Leveraging Nanocrystal HKUST-1 in Mixed-Matrix Membranes for Ethylene/Ethane Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Nanocrystal HKUST-1
2.3. Synthesis of ODPA-TMPDA Polymer
2.4. Synthesis of 6FDA-TMPDA Polymer
2.5. Membrane Fabrication
2.6. Characterization
2.6.1. Characterization of Nanocrystal HKUST-1
2.6.2. Characterization of Mixed-Matrix Membranes
2.6.3. Mixture Gas Permeation Analysis
2.6.4. C2H4 and C2H6 Adsorption Analysis of Membranes
3. Results and Discussion
3.1. Characterization of Nanocrystal HKUST-1
3.2. C2H4 and C2H6 Adsorption of Nanocrystal HKUST-1
3.3. Characterizaiton of Mixed-Matrix Membranes
3.4. Gas Permeation Behavior of Membranes
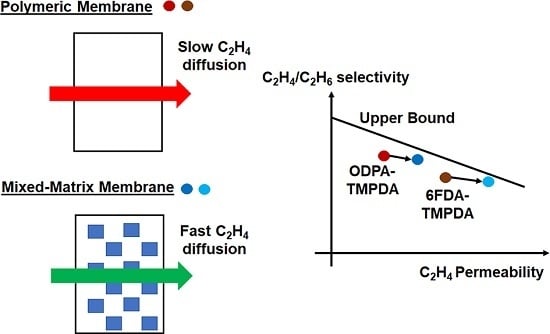
3.5. Comparison of Gas Separation Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| List of symbols | |
| , | Langmuir constant () |
| Change in mass per unit temperature () | |
| Diffusivity () | |
| Pressure () | |
| Permeability (Barrer or ) | |
| Isosteric heat of adsorption () | |
| Adsorbed amount (mmol g−1) | |
| , | Saturation loading (mmol g−1) |
| Molar gas constant () | |
| Solubility () | |
| Temperature () | |
| , | Mole fractions of the adsorbed phase |
| , | Mole fractions of the gas phase |
| Density () | |
| Abbreviations | |
| 6FDA | 4,4′-(hexafluoroisopropylidene) diphthalic anhydride |
| Ac2O | Acetic anhydride |
| BuI | 5-tert-butyl isophthalic acid |
| C2H4 | Ethylene |
| C2H6 | Ethane |
| CA | Cellulose acetate |
| Cu(NO3)2.3H2O | Copper(II) nitrate trihydrate |
| C9H6O6 | Trimesic acid |
| DAM (or TMPDA) | 2,4,6-trimethyl-m-phenylenediamine |
| DBzPBI | Substituted polybenzimidazole |
| DMAc | Dimethylacetamide |
| NMP | N-methyl-2-pyrrolidone |
| ODPA | 4,4′-oxydiphthalic anhydride |
| PBI | Polybenzimidazole |
| TEA | Triethylamine |
References
- Sholl, D.; Lively, R. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Cetinkaya, E.; Liu, N.; Jan Simons, T.; Wallach, J. Petrochemicals 2030: Reinventing the Way to Win in a Changing Industry. Available online: https://www.mckinsey.com/industries/chemicals/our-insights/petrochemicals-2030-reinventing-the-way-to-win-in-a-changing-industry# (accessed on 8 April 2020).
- Hays, K.; Cambeiro, M.; Dobashi, F.; Ang, S. New Capacities, Weaker Downstream Markets to Weigh on Ethylene in 2020. Available online: https://www.spglobal.com/platts/en/market-insights/latest-news/petrochemicals/112619-commodities-2020-new-capacities-weaker-downstream-markets-to-weigh-on-ethylene (accessed on 4 April 2020).
