An In Situ Incorporation of Acrylic Acid and ZnO Nanoparticles into Polyamide Thin Film Composite Membranes for Their Effect on Membrane pH Responsive Behavior
Abstract
:1. Introduction
2. Materials and Methods Experimental
2.1. Materials
2.2. Membrane Preparation
2.3. Characterization of Membranes
2.3.1. Contact Angle Analysis
2.3.2. Attenuated Total Reflectance-Fourier Transform Infra-Red Spectroscopic Analysis
2.3.3. Scanning Electron Microscopic Analysis
2.3.4. Atomic Force Microscopic Analysis
2.3.5. Thermal Gravimetric Analysis
2.3.6. X-ray Diffraction Spectroscopic Analysis
2.4. Membrane Performance Evaluation
2.4.1. Membrane Water Permeation and Solute Rejection Study
2.4.2. Membrane Swelling Studies
2.4.3. Ion Exchange Capacity Analysis
3. Results and Discussion
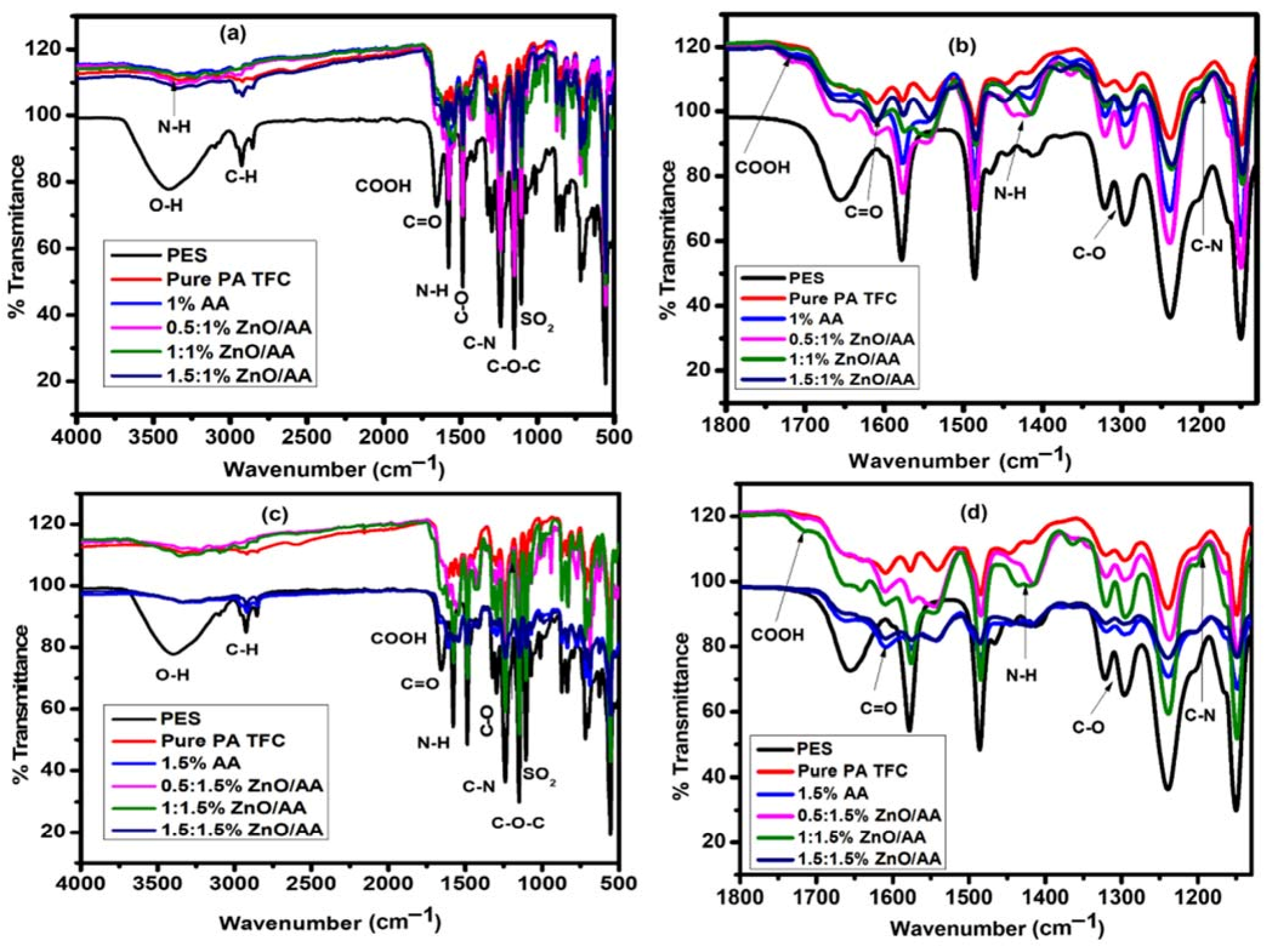
3.1. Attenuated Total Reflectance-Infra-Red Spectroscopic Analysis
3.2. Surface Morphological Study of Membranes
3.2.1. High-Resolution Scanning Electron Microscopic (HR-SEM) Analysis
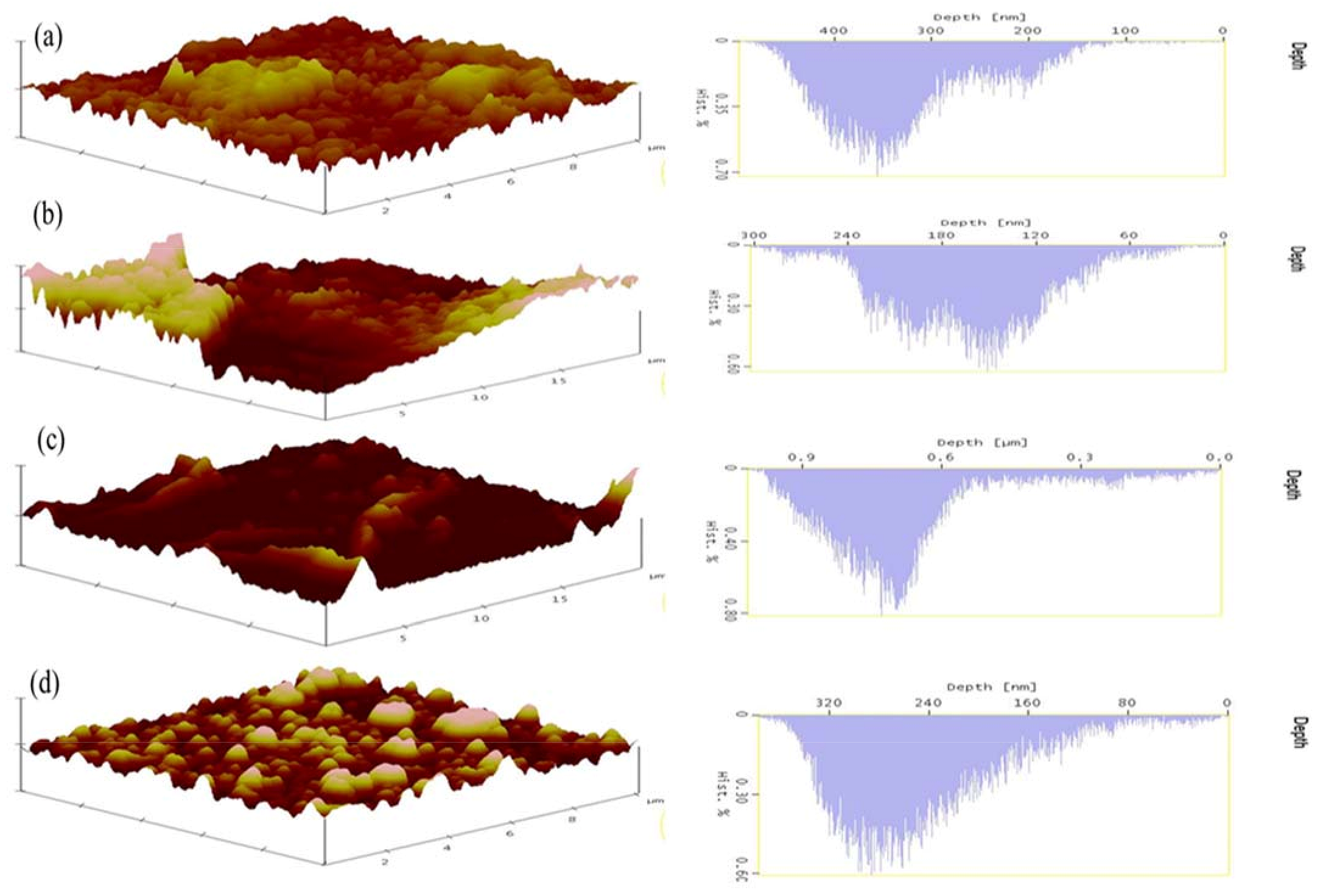
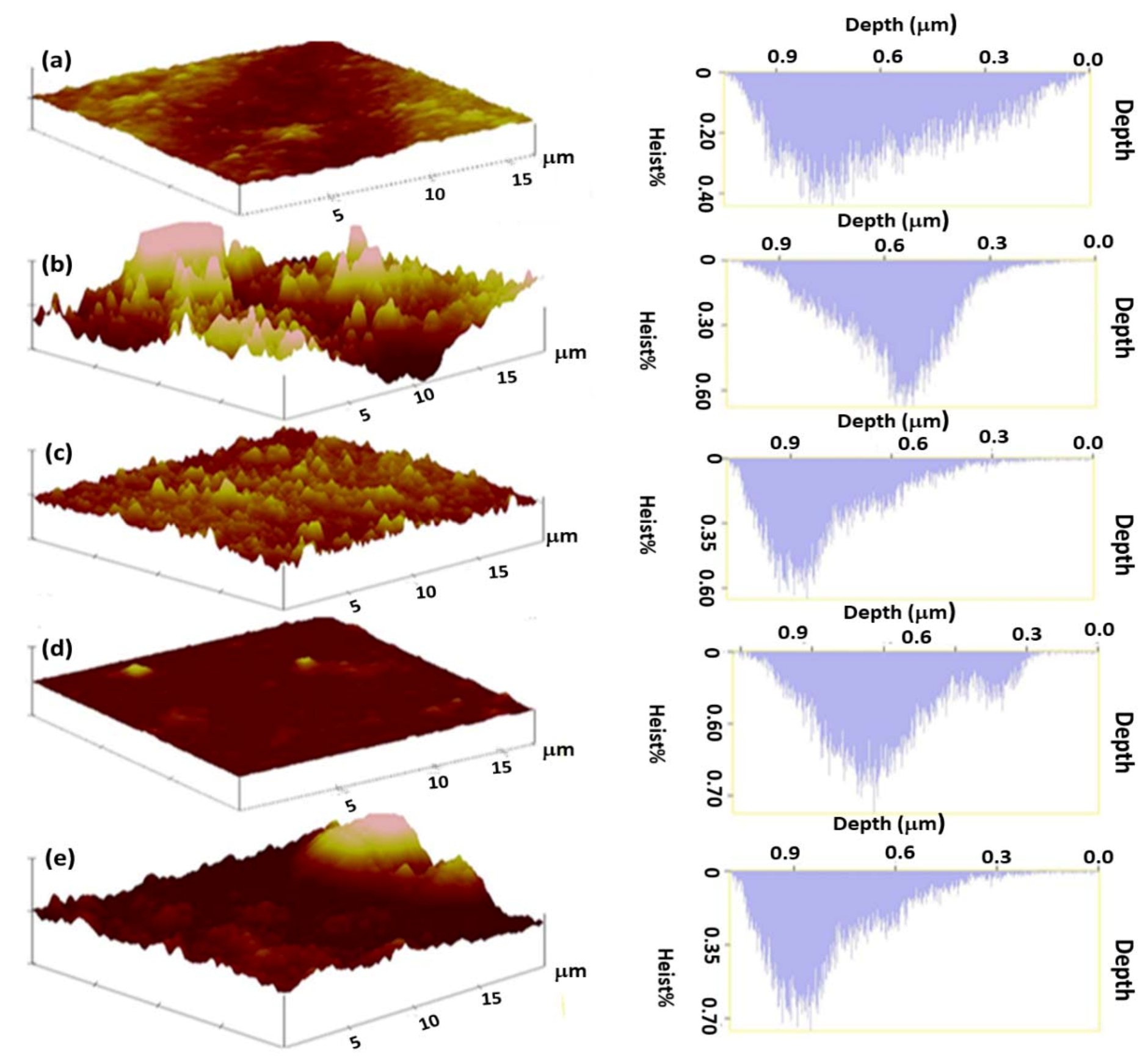
3.2.2. Atomic Force Microscopy Analysis
3.3. X-ray Diffraction Spectroscopic Analysis
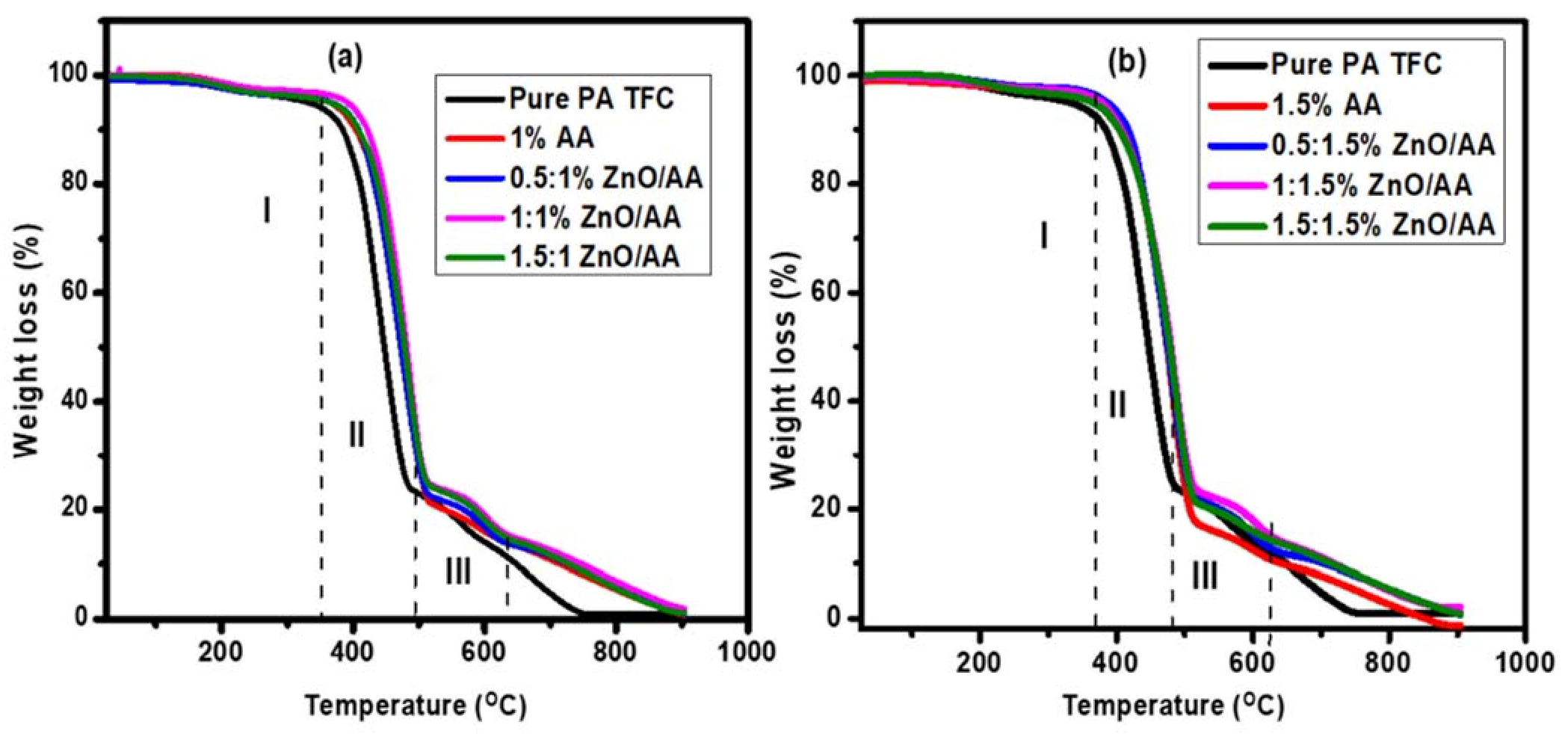
3.4. Thermal Gravimetric Analysis
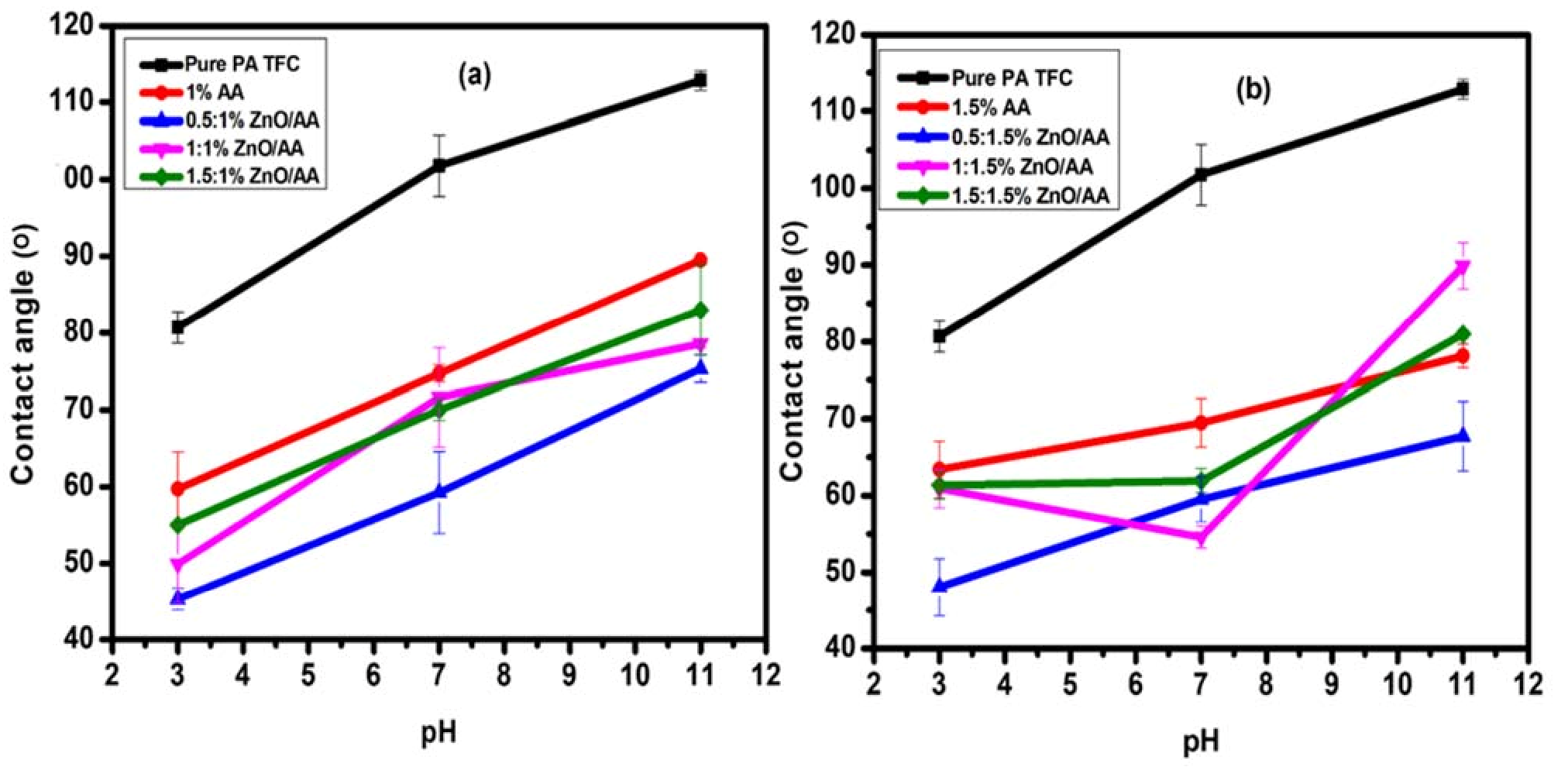
3.5. Membrane Hydrophilicity Evaluation
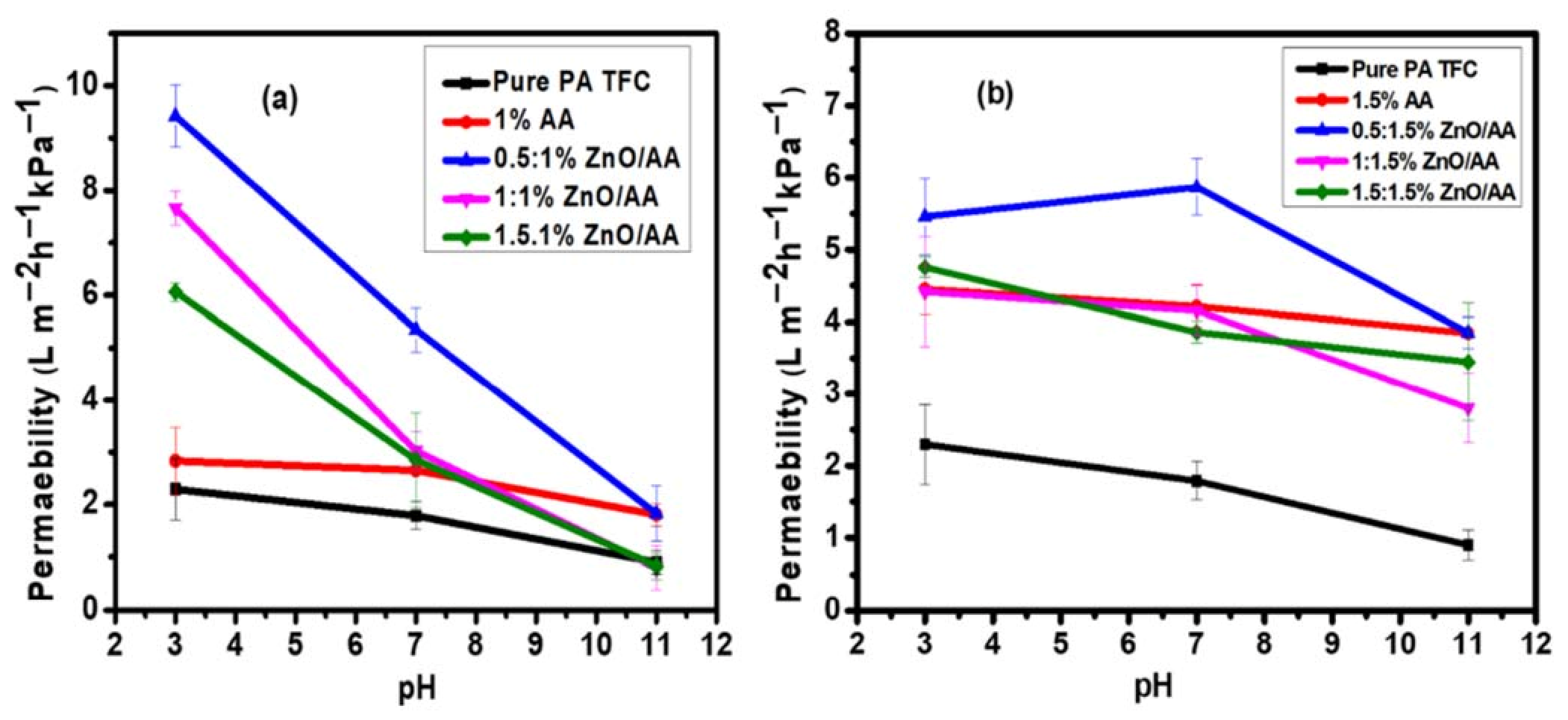
3.6. Water Permeability of the Membranes
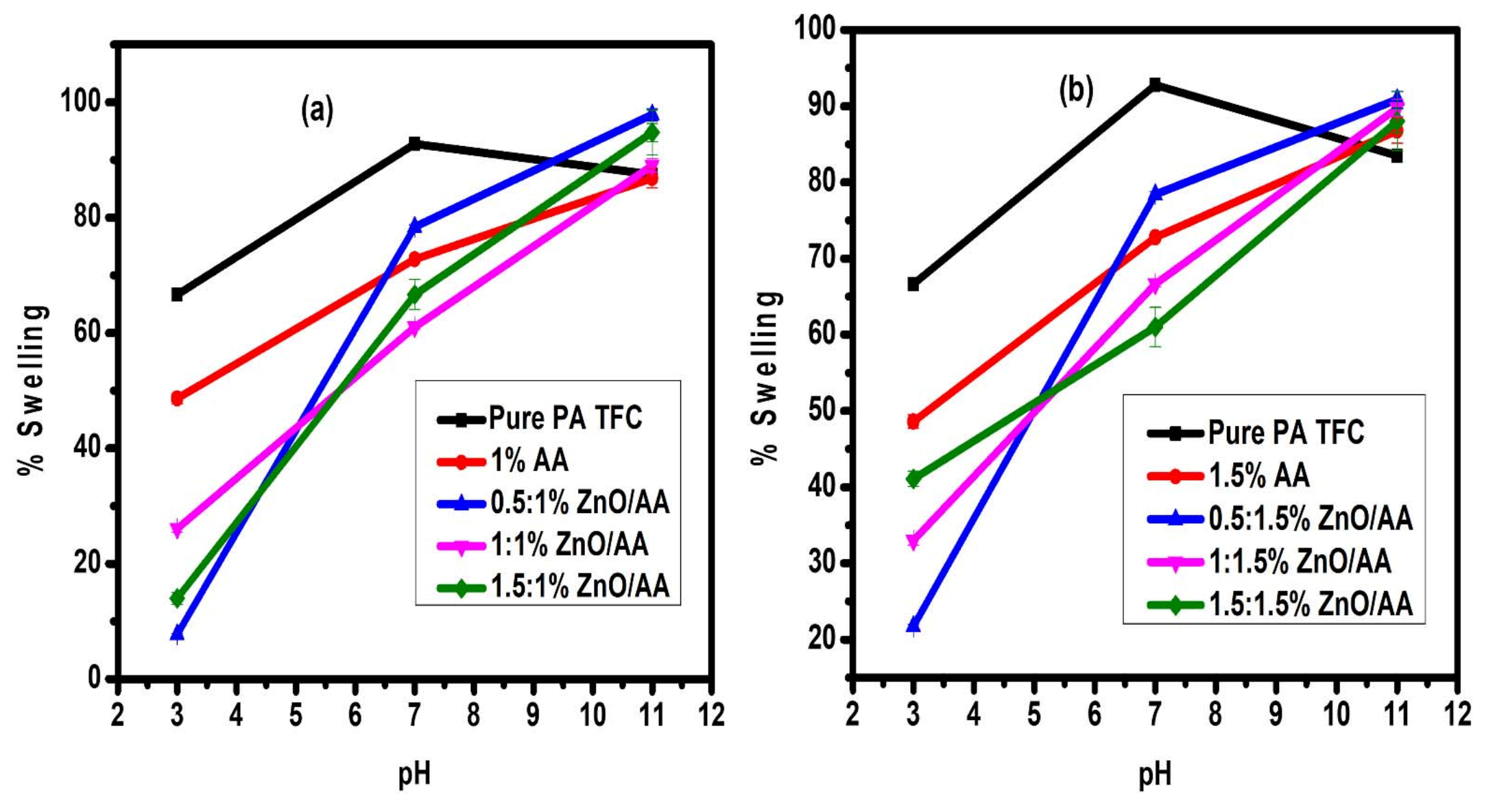
3.7. Membrane Swelling Evaluation
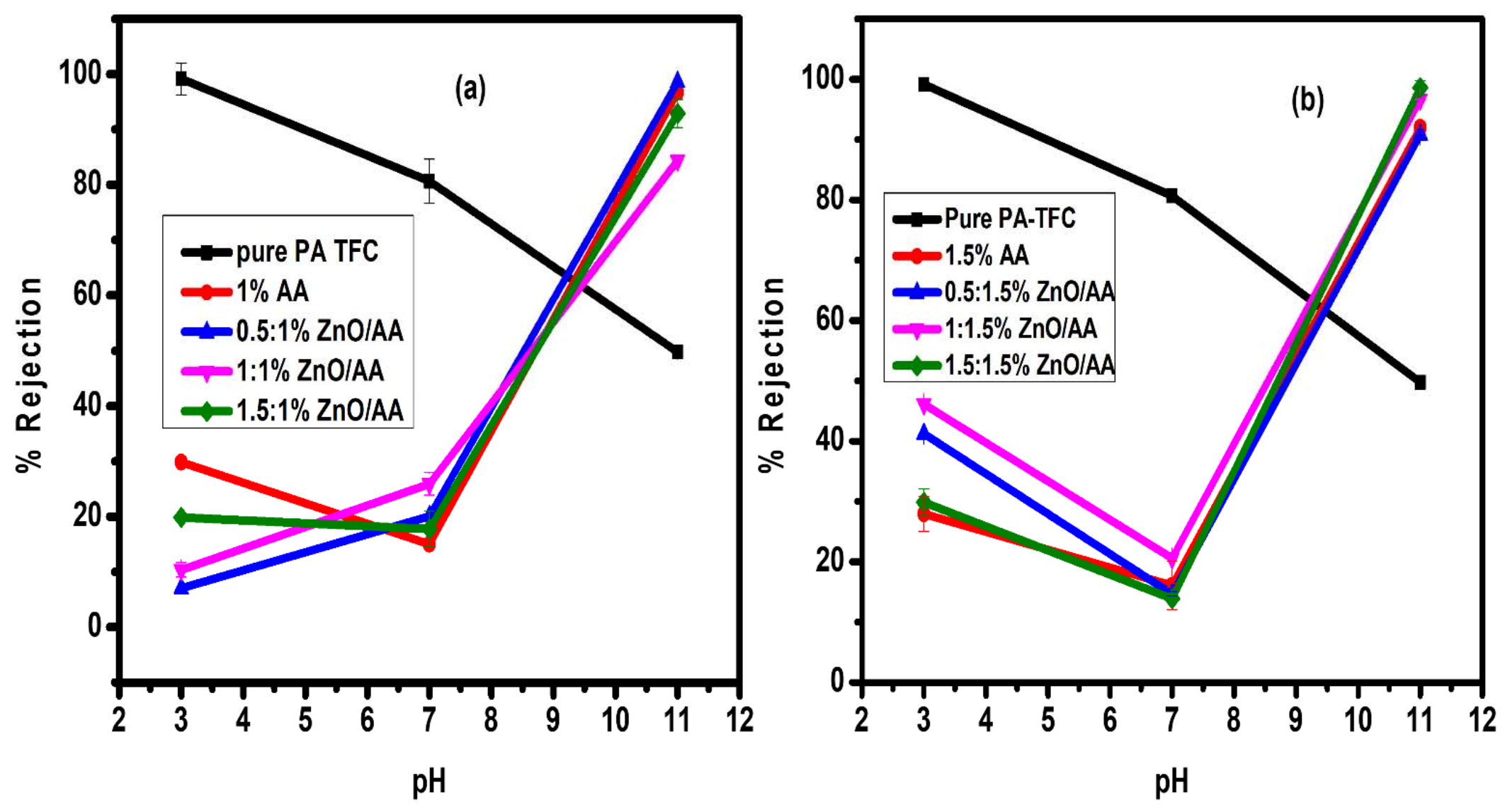
3.8. Pb(II) Rejection Analysis
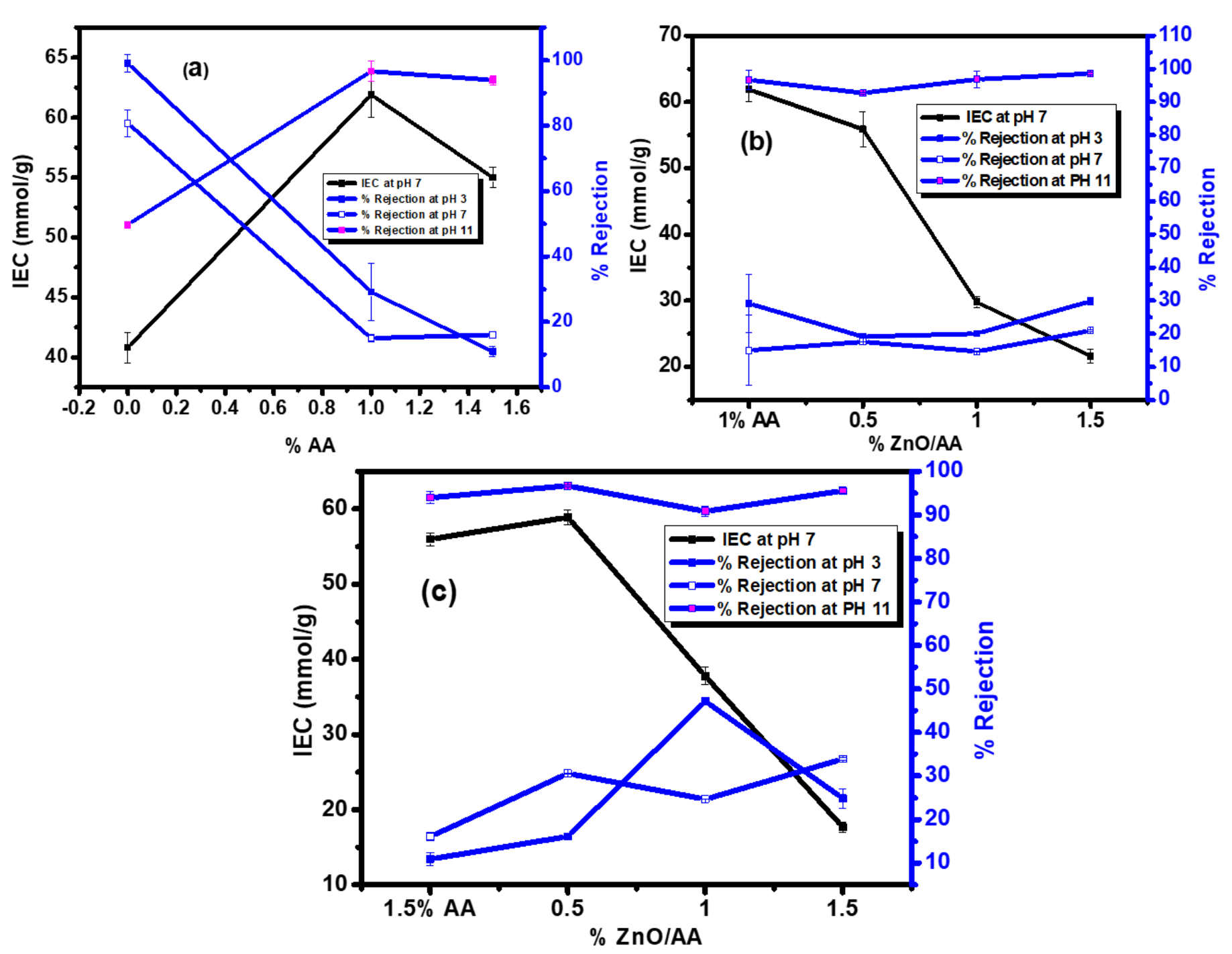
3.9. Ion Exchange Capacity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Yu, S.; Liu, M.; Liu, X.; Gao, C. Performance Enhancement in Interfacially Synthesized Thin-Film Composite Polyamide-Urethane Reverse Osmosis Membrane for Seawater Desalination. J. Membr. Sci. 2009, 342, 313–320. [Google Scholar] [CrossRef]
- Ibrahim, S.; Mohammadi Ghaleni, M.; Isloor, A.M.; Bavarian, M.; Nejati, S. Poly (Homopiperazine–Amide) Thin-Film Composite Membrane for Nanofiltration of Heavy Metal Ions. ACS Omega 2020, 5, 28749–28759. [Google Scholar] [CrossRef] [PubMed]
- Singto, S.; Sajomsang, W.; Ratanatawanate, C.; Zhang, F. Flexible and Hydrophilic Copolyamide Thin-Film Composites on Hollow Fiber Membranes for Enhanced Nanofiltration Performance. ACS Appl. Mater. Interfaces 2020, 12, 28624–28634. [Google Scholar] [CrossRef] [PubMed]
