Impact of MWCO and Dopamine/Polyethyleneimine Concentrations on Surface Properties and Filtration Performance of Modified Membranes
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
2.2. Methods
2.2.1. Membrane Modification
2.2.2. Membrane Characterization
2.2.3. Filtration Performance
2.3. Statistical Analysis
3. Results and Discussion
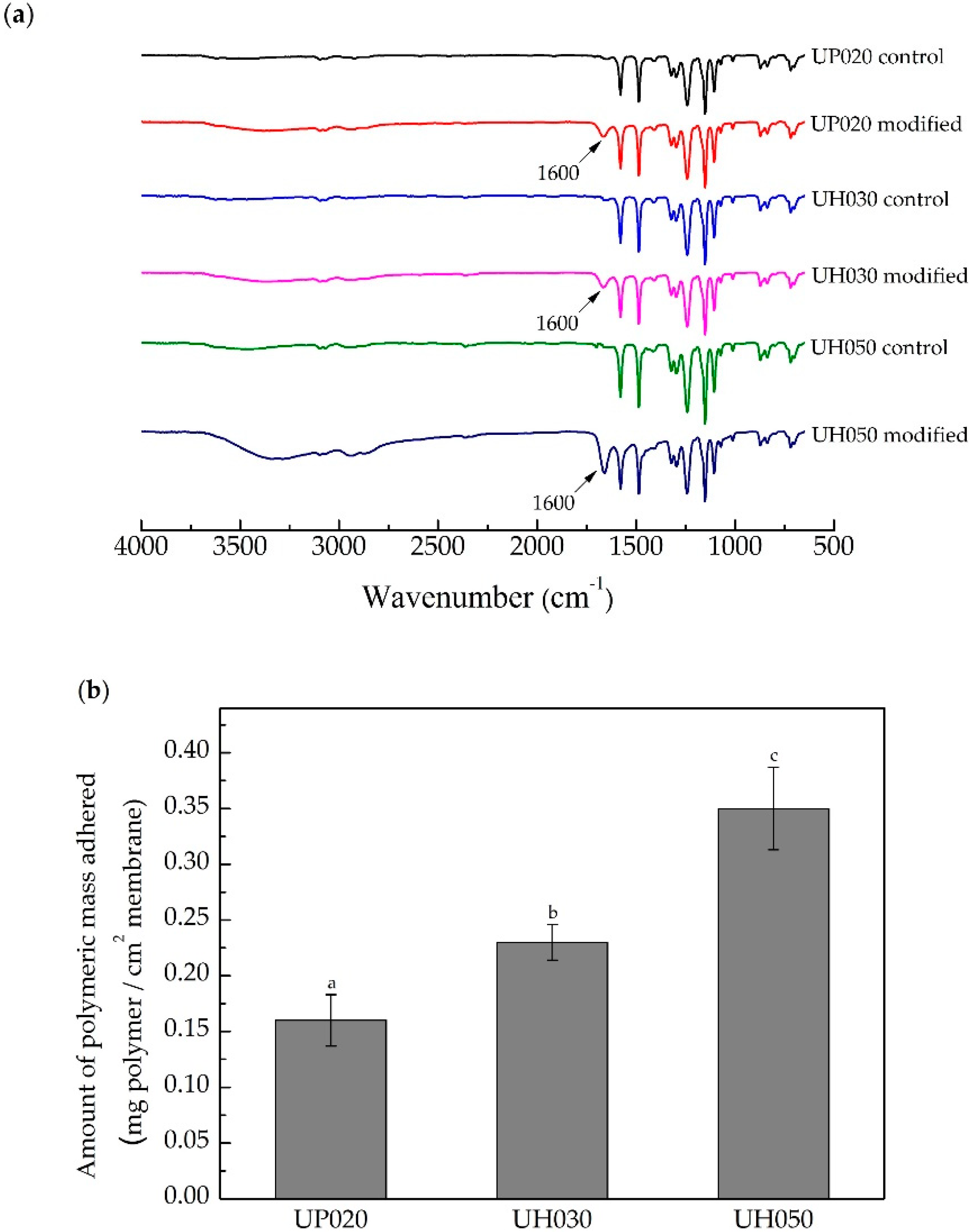
3.1. Influence of Membrane Molecular Weight Cut-off
3.1.1. Physicochemical Characteristics of Control and Modified Membranes
3.1.2. Membrane Filtration Performance of Control and Modified Membranes
3.2. Influence of DA and PEI Concentration
3.2.1. Effects of Different DA and PEI Concentrations
3.2.2. Influence of PEI Concentration
3.2.3. Membrane Regeneration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barclay, T.G.; Hegab, H.M.; Michelmore, A.; Weeks, M.; Ginic-markovic, M. Multidentate polyzwitterion attachment to polydopamine modified ultrafiltration membranes for dairy processing: Characterization, performance and durability. J. Ind. Eng. Chem. 2018, 61, 356–367. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, N.; Ranjan, R.; Kumar, S.; Bhat, Z.F.; Jeong, D.K. Perspective of membrane technology in dairy industry: A review, Asian-Australasian. J. Anim. Sci. 2013, 26, 1347–1358. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Tian, J.; Gao, S.; Shi, W.; Cui, F. Dopamine triggered one step polymerization and codeposition of reactive surfactant on PES membrane surface for antifouling modification. Sep. Purif. Technol. 2020, 249, 117148. [Google Scholar] [CrossRef]
- Gao, N.; Fan, W.; Xu, Z. Ceramic membrane with protein-resistant surface via dopamine/diglycolamine co-deposition. Sep. Purif. Technol. 2020, 234, 116135. [Google Scholar] [CrossRef]
- Bennani, C.F.; Ousji, B.; Ennigrou, D.J. Reclamation of dairy wastewater using ultrafiltration process. Desalin. Water Treat. 2015, 55, 297–303. [Google Scholar] [CrossRef]
- Tonon, R.V.; Bianca, A.; Couto, C.C.; Mellinger-silva, C.; Iraidy, A.; Brígida, S.; Cabral, L.M.C. Coupling of ultrafiltration and enzymatic hydrolysis aiming at valorizing shrimp wastewater. Food Chem. 2016, 198, 20–27. [Google Scholar] [CrossRef]
- Haberkamp, J.; Ernst, M.; Makdissy, G.; Huck, P.M. Protein fouling of ultrafiltration membranes—Investigation of several factors relevant for tertiary wastewater treatment. J. Environ. Eng. Sci. 2009, 660, 651–660. [Google Scholar] [CrossRef]
- Lech, M.; Niesobska, A.; Trusek-holownia, A. Dairy wastewater utilization: Separation of whey proteins in membrane and chromatographic processes. Desalin. Water Treat. 2016, 3994. [Google Scholar] [CrossRef]
- Sioutopoulos, D.; Karabelas, A. Membrane Fouling Due to Protein—Polysaccharide Mixtures in Dead-End Ultrafiltration; the Effect of Permeation Flux on Fouling Resistance. Membranes 2019, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Ren, P.; Yang, H.; Xu, Z. Fabrication of antifouling membrane surface by poly (sulfobetaine methacrylate)/polydopamine co-deposition. J. Memb. Sci. 2014, 466, 18–25. [Google Scholar] [CrossRef]