- Wang, X.; Wu, Y.; Zhou, X.; Xiao, J.; Xia, Q.; Wang, H.; Li, Z. Novel C-PDA adsorbents with high uptake and preferential adsorption of ethane over ethylene. Chem. Eng. Sci. 2016, 155, 338–347. [Google Scholar] [CrossRef]
- Naghsh, M.; Sadeghi, M.; Moheb, A.; Chenar, M.P.; Mohagheghian, M. Separation of ethylene/ethane and propylene/propane by cellulose acetate–silica nanocomposite membranes. J. Membr. Sci. 2012, 423, 97–106. [Google Scholar] [CrossRef]
- Lee, J.; Chuah, C.Y.; Kim, J.; Kim, Y.; Ko, N.; Seo, Y.; Kim, K.; Bae, T.H.; Lee, E. Separation of Acetylene from Carbon Dioxide and Ethylene by a Water-Stable Microporous Metal–Organic Framework with Aligned Imidazolium Groups inside the Channels. Angew. Chem. Int. Ed. 2018, 57, 7869–7873. [Google Scholar] [CrossRef] [PubMed]
- Nitzsche, R.; Budzinski, M.; Gröngröft, A. Techno-economic assessment of a wood-based biorefinery concept for the production of polymer-grade ethylene, organosolv lignin and fuel. Bioresour. Technol. 2016, 200, 928–939. [Google Scholar] [CrossRef]
- Santos-Panqueva, Y.; Guerrero-Fajardo, C.A.; Cuevas-Rodriguez, E.O.; Picos-Corrales, L.A.; Silva, C.M.; Contreras-Andrade, I. Production of bio-ethylene from wastes of microalgae to biodiesel biorefinery. Waste Biomass Valoriz. 2019, 10, 377–386. [Google Scholar] [CrossRef]
- De Jong, E.; Jungmeier, G. Chapter 1—Biorefinery Concepts in Comparison to Petrochemical Refineries. In Industrial Biorefineries & White Biotechnology; Pandey, A., Höfer, R., Taherzadeh, M., Nampoothiri, K.M., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–33. [Google Scholar] [CrossRef]
- Bennett, S.J.; Pearson, P.J.G. From petrochemical complexes to biorefineries? The past and prospective co-evolution of liquid fuels and chemicals production in the UK. Chem. Eng. Res. Des. 2009, 87, 1120–1139. [Google Scholar] [CrossRef]
- Alfano, S.; Berruti, F.; Denis, N.; Santagostino, A. The Future of Second-Generation Biomass. Available online: https://www.mckinsey.com/business-functions/sustainability/our-insights/the-future-of-second-generation-biomass (accessed on 8 April 2020).
- Liang, W.; Zhang, Y.; Wang, X.; Wu, Y.; Zhou, X.; Xiao, J.; Li, Y.; Wang, H.; Li, Z. Asphalt-derived high surface area activated porous carbons for the effective adsorption separation of ethane and ethylene. Chem. Eng. Sci. 2017, 162, 192–202. [Google Scholar] [CrossRef]
- Kunjattu, S.H.; Ashok, V.; Bhaskar, A.; Pandare, K.; Banerjee, R.; Kharul, U.K. ZIF-8@ DBzPBI-BuI composite membranes for olefin/paraffin separation. J. Membr. Sci. 2018, 549, 38–45. [Google Scholar] [CrossRef]
- Van Miltenburg, A.; Zhu, W.; Kapteijn, F.; Moulijn, J. Adsorptive separation of light olefin/paraffin mixtures. Chem. Eng. Res. Des. 2006, 84, 350–354. [Google Scholar] [CrossRef] [Green Version]
- Burns, R.L.; Koros, W.J. Defining the challenges for C3H6/C3H8 separation using polymeric membranes. J. Membr. Sci. 2003, 211, 299–309. [Google Scholar] [CrossRef]
- Sungpet, A.; Way, J.; Thoen, P.; Dorgan, J. Reactive polymer membranes for ethylene/ethane separation. J. Membr. Sci. 1997, 136, 111–120. [Google Scholar] [CrossRef]