- Khajouei, M.; Jahanshahi, M.; Peyravi, M. Biofouling Mitigation of TFC Membrane by in-Situ Grafting of PANI/Cu Couple Nanoparticle. J. Taiwan Inst. Chem. Eng. 2018, 85, 237–247. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.-N.; Leckie, J.O. Probing the Nano-and Micro-Scales of Reverse Osmosis Membranes—A Comprehensive Characterization of Physiochemical Properties of Uncoated and Coated Membranes by XPS, TEM, ATR-FTIR, and Streaming Potential Measurements. J. Membr. Sci. 2007, 287, 146–156. [Google Scholar] [CrossRef]
- Liu, F.; Wang, L.; Li, D.; Liu, Q.; Deng, B. Preparation and Characterization of Novel Thin Film Composite Nanofiltration Membrane with PVDF Tree-Like Nanofiber Membrane as Composite Scaffold. Mater. Des. 2020, 196, 109101. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, X.-Y.; Li, W.-X.; Ma, X.-H.; Tang, C.Y.; Xu, Z.-L. Thin-Film Nanocomposite Membranes Containing Tannic Acid-Fe3+ Modified MoS2 Nanosheets with Enhanced Nanofiltration Performance. J. Membr. Sci. 2020, 616, 118605. [Google Scholar] [CrossRef]
- Yang, T.; Wan, C.F.; Zhang, J.; Gudipati, C.; Chung, T.-S. Optimization of Interfacial Polymerization to Fabricate Thin-Film Composite Hollow Fiber Membranes in Modules for Brackish Water Reverse Osmosis. J. Membr. Sci. 2021, 626, 119187. [Google Scholar] [CrossRef]
- Efa, M.T.; Imae, T. Hybridization of Carbon-Dots with ZnO Nanoparticles of Different Sizes. J. Taiwan Inst. Chem. Eng. 2018, 92, 112–117. [Google Scholar] [CrossRef]
- Heidari, A.A.; Mahdavi, H. Polyethylene Coated with Mno2 Nanoparticles as Thin Film Composite Membranes for Organic Solvent Nanofiltration. ACS Appl. Nano. Mater. 2021, 4, 2768–2782. [Google Scholar] [CrossRef]
- Zhao, B.; Guo, Z.; Wang, H.; Wang, L.; Qian, Y.; Long, X.; Ma, C.; Zhang, Z.; Li, J.; Zhang, H. Enhanced Water Permeance of a Polyamide Thin-Film Composite Nanofiltration Membrane with a Metal-Organic Framework Interlayer. J. Membr. Sci. 2021, 625, 119154. [Google Scholar] [CrossRef]
- El-Sayed, M.E. Nanoadsorbents for Water and Wastewater Remediation. Sci. Total Environ. 2020, 739, 139903. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Aktij, S.A.; Dabaghian, Z.; Firouzjaei, M.D.; Rahimpour, A.; Eke, J.; Escobar, I.C.; Abolhassani, M.; Greenlee, L.F.; Esfahani, A.R. Nanocomposite Membranes for Water Separation and Purification: Fabrication, Modification, and Applications. Sep. Purif. Technol. 2019, 213, 465–499. [Google Scholar] [CrossRef]