- Tripathi, B.P.; Das, P.; Simon, F.; Stamm, M. Ultralow fouling membranes by surface modi fi cation with functional polydopamine. Eur. Polym. J. 2018, 99, 80–89. [Google Scholar] [CrossRef]
- Yu, C.; Gao, B.; Wang, W.; Xu, X.; Yue, Q. Chemosphere Alleviating membrane fouling of modified polysulfone membrane via coagulation pretreatment/ultrafiltration hybrid process. Chemosphere. 2019, 235, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Nthunya, L.N.; Gutierrez, L.; Lapeire, L.; Verbeken, K.; Zaouri, N.; Nxumalo, E.N.; Mamba, B.B.; Verliefde, A.R.; Mhlanga, S.D. Fouling-resistant PVDF nano fi bre membranes for the desalination of brackish water in membrane distillation. Sep. Purif. Technol. 2019, 228, 115793. [Google Scholar] [CrossRef]
- Salama, A.; Zoubeik, M.; Henni, A.; El, M. A new modeling approach for flux declining behavior during the filtration of oily-water systems due to coalescence and clustering of oil droplets: Experimental and multicontinuum investigation. Sep. Purif. Technol. 2019, 227, 115688. [Google Scholar] [CrossRef]
- Cheng, L.; Shaikh, A.R.; Fang, L.; Jeon, S.; Liu, C.; Zhang, L.; Wu, H.; Wang, D.; Matsuyama, H. Fouling-Resistant and Self-Cleaning Aliphatic Polyketone Membrane for Sustainable Oil − Water Emulsion Separation. ACS Appl. Mater. Interfaces. 2018, 10, 44880–44889. [Google Scholar] [CrossRef]
- Li, Y.; Shi, S.; Cao, H.; Zhao, Z.; Su, C.; Wen, H. Improvement of the antifouling performance and stability of an anion exchange membrane by surface modi fi cation with graphene oxide (GO) and polydopamine (PDA). J. Memb. Sci. 2018, 566, 44–53. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, P.; Wei, J. New insight into the fouling behavior of hydrophobic and hydrophilic polypropylene membranes in integrated membrane bioreactors. J. Environ. Technol. 2018, 3330. [Google Scholar] [CrossRef]
- Huner, I.D.; Gulec, H.A. Fouling behavior of poly (ether) sulfone ultrafiltration membrane during concentration of whey proteins: Effect of hydrophilic modification using atmospheric pressure argon jet plasma. Colloids Surfaces B Biointerfaces. 2017, 160, 510–519. [Google Scholar] [CrossRef]
- Shen, L.; Wang, H.; Zhang, Y.; Li, R.; Fabien, B.; Yu, G.; Lin, H.; Liao, B. New strategy of grafting hydroxyethyl acrylate (HEA) via γ ray radiation to modify polyvinylidene fluoride (PVDF) membrane: Thermodynamic mechanisms of the improved antifouling performance. Sep. Purif. Technol. 2018, 207, 83–91. [Google Scholar] [CrossRef]
- Rajakumaran, R.; Boddu, V.; Kumar, M.; Shalaby, M.S.; Abdallah, H. Effect of ZnO morphology on GO-ZnO modified polyamide reverse osmosis membranes for desalination. Desalination. 2019, 467, 245–256. [Google Scholar] [CrossRef]