- Staudt-Bickel, C.; Koros, W.J. Olefin/paraffin gas separations with 6FDA-based polyimide membranes. J. Membr. Sci. 2000, 170, 205–214. [Google Scholar] [CrossRef]
- Wang, H.; Werth, S.; Schiestel, T.; Caro, J. Perovskite Hollow-Fiber Membranes for the Production of Oxygen-Enriched Air. Angew. Chem. Int. Ed. 2005, 44, 6906–6909. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Chuah, C.Y.; Yang, Y.; Bae, T.-H. High performance composite membranes comprising Zn(pyrz)2(SiF6) nanocrystals for CO2/CH4 separation. J. Ind. Eng. Chem. 2018, 60, 279–285. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Kim, K.; Lee, J.; Koh, D.-Y.; Bae, T.-H. CO2 absorption using membrane contactors: Recent progress and future perspective. Ind. Eng. Chem. Res. 2019. [Google Scholar] [CrossRef]
- Jiang, X.; Kumar, A. Performance of silicone-coated polymeric membrane in separation of hydrocarbons and nitrogen mixtures. J. Membr. Sci. 2005, 254, 179–188. [Google Scholar] [CrossRef]
- Pinnau, I.; Toy, L.G. Solid polymer electrolyte composite membranes for olefin/paraffin separation. J. Membr. Sci. 2001, 184, 39–48. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Goh, K.; Yang, Y.; Gong, H.; Li, W.; Karahan, H.E.; Guiver, M.D.; Wang, R.; Bae, T.-H. Harnessing filler materials for enhancing biogas separation membranes. Chem. Rev. 2018, 118, 8655–8769. [Google Scholar] [CrossRef]
- Rungta, M.; Zhang, C.; Koros, W.J.; Xu, L. Membrane-based ethylene/ethane separation: The upper bound and beyond. AIChE J. 2013, 59, 3475–3489. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Zhang, C.; Dai, Y.; Johnson, J.R.; Karvan, O.; Koros, W.J. High performance ZIF-8/6FDA-DAM mixed matrix membrane for propylene/propane separations. J. Membr. Sci. 2012, 389, 34–42. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Yang, Y.; Chuah, C.Y.; Nie, L.; Bae, T.-H. Enhancing the mechanical strength and CO2/CH4 separation performance of polymeric membranes by incorporating amine-appended porous polymers. J. Membr. Sci. 2019, 569, 149–156. [Google Scholar] [CrossRef]
- Yang, Y.; Goh, K.; Weerachanchai, P.; Bae, T.-H. 3D covalent organic framework for morphologically induced high-performance membranes with strong resistance toward physical aging. J. Membr. Sci. 2019, 574, 235–242. [Google Scholar] [CrossRef]
- Pera-Titus, M. Porous inorganic membranes for CO2 capture: Present and prospects. Chem. Rev. 2013, 114, 1413–1492. [Google Scholar] [CrossRef]
- Usman, M.; Ahmed, A.; Yu, B.; Peng, Q.; Shen, Y.; Cong, H. A review of different synthetic approaches of amorphous intrinsic microporous polymers and their potential applications in membrane-based gases separation. Eur. Polym. J. 2019, 120, 109262. [Google Scholar] [CrossRef]
- Álvarez, C.; Lozano, Á.E.; Juan-y-Seva, M.; de la Campa, J.G. Gas separation properties of aromatic polyimides with bulky groups. Comparison of experimental and simulated results. J. Membr. Sci. 2020, 602, 117959. [Google Scholar] [CrossRef]
- Ploegmakers, J.; Japip, S.; Nijmeijer, K. Mixed matrix membranes containing MOFs for ethylene/ethane separation—Part B: Effect of Cu3BTC2 on membrane transport properties. J. Membr. Sci. 2013, 428, 331–340. [Google Scholar] [CrossRef]