- Parhizkar, N.; Shahrabi, T.; Ramezanzadeh, B. Synthesis and Characterization of a Unique Isocyanate Silane Reduced Graphene Oxide Nanosheets; Screening the Role of Multifunctional Nanosheets on the Adhesion and Corrosion Protection Performance of an Amido-Amine Cured Epoxy Composite. J. Taiwan Inst. Chem. Eng. 2018, 82, 281–299. [Google Scholar] [CrossRef]
- Soni, R.; Pal, A.K.; Tripathi, P.; Lal, J.A.; Kesari, K.; Tripathi, V. An Overview of Nanoscale Materials on the Removal of Wastewater Contaminants. Appl. Water Sci. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, Y.; Xu, J.; Han, Y.; Xu, X. Preparation, Performances of PVDF/ZnO Hybrid Membranes and Their Applications in the Removal of Copper Ions. Appl. Surf. Sci. 2014, 316, 333–340. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, F.; Xue, J.; Chen, S.; Wang, J.; Yang, Y. Enhanced Removal of Heavy Metal Ions from Aqueous Solution Using Manganese Dioxide-Loaded Biochar: Behavior and Mechanism. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mustapha, S.; Tijani, J.; Ndamitso, M.; Abdulkareem, S.; Shuaib, D.; Mohammed, A.; Sumaila, A. The Role of Kaolin and Kaolin/Zno Nanoadsorbents in Adsorption Studies for Tannery Wastewater Treatment. Sci. Rep. 2020, 10, 1–22. [Google Scholar] [CrossRef]
- Xu, G.-R.; Wang, J.-N.; Li, C.-J. Strategies for Improving the Performance of the Polyamide Thin Film Composite (PA–TFC) Reverse Osmosis (RO) Membranes: Surface Modifications and Nanoparticles Incorporations. Desalination 2013, 328, 83–100. [Google Scholar] [CrossRef]
- Al-Hobaib, A.; El Ghoul, J.; Ghiloufi, I.; El Mir, L. Synthesis and Characterization of Polyamide Thin-Film Nanocomposite Membrane Reached by Aluminum Doped ZnO Nanoparticles. Mater. Sci. Semicond. Process 2016, 42, 111–114. [Google Scholar] [CrossRef]
- Himstedt, H.H.; Sengupta, A.; Qian, X.; Wickramasinghe, S.R. Magnetically Responsive Nano Filtration Membranes for Treatment of Coal Bed Methane Produced Water. J. Taiwan Inst. Chem. Eng. 2019, 94, 97–108. [Google Scholar] [CrossRef]
- Kotlhao, K.; Lawal, I.A.; Moutloali, R.M.; Klink, M.J. Antifouling Properties of Silver-Zinc Oxide Polyamide Thin Film Composite Membrane and Rejection of 2-Chlorophenol and 2, 4-Dichlorophenol. Membranes 2019, 9, 96. [Google Scholar] [CrossRef] [Green Version]
- Namvar-Mahboub, M.; Pakizeh, M. Development of a Novel Thin Film Composite Membrane by Interfacial Polymerization on Polyetherimide/Modified SiO2 Support for Organic Solvent Nanofiltration. Sep. Purif. Technol. 2013, 119, 35–45. [Google Scholar] [CrossRef]