- Davenport, D.M.; Lee, J.; Elimelech, M. Efficacy of antifouling modi fi cation of ultra fi ltration membranes by grafting zwitterionic polymer brushes. Sep. Purif. Technol. 2017, 189, 389–398. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Suo, X.; Huang, J.; Gong, Y.; Liu, Y.; Li, H. Effect of surface topological structure and chemical modification of flame sprayed aluminum coatings on the colonization of Cylindrotheca closterium on their surfaces. Appl. Surf. Sci. 2016, 388, 385–391. [Google Scholar] [CrossRef]
- Nguyen, A.; Azari, S.; Zou, L. Coating zwitterionic amino acid l-DOPA to increase fouling resistance of forward osmosis membrane. Desalination 2013, 312, 82–87. [Google Scholar] [CrossRef]
- Chang, X.; Wang, Z.; Quan, S.; Xu, Y.; Jiang, Z.; Shao, L. Exploring the synergetic effects of graphene oxide (GO) and polyvinylpyrrodione (PVP) on poly (vinylylidenefluoride)(PVDF) ultrafiltration membrane performance. Appl. Surf. Sci. 2014, 316, 537–548. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhang, H.; Zhu, B.; Xu, Y. Antifouling and antimicrobial polymer membranes based on bioinspired polydopamine and strong hydrogen-bonded poly(n -vinyl pyrrolidone). ACS Appl. Mater. Interfaces. 2013, 5, 12895–12904. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Wu, M.B.; Li, Y.J.; Chen, Y.F.; Wan, L.S.; Xu, Z.K. Effects of polyethyleneimine molecular weight and proportion on the membrane hydrophilization by codepositing with dopamine. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, H.; Liang, H.; Wan, L.; Xu, Z. Nanofiltration membranes via co-deposition of polydopamine/polyethylenimine followed by cross-linking. J. Memb. Sci. 2015, 476, 50–58. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Lau, C.-H.; Zhang, N.-Q.; Bai, Y.-P.; Shao, L. Mussel-inspired tailoring of membrane wettability for harsh water treatment. J. Mater. Chem. A. 2015, 3, 2650–2657. [Google Scholar] [CrossRef]
- Li, J.; Yuan, S.; Wang, J.; Zhu, J.; Shen, J. Mussel-inspired modi fi cation of ion exchange membrane for monovalent separation. J. Memb. Sci. 2018, 553, 139–150. [Google Scholar] [CrossRef]
- Shi, H.; He, Y.; Pan, Y.; Di, H.; Zeng, G.; Zhang, L.; Zhang, C. A modified mussel-inspired method to fabricate TiO2 decorated superhydrophilic PVDF membrane for oil/water separation. J. Memb. Sci. 2016, 506, 60–70. [Google Scholar] [CrossRef]
- Kasemset, S.; Wang, L.; He, Z.; Miller, D.J.; Kirschner, A.; Freeman, B.D.; Sharma, M.M. Influence of polydopamine deposition conditions on hydraulic permeability, sieving coefficients, pore size and pore size distribution for a polysulfone ultrafiltration membrane. J. Memb. Sci. 2017, 522, 100–115. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Liu, J.; Shi, A.; Luo, X.; Lin, J.; Zheng, R.; Fan, H.; Selasie, S.V.; Lin, H. A facile method to modify polypropylene membrane by polydopamine coating via inkjet printing technique for superior performance. J. Colloid Interface Sci. 2019, 552, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wu, Y.; Shen, L.; Chen, J.; Lin, H. A novel strategy to develop antifouling and antibacterial conductive Cu/polydopamine/polyvinylidene fluoride membranes for water treatment. J. Colloid Interface Sci. 2018, 531, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhai, Y.; Han, X.; Liu, H.; Hu, Y. Surface chemistry-dominated underwater superoleophobic mesh with mussel-inspired zwitterionic coatings for oil/water separation and self- cleaning. Appl. Surf. Sci. 2019, 483, 399–408. [Google Scholar] [CrossRef]
- Xia, Y.; Dai, X.; Gai, J. Preparation of high-performance reverse osmosis membrane by zwitterionic polymer coating in a facile one-step way. J. Appl. Polym. Sci. 2019, 48355, 1–11. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Li, S.; Zhao, W.; Wei, Q.; Nie, S.; Sun, S.; Zhao, C. The hydrodynamic permeability and surface property of polyethersulfone ultrafiltration membranes with mussel-inspired polydopamine coatings. J. Memb. Sci. 2012, 417–418, 228–236. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, F.; Xue, L. Under seawater superoleophobic PVDF membrane inspired by polydopamine for efficient oil/seawater separation. J. Memb. Sci. 2015, 476, 321–329. [Google Scholar] [CrossRef]
- Wang, R.; Song, X.; Xiang, T.; Liu, Q.; Su, B.; Zhao, W.; Zhao, C. Mussel-inspired chitosan-polyurethane coatings for improving the antifouling and antibacterial properties of polyethersulfone membranes. Carbohydr. Polym. 2017, 168, 310–319. [Google Scholar] [CrossRef]
- Kasemset, S.; Lee, A.; Miller, D.J.; Freeman, B.D.; Sharma, M.M. Effect of polydopamine deposition conditions on fouling resistance, physical properties, and permeation properties of reverse osmosis membranes in oil/water separation. J. Memb. Sci. 2013, 426, 208–216. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Wang, Y.; Long, Y.; Gao, H.; Zhang, X.; Zhao, N.; Cai, Y.; Xu, J. Mussel-inspired chemistry for robust and surface-modifiable multilayer films. Langmuir. 2011, 27, 13684–13691. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.Y.; Xu, Y.Y.; Zhu, L.P.; Wang, Y.; Zhu, B.K. A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly(DOPA) and poly(dopamine). J. Memb. Sci. 2009, 327, 244–253. [Google Scholar] [CrossRef]
- Xue, Q.; Cao, H.; Meng, F.; Quan, M.; Gong, Y.K. Cell membrane mimetic coating immobilized by mussel-inspired adhesion on commercial ultrafiltration membrane to enhance antifouling performance. J. Memb. Sci. 2017, 528, 1–11. [Google Scholar] [CrossRef]
- Yang, H.-C.; Luo, J.; Lv, Y.; Shen, P.; Xu, Z.-K. Surface Engineering of Polymer Membranes. J. Memb. Sci. 2015, 483, 42–59. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Lin, W.; Sun, H.; Wu, L.; Chen, S. A facile method for polyamide membrane modification by poly(sulfobetaine methacrylate) to improve fouling resistance. J. Memb. Sci. 2013, 446, 164–170. [Google Scholar] [CrossRef]