- Zamaro, J.M.; Pérez, N.C.; Miró, E.E.; Casado, C.; Seoane, B.; Téllez, C.; Coronas, J. HKUST-1 MOF: A matrix to synthesize CuO and CuO–CeO2 nanoparticle catalysts for CO oxidation. Chem. Eng. J. 2012, 195, 180–187. [Google Scholar] [CrossRef]
- Casado-Coterillo, C.; Fernández-Barquín, A.; Zornoza, B.; Téllez, C.; Coronas, J.; Irabien, Á. Synthesis and characterisation of MOF/ionic liquid/chitosan mixed matrix membranes for CO2/N2 separation. RSC Adv. 2015, 5, 102350–102361. [Google Scholar] [CrossRef] [Green Version]
- Pu, S.; Wang, J.; Li, L.; Zhang, Z.; Bao, Z.; Yang, Q.; Yang, Y.; Xing, H.; Ren, Q. Performance comparison of metal–organic framework extrudates and commercial zeolite for ethylene/ethane separation. Ind. Eng. Chem. Res. 2018, 57, 1645–1654. [Google Scholar] [CrossRef]
- Krokidas, P.; Moncho, S.; Brothers, E.N.; Castier, M.; Economou, I.G. Tailoring the gas separation efficiency of metal organic framework ZIF-8 through metal substitution: A computational study. Phys. Chem. Chem. Phys. 2018, 20, 4879–4892. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Samarasinghe, S.; Chuah, C.Y.; Yang, Y.; Bae, T.-H. Tailoring CO2/CH4 separation properties of mixed-matrix membranes via combined use of two-and three-dimensional metal-organic frameworks. J. Membr. Sci. 2018, 557, 30–37. [Google Scholar] [CrossRef]
- Mueller, R.; Hariharan, V.; Zhang, C.; Lively, R.; Vasenkov, S. Relationship between mixed and pure gas self-diffusion for ethane and ethene in ZIF-8/6FDA-DAM mixed-matrix membrane by pulsed field gradient NMR. J. Membr. Sci. 2016, 499, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Montoro, C.; García, E.; Calero, S.; Pérez-Fernández, M.A.; López, A.L.; Barea, E.; Navarro, J.A. Functionalisation of MOF open metal sites with pendant amines for CO2 capture. J. Mater. Chem. 2012, 22, 10155–10158. [Google Scholar] [CrossRef]
- Planas, N.; Dzubak, A.L.; Poloni, R.; Lin, L.-C.; McManus, A.; McDonald, T.M.; Neaton, J.B.; Long, J.R.; Smit, B.; Gagliardi, L. The mechanism of carbon dioxide adsorption in an alkylamine-functionalized metal–organic framework. J. Am. Chem. Soc. 2013, 135, 7402–7405. [Google Scholar] [CrossRef]
- Duval, J.M.; Kemperman, A.; Folkers, B.; Mulder, M.; Desgrandchamps, G.; Smolders, C. Preparation of zeolite filled glassy polymer membranes. J. Appl. Polym. Sci. 1994, 54, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Goh, K.; Wang, R.; Bae, T.-H. High-performance nanocomposite membranes realized by efficient molecular sieving with CuBDC nanosheets. Chem. Commun. 2017, 53, 4254–4257. [Google Scholar] [CrossRef]
- Guücuüyener, C.; van den Bergh, J.; Gascon, J.; Kapteijn, F. Ethane/ethene separation turned on its head: Selective ethane adsorption on the metal− organic Framework ZIF-7 through a gate-opening mechanism. J. Am. Chem. Soc. 2010, 132, 17704–17706. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.O.; Pinto, M.S.L.; Saini, V.K. Ethane selective IRMOF-8 and its significance in ethane–ethylene separation by adsorption. ACS Appl. Mater. Interfaces 2014, 6, 12093–12099. [Google Scholar] [CrossRef] [PubMed]
- Boöhme, U.; Barth, B.; Paula, C.; Kuhnt, A.; Schwieger, W.; Mundstock, A.; Caro, J.R.; Hartmann, M. Ethene/ethane and propene/propane separation via the olefin and paraffin selective metal–organic framework adsorbents CPO-27 and ZIF-8. Langmuir 2013, 29, 8592–8600. [Google Scholar] [CrossRef]