- Tavares, C.J.; Marques, S.; Rebouta, L.; Lanceros-Méndez, S.; Sencadas, V.; Costa, C.M.; Alves, E.; Fernandes, A. PVDF-Grown Photocatalytic TiO2 Thin Films on PVDF Substrates for Sensors and Actuators Applications. Thin Solid Films 2008, 517, 1161–1166. [Google Scholar] [CrossRef]
- Khulbe, K.; Feng, C.; Matsuura, T. The Art of Surface Modification of Synthetic Polymeric Membranes. J. Appl. Polym. Sci. 2010, 115, 855–895. [Google Scholar] [CrossRef]
- Lavrador, P.; Esteves, M.R.; Gaspar, V.M.; Mano, J.F. Stimuli-Responsive Nanocomposite Hydrogels for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2005941. [Google Scholar] [CrossRef]
- Li, J.; Zeng, J.; Jia, X.; Liu, L.; Zhou, T.; Liu, P. pH, Temperature and Reduction Multi-Responsive Polymeric Microspheres as Drug Delivery System for Anti-Tumor Drug: Effect of Middle Hollow Layer between pH and Reduction Dual-Responsive Cores and Temperature Sensitive Shells. J. Taiwan Inst. Chem. Eng. 2017, 74, 238–245. [Google Scholar] [CrossRef]
- Reinhardt, M.; Dzubiella, J.; Trapp, M.; Gutfreund, P.; Kreuzer, M.; Gröschel, A.H.; Müller, A.H.; Ballauff, M.; Steitz, R. Fine-Tuning the Structure of Stimuli-Responsive Polymer Films by Hydrostatic Pressure and Temperature. Macromolecules 2013, 46, 6541–6547. [Google Scholar] [CrossRef]
- Shao, W.; Liu, H.; Wu, J.; Wang, S.; Liu, X.; Huang, M.; Xu, P. Preparation, Antibacterial Activity and pH-Responsive Release Behavior of Silver Sulfadiazine Loaded Bacterial Cellulose for Wound Dressing Applications. J. Taiwan Inst. Chem. Eng. 2016, 63, 404–410. [Google Scholar] [CrossRef]
- Dolatkhah, A.; Wilson, L.D. Magnetite/Polymer Brush Nanocomposites with Switchable Uptake Behavior toward Methylene Blue. ACS Appl. Mater. Interfaces 2016, 8, 5595–5607. [Google Scholar] [CrossRef]
- Wandera, D.; Wickramasinghe, S.R.; Husson, S.M. Stimuli-Responsive Membranes. J. Membr. Sci. 2010, 357, 6–35. [Google Scholar] [CrossRef]
- Mahdavi, S.; Jalali, M.; Afkhami, A. Removal of Heavy Metals from Aqueous Solutions Using Fe3o4, ZnO, and CuO Nanoparticles. Nanotechnol. Sustain. Dev. 2012, 14, 177–178. [Google Scholar] [CrossRef]
- Elsherbiny, I.M.; Khalil, A.S.; Ulbricht, M. Tailoring Surface Characteristics of Polyamide Thin-Film Composite Membranes toward Pronounced Switchable Wettability. Adv. Mater. Interfaces 2019, 6, 1801408. [Google Scholar] [CrossRef]