- Yang, J.; Xu, H.; Zhang, L.; Zhong, Y.; Sui, X.; Mao, Z. Lasting superhydrophobicity and antibacterial activity of Cu nanoparticles immobilized on the surface of dopamine modified cotton fabrics. Surf. Coatings Technol. 2017, 309, 149–154. [Google Scholar] [CrossRef]
- He, Y.; Xu, L.; Feng, X.; Zhao, Y.; Chen, L. Dopamine-induced nonionic polymer coatings for significantly enhancing separation and antifouling properties of polymer membranes: Codeposition versus sequential deposition. J. Memb. Sci. 2017, 539, 421–431. [Google Scholar] [CrossRef]
- Jiang, J.H.; Zhu, L.P.; Li, X.L.; Xu, Y.Y.; Zhu, B.K. Surface modification of PE porous membranes based on the strong adhesion of polydopamine and covalent immobilization of heparin. J. Memb. Sci. 2010, 364, 194–202. [Google Scholar] [CrossRef]
- Li, M.; Xu, J.; Chang, C.; Feng, C.; Zhang, L. Bioinspired fabrication of composite nano fi ltration membrane based on the formation of DA/PEI layer followed by cross-linking. J. Memb. Sci. 2014, 459, 62–71. [Google Scholar] [CrossRef]
- McCloskey, B.D.; Park, H.B.; Ju, H.; Rowe, B.W.; Miller, D.J.; Chun, B.J.; Kin, K.; Freeman, B.D. Influence of polydopamine deposition conditions on pure water flux and foulant adhesion resistance of reverse osmosis, ultrafiltration, and microfiltration membranes. Polymer. 2010, 51, 3472–3485. [Google Scholar] [CrossRef]
- Oymaci, P.; Nijmeijer, K.; Borneman, Z. Development of Polydopamine Forward Osmosis Membranes with Low Reverse Salt Flux. Membranes 2020, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-C.; Liao, K.-J.; Huang, H.; Wu, Q.-Y.; Wan, L.-S.; Xu, Z.-K. Mussel-inspired modification of a polymer membrane for ultra-high water permeability and oil-in-water emulsion separation. J. Mater. Chem. A. 2014, 2, 10225–10230. [Google Scholar] [CrossRef]
- Xue, S.; Li, C.; Li, J.; Zhu, H.; Guo, Y. A catechol-based biomimetic strategy combined with surface mineralization to enhance hydrophilicity and anti-fouling property of PTFE flat membrane. J. Membr. 2017, 524, 409–418. [Google Scholar] [CrossRef]
- Shi, H.; Xue, L.; Gao, A.; Fu, Y.; Zhou, Q.; Zhu, L. Fouling-resistant and adhesion-resistant surface modification of dual layer PVDF hollow fiber membrane by dopamine and quaternary polyethyleneimine. J. Memb. Sci. 2016, 498, 39–47. [Google Scholar] [CrossRef]
- Xu, Y.C.; Wang, Z.X.; Cheng, X.Q.; Xiao, Y.C.; Shao, L. Positively charged nanofiltration membranes via economically mussel-substance-simulated co-deposition for textile wastewater treatment. Chem. Eng. J. 2016, 303, 555–564. [Google Scholar] [CrossRef]