- Chanut, N.; Bourrelly, S.; Kuchta, B.; Serre, C.; Chang, J.S.; Wright, P.A.; Llewellyn, P.L. Screening the effect of water vapour on gas adsorption performance: Application to CO2 capture from flue gas in metal–organic frameworks. ChemSusChem 2017, 10, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chuah, C.Y.; Yang, Y.; Bae, T.-H. Nanocomposites formed by in situ growth of NiDOBDC nanoparticles on graphene oxide sheets for enhanced CO2 and H2 storage. Microporous Mesoporous Mater. 2018, 265, 35–42. [Google Scholar] [CrossRef]
- Bae, T.H.; Lee, J.S.; Qiu, W.; Koros, W.J.; Jones, C.W.; Nair, S. A high-performance gas-separation membrane containing submicrometer-sized metal–organic framework crystals. Angew. Chem. Int. Ed. 2010, 122, 10059–10062. [Google Scholar] [CrossRef]
- Davoodi, S.M.; Sadeghi, M.; Naghsh, M.; Moheb, A. Olefin–paraffin separation performance of polyimide Matrimid®/silica nanocomposite membranes. RSC Adv. 2016, 6, 23746–23759. [Google Scholar] [CrossRef]
- Ploegmakers, J.; Japip, S.; Nijmeijer, K. Mixed matrix membranes containing MOFs for ethylene/ethane separation Part A: Membrane preparation and characterization. J. Membr. Sci. 2013, 428, 445–453. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Goh, K.; Bae, T.-H. Hierarchically structured HKUST-1 nanocrystals for enhanced SF6 capture and recovery. J. Phys. Chem. C 2017, 121, 6748–6755. [Google Scholar] [CrossRef]
- Duan, C.; Jie, X.; Liu, D.; Cao, Y.; Yuan, Q. Post-treatment effect on gas separation property of mixed matrix membranes containing metal organic frameworks. J. Membr. Sci. 2014, 466, 92–102. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Yu, S.; Na, K.; Bae, T.-H. Enhanced SF6 recovery by hierarchically structured MFI zeolite. J. Ind. Eng. Chem. 2018, 62, 64–71. [Google Scholar] [CrossRef]
- Myers, A.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- Mason, J.A.; Sumida, K.; Herm, Z.R.; Krishna, R.; Long, J.R. Evaluating metal-organic frameworks for post-combustion carbon dioxide capture via temperature swing adsorption. Energy Environ. Sci. 2011, 4, 3030–3040. [Google Scholar] [CrossRef]
- Mathias, P.M.; Kumar, R.; Moyer, J.D.; Schork, J.M.; Srinivasan, S.R.; Auvil, S.R.; Talu, O. Correlation of multicomponent gas adsorption by the dual-site Langmuir model. Application to nitrogen/oxygen adsorption on 5A-zeolite. Ind. Eng. Chem. Res. 1996, 35, 2477–2483. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Yang, Y.; Bae, T.-H. Hierarchically porous polymers containing triphenylamine for enhanced SF6 separation. Microporous Mesoporous Mater. 2018, 272, 232–240. [Google Scholar] [CrossRef]
- Bachman, J.E.; Smith, Z.P.; Li, T.; Xu, T.; Long, J.R. Enhanced ethylene separation and plasticization resistance in polymer membranes incorporating metal–organic framework nanocrystals. Nat. Mater. 2016, 15, 845. [Google Scholar] [CrossRef] [PubMed]
- Bereciartua, P.J.; Cantín, Á.; Corma, A.; Jordá, J.L.; Palomino, M.; Rey, F.; Valencia, S.; Corcoran, E.W.; Kortunov, P.; Ravikovitch, P.I. Control of zeolite framework flexibility and pore topology for separation of ethane and ethylene. Science 2017, 358, 1068–1071. [Google Scholar] [CrossRef]