- Franklin, D.; Guhanathan, S. Investigation of Citric Acid–Glycerol Based pH-Sensitive Biopolymeric Hydrogels for Dye Removal Applications: A Green Approach. Ecotoxicol. Environ. Saf. 2015, 121, 80–86. [Google Scholar] [CrossRef]
- Mondal, S.; Wickramasinghe, S. Photo-Induced Graft Polymerization of N-Isopropyl Acrylamide on Thin Film Composite Membrane: Produced Water Treatment and Antifouling Properties. Sep. Purif. Technol. 2012, 90, 231–238. [Google Scholar] [CrossRef]
- Yu, S.; Lü, Z.; Chen, Z.; Liu, X.; Liu, M.; Gao, C. Surface Modification of Thin-Film Composite Polyamide Reverse Osmosis Membranes by Coating N-Isopropylacrylamide-Co-Acrylic Acid Copolymers for Improved Membrane Properties. J. Membr. Sci. 2011, 371, 293–306. [Google Scholar] [CrossRef]
- Xie, W.; Geise, G.M.; Freeman, B.D.; Lee, H.-S.; Byun, G.; Mcgrath, J.E. Polyamide Interfacial Composite Membranes Prepared from M-Phenylene Diamine, Trimesoyl Chloride and a New Disulfonated Diamine. J. Membr. Sci. 2012, 403, 152–161. [Google Scholar] [CrossRef]
- Miron, S.M.; Dutournié, P.; Ponche, A. Filtration of Uncharged Solutes: An Assessment of Steric Effect by Transport and Adsorption Modelling. Water 2019, 11, 2173. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.Y.; Kwon, Y.-N.; Leckie, J.O. Effect of Membrane Chemistry and Coating Layer on Physiochemical Properties of Thin Film Composite Polyamide RO and NF Membranes: I. FTIR and XPS Characterization of Polyamide and Coating Layer Chemistry. Desalination 2009, 242, 149–167. [Google Scholar] [CrossRef]
- Sinha, M.; Purkait, M. Preparation and Characterization of Stimuli-Responsive Hydrophilic Polysulfone Membrane Modified with Poly (N-Vinylcaprolactam-Co-Acrylic Acid). Desalination 2014, 348, 16–25. [Google Scholar] [CrossRef]
- Bui, N.-N.; Lind, M.L.; Hoek, E.M.; Mccutcheon, J.R. Electrospun Nanofiber Supported Thin Film Composite Membranes for Engineered Osmosis. J. Membr. Sci. 2011, 385, 10–19. [Google Scholar] [CrossRef]
- Kwon, Y.-N.; Leckie, J.O. Hypochlorite Degradation of Crosslinked Polyamide Membranes: II. Changes in Hydrogen Bonding Behavior and Performance. J. Membr. Sci. 2006, 282, 456–464. [Google Scholar] [CrossRef]
- Akin, O.; Temelli, F. Probing the Hydrophobicity of Commercial Reverse Osmosis Membranes Produced by Interfacial Polymerization Using Contact Angle, XPS, FTIR, FE-SEM AND AFM. Desalination 2011, 278, 387–396. [Google Scholar] [CrossRef]
