel ceramic MF and PP particles using water backwashing. The DOM decay effectiveness for air backwashing was higher below HA of 8 mg/L; nevertheless, it showed lower in HA of 10 mg/L than that for water backwashing. It proved that the air backwashing was more effective for DOM decay than the water backwashing in this integrated system.
As presented in
Table 8, the decay effectiveness of DOM showed just about persistent values in the range of 33.1% and 34.6%, while HA amplified from 2 to 6 mg/L; however, it increased to 40.9% at 8 mg/L, and displayed the maximum of 42.5% at 10 mg/L. The treated water quality of DOM improved a little faster than the feed water quality, when HA changed from 2 to 6 mg/L, for the reason that HA might not be attached enough via PP particles and decomposed by UV photo-oxidation. On the other hand, the treated water quality of DOM enlarged more slowly than the feed water quality beyond HA 6 mg/L, since organic materials could be rejected by the secondary membrane formed on the membrane surface via sturdy membrane fouling.
3.3. The Influence of Pure PP Particles on Decay Effectiveness and Membrane Fouling
As compared in
Figure 4a, the R
f presented the highest at 0 g/L of pure PP particles, and the lowest at 50 g/L during 180 min in this integrated system of multichannel ceramic membrane and PP particles using air backwashing. It means that the more amount of PP particles could inhibit more tremendously the membrane fouling in the integrated water treatment system.
As arranged in
Table 9, the R
f,180 after 3 h operation presented the maximum (i.e., 7.045 × 10
9 kg/m
2/s) at 0 g/L of PP particles, which was 3.65 times more than 1.932 × 10
9 kg/m
2/s of the R
f,180 at 30 g/L of PP particles. The R
rf was the lowest, 0.353 × 10
9 kg/m
2/s, at 30 g/L, and the maximal, 3.654 × 10
9 kg/m
2/s, at 0 g/L of PP particles; nevertheless, the R
if showed the minimal, 1.144 × 10
9 kg/m
2/s, at 50 g/L and the maximal, 3.390 × 10
9 kg/m
2/s, at 0 g/L of PP particles. The R
b presented the lowest at 30 g/L and the highest at 0 g/L. It proved that 30 g/L of PP particles might be the optimal condition in the integrated system, due to the lowest reversible membrane fouling and concentration polarization; however, the irreversible membrane fouling might be prevented at 50 g/L of PP particles, which was the highest quantity of PP particles in our experimental range. The concentration polarization at the membrane surface could be checked by the resistance of the boundary layer (R
b).
In former research via [
26], about the integrated system using water backwashing, as compared in
Table 9, the R
f,180 after 3 h was the maximal, 12.94 × 10
9 kg/m
2/s, at 50 g/L, which was 3.06 times more than 4.23 × 10
9 kg/m
2/s at 0 g/L of PP particles. The R
rf presented a growing tendency when PP particles concentration was increased from 0 to 50 g/L; on the other hand, the least R
if was at 40 g/L and the extreme was at 0 g/L of PP particles. The results were in agreement with the research results using air backwashing. It means that the backwashing media did not affect the membrane fouling in the integrated system of multichannel ceramic membrane and PP particles.
As compared in
Figure 4b, the J/J
0 presented the peak at 50 g/L of PP particles for the duration of the 3 h process, and the least in the range of 0–10 g/L in the integrated system. As arranged in
Table 9, the J
0 and J
180 presented the extreme at 30 g/L of PP particles, and J
180 amplified, with the increasing of PP particles concentration; nevertheless, after the 3 h process, the J
180/J
0 on the PP particles of 50 g/L was the maximal, 0.304, due to the least membrane fouling. In conclusion, the V
T was the best, 2.09 L, for PP particles of 30 and 50 g/L, for the reason that J was preserved greater during the 3 h process on PP particles of 30 g/L. It means that the best condition of PP particles might be 30 g/L, due to the highest J
180/J
0 and V
T.
In the former research by [
26], for the integrated system using water backwashing, as paralleled in
Table 9, the J
0 and J
180 presented a reducing tendency, from 0 to 40 g/L and 50 g/L, of the PP particles, respectively. This phenomenon came from the reason that the R
b and R
f displayed an increasing trend from 0 to 40 g/L and to 50 g/L of the PP particles, correspondingly. As a final point, after the 3 h procedure, the J
180/J
0 on the PP particles of 0 g/L presented the extreme result, 0.230, which was 2.13 times more than 0.108 at 50 g/L. On the other hand, the V
T displayed the maximum 11.75 L at the PP particles of 5 g/L. It came from the reason that J was preserved greater throughout the procedure than those of other PP particles situations. It proved that the backwashing media could affect the treated flux in the integrated system of multichannel ceramic membrane and PP particles.
As prepared in
Table 10, the turbidity decay effectiveness showed an increasing tendency, while increasing the PP particles concentration; however, it presented the maximal 95.7% on the PP particles at 50 g/L. It proved that the ideal PP particles concentration to treat the turbid components, such as kaolin, could be 50 g/L in the integrated system, because the PP particles adsorption was the most effective on the PP particles at 50 g/L.
In the former study by [
26], about the integrated system using water backwashing, as paralleled in
Table 10, the turbidity decay efficiencies presented nearly consistent results at the scope of 97.5% and 98.9%, unrestricted by the PP particles concentration. It proved that the turbid components might be rejected excellently, unrestricted by PP particles concentration in the integrated water treatment system. It verified that the backwashing media could effect the decay efficiencies of turbidity, because the result with water backwashing did not agree with this study with air backwashing.
As paralleled in
Table 11, the DOM decay effectiveness did not show a special trend as the PP particles concentration was amplified; however, it was the best, 56.8%, at 40 g/L of PP particles and dropped to the lowest, 37.8%, at 50 g/L. It verified that the DOM could be treated the most excellently on the PP particles of 40 g/L, because UV for photo-oxidation could transfer enough to the module inside on the PP particles of 40 g/L. Finally, the optimal condition could be 40 g/L for treating turbid matter and DOM in the integrated system of multichannel alumina membrane and PP particles.
In the former research via [
26], about the integrated system using water backwashing, as paralleled in
Table 11, the DOM decay effectiveness did not display a tendency; on the other hand, it presented the best, 51.3%, on the PP particles of 5 g/L. It proved that the ideal PP particles concentration could be 5 g/L to decay DOM in the integrated water treatment system using water backwashing. In addition, it verified that the backwashing media could best affect the PP particles concentration for the DOM decay in the integrated system.
This integrated system could be applied as a tertiary process in water or wastewater treatment. A total 6.60 g of PP particles was supplied for the PP particles of 50 g/L in this integrated system of 1.0 L/min (60 L/h), because the volume between the ceramic membrane outside and the module inside was 0.165 L. For water treatment of 1 m3/h, PP particles of 110 g would be used, if this integrated system of 60 L/h was scaled up to a 16.7 times (= (1000 L/h)/(60 L/h)) practical system.