- Sakai, M.; Sasaki, Y.; Tomono, T.; Seshimo, M.; Matsukata, M. Olefin selective Ag-exchanged X-type zeolite membrane for propylene/propane and ethylene/ethane separation. ACS Appl. Mater. Interfaces 2019, 11, 4145–4151. [Google Scholar] [CrossRef]
- Wee, L.H.; Lohe, M.R.; Janssens, N.; Kaskel, S.; Martens, J.A. Fine tuning of the metal–organic framework Cu3(BTC)2 HKUST-1 crystal size in the 100 nm to 5 micron range. J. Mater. Chem. 2012, 22, 13742–13746. [Google Scholar] [CrossRef]
- Xin, C.; Zhan, H.; Huang, X.; Li, H.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. Effect of various alkaline agents on the size and morphology of nano-sized HKUST-1 for CO2 adsorption. RSC Adv. 2015, 5, 27901–27911. [Google Scholar] [CrossRef]
- Loera-Serna, S.; Solis, H.; Ortiz, E.; Martínez-Hernandéz, A.; Noreña, L. Elimination of Methylene Blue and Reactive Black 5 from Aqueous Solution Using HKUST-1. Ind. J. Environ. Sci. Dev. 2017, 8, 241. [Google Scholar] [CrossRef] [Green Version]
- Chuah, C.Y.; Bae, T.-H. Incorporation of Cu3BTC2 nanocrystals to increase the permeability of polymeric membranes in O2/N2 separation. BMC Chem. Eng. 2019, 1, 2. [Google Scholar] [CrossRef]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Geier, S.J.; Mason, J.A.; Bloch, E.D.; Queen, W.L.; Hudson, M.R.; Brown, C.M.; Long, J.R. Selective adsorption of ethylene over ethane and propylene over propane in the metal–organic frameworks M2(dobdc)(M = Mg, Mn, Fe, Co, Ni, Zn). Chem. Sci. 2013, 4, 2054–2061. [Google Scholar] [CrossRef]
- Ziegler, T.; Rauk, A. A theoretical study of the ethylene-metal bond in complexes between copper (1+), silver (1+), gold (1+), platinum (0) or platinum (2+) and ethylene, based on the Hartree-Fock-Slater transition-state method. Inorg. Chem. 1979, 18, 1558–1565. [Google Scholar] [CrossRef]
- Li, W.; Samarasinghe, S.; Bae, T.-H. Enhancing CO2/CH4 separation performance and mechanical strength of mixed-matrix membrane via combined use of graphene oxide and ZIF-8. J. Ind. Eng. Chem. 2018, 67, 156–163. [Google Scholar] [CrossRef]
- Samarasinghe, S.; Chuah, C.Y.; Li, W.; Sethunga, G.; Wang, R.; Bae, T.-H. Incorporation of CoIII acetylacetonate and SNW-1 nanoparticles to tailor O2/N2 separation performance of mixed-matrix membrane. Sep. Purif. Technol. 2019, 223, 133–141. [Google Scholar] [CrossRef]
- Fernández-Barquín, A.; Casado-Coterillo, C.; Palomino, M.; Valencia, S.; Irabien, A. LTA/Poly (1-trimethylsilyl-1-propyne) Mixed-Matrix Membranes for High-Temperature CO2/N2 Separation. Chem. Eng. Technol. 2015, 38, 658–666. [Google Scholar] [CrossRef]
- Li, W.; Chuah, C.Y.; Nie, L.; Bae, T.-H. Enhanced CO2/CH4 selectivity and mechanical strength of mixed-matrix membrane incorporated with NiDOBDC/GO composite. J. Ind. Eng. Chem. 2019, 74, 118–125. [Google Scholar] [CrossRef]
- Goh, P.; Ismail, A.; Sanip, S.; Ng, B.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Gong, H.; Nguyen, T.H.; Wang, R.; Bae, T.-H. Separations of binary mixtures of CO2/CH4 and CO2/N2 with mixed-matrix membranes containing Zn(pyrz)2(SiF6) metal-organic framework. J. Membr. Sci. 2015, 495, 169–175. [Google Scholar] [CrossRef]