- Alsvik, I.L.; Hägg, M.-B. Preparation of Thin Film Composite Membranes with Polyamide Film on Hydrophilic Supports. J. Membr. Sci. 2013, 428, 225–231. [Google Scholar] [CrossRef]
- Son, S.H.; Jegal, J. Preparation and Characterization of Polyamide Reverse-Osmosis Membranes with Good Chlorine Tolerance. J. Appl. Polym. Sci. 2011, 120, 1245–1252. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, F.; Chung, T.-S. Substrate Modifications and Alcohol Treatment on Thin Film Composite Membranes for Osmotic Power. Chem. Eng. Sci. 2013, 87, 40–50. [Google Scholar] [CrossRef]
- Sagle, A.C.; Van Wagner, E.M.; Ju, H.; Mccloskey, B.D.; Freeman, B.D.; Sharma, M.M. Peg-Coated Reverse Osmosis Membranes: Desalination Properties and Fouling Resistance. J. Membr. Sci. 2009, 340, 92–108. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface Modifications for Antifouling Membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef]
- Feng, C.; Khulbe, K.; Matsuura, T. Recent Progress in the Preparation, Characterization, and Applications of Nanofibers and Nanofiber Membranes via Electrospinning/Interfacial Polymerization. J. Appl. Polym. Sci. 2010, 115, 756–776. [Google Scholar] [CrossRef]
- Misdan, N.; Lau, W.; Ismail, A.; Matsuura, T. Formation of Thin Film Composite Nanofiltration Membrane: Effect of Polysulfone Substrate Characteristics. Desalination 2013, 329, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Mbareck, C.; Nguyen, Q.T.; Alaoui, O.T.; Barillier, D. Elaboration, Characterization and Application of Polysulfone and Polyacrylic Acid Blends as Ultrafiltration Membranes for Removal of Some Heavy Metals from Water. J. Hazard Mater. 2009, 171, 93–101. [Google Scholar] [CrossRef]
- Hu, S.Y.; Zhang, Y.; Lawless, D.; Feng, X. Composite Membranes Comprising of Polyvinylamine-Poly (Vinyl Alcohol) Incorporated with Carbon Nanotubes for Dehydration of Ethylene Glycol by Pervaporation. J. Membr. Sci. 2012, 417, 34–44. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Wu, R.-J.; Cheng, H.-Y.; Li, S.-C.; Siao, Y.-Y.; Kong, D.-C.; Jang, G.-W. Crystallinity and Dimensional Stability of Biaxial Oriented Poly (Lactic Acid) Films. Polym. Degrad. Stab. 2010, 95, 1292–1298. [Google Scholar] [CrossRef]
- Wagh, S.; Dhumal, S.; Suresh, A. An Experimental Study of Polyurea Membrane Formation by Interfacial Polycondensation. J. Membr. Sci. 2009, 328, 246–256. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, F.; Arshi, N.; Chaman, M.; Naqvi, A. Formation and Characterization of Zno Nanopowder Synthesized by Sol–Gel Method. J. Alloy. Compd. 2010, 496, 399–402. [Google Scholar] [CrossRef]