- Yang, Q.; Luo, J.; Guo, S.; Hang, X.; Chen, X.; Wan, Y. Threshold flux in concentration mode: Fouling control during clarification of molasses by ultrafiltration. J. Memb. Sci. 2019, 586, 130–139. [Google Scholar] [CrossRef]
- Zin, G.; Wu, J.; Rezzadori, K.; Petrus, J.C.C.; Di Luccio, M.; Li, Q. Modification of hydrophobic commercial PVDF microfiltration membranes into superhydrophilic membranes by the mussel-inspired method with dopamine and polyethyleneimine. Sep. Purif. Technol. 2019, 212, 641–649. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 254, 248–254. [Google Scholar] [CrossRef]
- Li, F.; Meng, J.; Ye, J.; Yang, B.; Tian, Q.; Deng, C. Surface modification of PES ultrafiltration membrane by polydopamine coating and poly(ethyleneglycol) grafting: Morphology stability, and anti-fouling. Desalination 2014, 344, 422–430. [Google Scholar] [CrossRef]
- Guan, N.; Chew, P.; Zhao, S.; Malde, C.; Wang, R. Superoleophobic surface modification for robust membrane distillation performance. J. Memb. Sci. 2017, 541, 162–173. [Google Scholar] [CrossRef]
- Xu, F.; Wei, M.; Zhang, X.; Song, Y.; Zhou, W.; Wang, Y. How Pore Hydrophilicity Influences Water Permeability? Research 2019, 2019, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Azari, S.; Zou, L. Using zwitterionic amino acid l-DOPA to modify the surface of thin film composite polyamide reverse osmosis membranes to increase their fouling resistance. J. Memb. Sci. 2012, 401–402, 68–75. [Google Scholar] [CrossRef]









| DA (mg mL−1) | PEI (mg mL−1) |
|---|---|
| 2.0 | 0.5, 2.0 |
| 0.5 | 1.0, 2.0, 4.0, 8.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proner, M.C.; Ramalho Marques, I.; Ambrosi, A.; Rezzadori, K.; da Costa, C.; Zin, G.; Tres, M.V.; Di Luccio, M. Impact of MWCO and Dopamine/Polyethyleneimine Concentrations on Surface Properties and Filtration Performance of Modified Membranes. Membranes 2020, 10, 239. https://doi.org/10.3390/membranes10090239
Proner MC, Ramalho Marques I, Ambrosi A, Rezzadori K, da Costa C, Zin G, Tres MV, Di Luccio M. Impact of MWCO and Dopamine/Polyethyleneimine Concentrations on Surface Properties and Filtration Performance of Modified Membranes. Membranes. 2020; 10(9):239. https://doi.org/10.3390/membranes10090239
Chicago/Turabian StyleProner, Mariane Carolina, Ingrid Ramalho Marques, Alan Ambrosi, Katia Rezzadori, Cristiane da Costa, Guilherme Zin, Marcus Vinícius Tres, and Marco Di Luccio. 2020. "Impact of MWCO and Dopamine/Polyethyleneimine Concentrations on Surface Properties and Filtration Performance of Modified Membranes" Membranes 10, no. 9: 239. https://doi.org/10.3390/membranes10090239
APA StyleProner, M. C., Ramalho Marques, I., Ambrosi, A., Rezzadori, K., da Costa, C., Zin, G., Tres, M. V., & Di Luccio, M. (2020). Impact of MWCO and Dopamine/Polyethyleneimine Concentrations on Surface Properties and Filtration Performance of Modified Membranes. Membranes, 10(9), 239. https://doi.org/10.3390/membranes10090239