- Ge, L.; Zhou, W.; Rudolph, V.; Zhu, Z. Mixed matrix membranes incorporated with size-reduced Cu-BTC for improved gas separation. J. Mater. Chem. A 2013, 1, 6350–6358. [Google Scholar] [CrossRef]
- Zhang, C.; Lively, R.P.; Zhang, K.; Johnson, J.R.; Karvan, O.; Koros, W.J. Unexpected molecular sieving properties of zeolitic imidazolate framework-8. J. Phys. Chem. Lett. 2012, 3, 2130–2134. [Google Scholar] [CrossRef] [PubMed]








| Membrane | C2H4 Permeability (Barrer) | C2H4/C2H6 Selectivity |
|---|---|---|
| ODPA-TMPDA | 6.3 ± 0.4 | 3.9 ± 0.4 |
| ODPA-TMPDA + 10 wt% HKUST-1 | 13.6 ± 1.7 | 3.6 ± 0.3 |
| ODPA-TMPDA + 20 wt% HKUST-1 | 16.0 ± 0.2 | 3.4 ± 0.2 |
| 6FDA-TMPDA | 108 ± 7.2 | 2.5 ± 0.1 |
| 6FDA-TMPDA + 10 wt% HKUST-1 | 148 ± 11.8 | 2.5 ± 0.5 |
| 6FDA-TMPDA + 20 wt% HKUST-1 | 183 ± 3.8 | 2.4 ± 0.1 |
| Membrane | Density (g cm−3) | C2H4 Solubility, × 103 (mol m−3 bar−1) | C2H4 Diffusivity, × 10−13 (m2 s−1) | C2H6 Solubility, × 103 (mol m−3 bar−1) | C2H6 Diffusivity, × 10−13 (m2 s−1) | Solubility Selectivity | Diffusivity Selectivity |
|---|---|---|---|---|---|---|---|
| ODPA-TMPDA | 1.25 | 1.21 | 0.394 | 0.989 | 0.124 | 1.22 | 3.17 |
| ODPA-TMPDA + 20 wt% HKUST-1 | 1.24 | 1.77 | 0.688 | 1.46 | 0.244 | 1.21 | 2.82 |
| 6FDA-TMPDA | 1.26 | 1.19 | 0.687 | 1.28 | 2.57 | 0.933 | 2.68 |
| 6FDA-TMPDA + 20 wt% HKUST-1 | 1.22 | 1.58 | 0.880 | 1.69 | 3.50 | 0.934 | 2.51 |
| Filler | Polymer | Filler Loading (wt%) | Separation Performance | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Testing Condition | P(C2H4) (Barrer) | Permeability Enhancement (%) | α(C2H4/C2H6) | Selectivity Enhancement (%) | |||||
| Pressure (bar) | Temp. (°C) | ||||||||
| Silica nanoparticles | CA | 30 | 2 | 35 | 0.11 | 100 | 4.1 | 173 | [5] |
| Silica nanoparticles | Matrimid® | 20 | 3 | 30 | 0.19 | 137.5 | 3.2 | 98.1 | [51] |
| ZIF-8 | 6FDA-DAM | 23.8 | 2 | 35 | 72.9 | 85.0 | 3.2 | −3.1 | [77] |
| ZIF-8 | DBzPBI-BuI | 30 | 2.7 | 35 | 111 | 2953 | 2.6 | −2.29 | [13] |
| HKUST-1 b | ODPA-TMPDA | 20 | 1 | 35 | 16 | 155.2 | 3.4 | −11.6 | This work |
| HKUST-1 b | 6FDA-TMPDA | 20 | 1 | 35 | 183 | 69.4 | 2.4 | −6.0 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuah, C.Y.; Samarasinghe, S.A.S.C.; Li, W.; Goh, K.; Bae, T.-H. Leveraging Nanocrystal HKUST-1 in Mixed-Matrix Membranes for Ethylene/Ethane Separation. Membranes 2020, 10, 74. https://doi.org/10.3390/membranes10040074
Chuah CY, Samarasinghe SASC, Li W, Goh K, Bae T-H. Leveraging Nanocrystal HKUST-1 in Mixed-Matrix Membranes for Ethylene/Ethane Separation. Membranes. 2020; 10(4):74. https://doi.org/10.3390/membranes10040074
Chicago/Turabian StyleChuah, Chong Yang, S.A.S.C. Samarasinghe, Wen Li, Kunli Goh, and Tae-Hyun Bae. 2020. "Leveraging Nanocrystal HKUST-1 in Mixed-Matrix Membranes for Ethylene/Ethane Separation" Membranes 10, no. 4: 74. https://doi.org/10.3390/membranes10040074
APA StyleChuah, C. Y., Samarasinghe, S. A. S. C., Li, W., Goh, K., & Bae, T. -H. (2020). Leveraging Nanocrystal HKUST-1 in Mixed-Matrix Membranes for Ethylene/Ethane Separation. Membranes, 10(4), 74. https://doi.org/10.3390/membranes10040074