- Hong, R.; Li, J.; Chen, L.; Liu, D.; Li, H.Z.; Zheng, Y.; Ding, J. Synthesis, Surface Modification and Photocatalytic Property of Zno Nanoparticles. Powder Technol. 2009, 189, 426–432. [Google Scholar] [CrossRef]
- Amin, M.C.I.M.; Ahmad, N.; Halib, N.; Ahmad, I. Synthesis and Characterization of Thermo-and pH-Responsive Bacterial Cellulose/Acrylic Acid Hydrogels for Drug Delivery. Carbohydr. Polym. 2012, 88, 465–473. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Mandre, N.; Panda, A.; Pal, S. Amylopectin Grafted with Poly (Acrylic Acid): Development and Application of a High-Performance Flocculant. Carbohydr. Polym. 2013, 95, 753–759. [Google Scholar] [CrossRef]
- ] Lin, C.-L.; Lee, C.-F.; Chiu, W.-Y. Preparation and Properties of Poly (Acrylic Acid) Oligomer Stabilized Superparamagnetic Ferrofluid. J. Colloid Interface Sci. 2005, 291, 411–420. [Google Scholar] [CrossRef]
- Wei, J.; Qiu, C.; Wang, Y.-N.; Wang, R.; Tang, C.Y. Comparison of NF-Like and RO-Like Thin Film Composite Osmotically-Driven Membranes—Implications for Membrane Selection and Process Optimization. J. Membr. Sci. 2013, 427, 460–471. [Google Scholar] [CrossRef]
- Himstedt, H.H.; Marshall, K.M.; Wickramasinghe, S.R. Ph-Responsive Nanofiltration Membranes by Surface Modification. J. Membr. Sci. 2011, 366, 373–381. [Google Scholar] [CrossRef]
- Dražević, E.; Košutić, K.; Freger, V. Permeability and Selectivity of Reverse Osmosis Membranes: Correlation to Swelling Revisited. Water Res. 2014, 49, 444–452. [Google Scholar] [CrossRef] [PubMed]
- González, M.; Saucedo, I.; Navarro, R.; Prádanos, P.; Palacio, L.; Martínez, F.; Martín, A.; Hernández, A. Effect of Phosphoric and Hydrofluoric Acid on the Structure and Permeation of a Nanofiltration Membrane. J. Membr. Sci. 2006, 281, 177–185. [Google Scholar] [CrossRef]
- Lejarazu-Larrañaga, A.; Ortiz, J.M.; Molina, S.; Zhao, Y.; García-Calvo, E. Nitrate-Selective Anion Exchange Membranes Prepared Using Discarded Reverse Osmosis Membranes as Support. Membranes 2020, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, Z.; Yuan, S.; Zhu, J.; Van Der Bruggen, B. High-Performance Thin-Film-Nanocomposite Cation Exchange Membranes Containing Hydrophobic Zeolitic Imidazolate Framework for Monovalent Selectivity. Appl. Sci. 2018, 8, 759. [Google Scholar] [CrossRef] [Green Version]



| MPDA (% m/v) | AA (% m/v) | ZnO (% m/v) |
|---|---|---|
| 2.00 | 0.00 | 0.00 |
| 1.00 | 1.00 | 0.50 |
| 1.00 | 1.00 | 1.00 |
| 1.00 | 1.00 | 1.50 |
| 0.50 | 1.50 | 0.50 |
| 0.50 | 1.50 | 1.00 |
| 0.50 | 1.50 | 1.50 |
| Membrane Type | Rq (nm) | Ra (nm) | Sy (nm) | Hd (nm) | Pn |
|---|---|---|---|---|---|
| Unmodified Membranes | 218.94 | 183.11 | 26.00 | 712.89 | 141 |
| 1.00% AA | 11.85 | 77.63 | 17.59 | 693.04 | 169 |
| 0.50:1.00% ZnO/AA | 354.25 | 218.80 | 38.28 | 850.92 | 170 |
| 1.00:1.00% ZnOAA | 77.59 | 56.98 | 4.85 | 355.85 | 139 |
| 1.50:1.00% ZnO/AA | 84.93 | 60.19 | 5.31 | 151.10 | 157 |
| 1.50% AA | 62.85 | 50.13 | 4.02 | 250.83 | 150 |
| 0.50:1.50% ZnO/AA | 111.85 | 208.80 | 2.18 | 727.14 | 163 |
| 1.00:1.50% ZnO/AA | 193.40 | 146.56 | 30.03 | 151.10 | 152 |
| 1.50:1.50% ZnO/AA | 71.72 | 56.23 | 7.43 | 286.11 | 156 |
| Names | Initial Stage | Degradation Stage | Total wt. Loss (%) | ||||
|---|---|---|---|---|---|---|---|
| ZnO/AA Concentration (%) | Phase I | Phase II | Phase III | ||||
| Initial Temp. (°C) | %wt. Loss | 1st Deg Temp (°C) | %wt. Loss | 2nd Deg Temp (°C) | %wt. Loss | ||
| 0.00 | 223–382 | 1.84 | 505–382 | 82.20 | 494–580 | 7.51 | 91.55 |
| 1.00 AA | 224–381 | 2.25 | 381–511 | 78.89 | 516–642 | 6.83 | 87.97 |
| 0.50:1.00 | 260–400 | 4.26 | 400–510 | 72.20 | 520–612 | 8.06 | 84.52 |
| 1.00:1.00 | 244–387 | 2.94 | 387–518 | 74.03 | 522–619 | 15.48 | 92.45 |
| 1.50:1.00 | 246–392 | 4.00 | 392–516 | 71.10 | 522–623 | 8.06 | 83.16 |
| 1.50 AA | 225–382 | 2.12 | 382–515 | 76.61 | 515–624 | 8.07 | 86.8 |
| 0.50:1.50 | 234–357 | 2.05 | 357–512 | 74.42 | 516–631 | 10.14 | 86.61 |
| 1.00:1.50 | 220–385 | 2.02 | 385–518 | 74.30 | 520–625 | 6.84 | 83.16 |
| 1.50:1.50 | 248–379 | 0.70 | 379–525 | 75.28 | 520–613 | 5.58 | 81.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malatjie, K.I.; Mbuli, B.S.; Moutloali, R.M.; Ngila, C.J. An In Situ Incorporation of Acrylic Acid and ZnO Nanoparticles into Polyamide Thin Film Composite Membranes for Their Effect on Membrane pH Responsive Behavior. Membranes 2021, 11, 910. https://doi.org/10.3390/membranes11120910
Malatjie KI, Mbuli BS, Moutloali RM, Ngila CJ. An In Situ Incorporation of Acrylic Acid and ZnO Nanoparticles into Polyamide Thin Film Composite Membranes for Their Effect on Membrane pH Responsive Behavior. Membranes. 2021; 11(12):910. https://doi.org/10.3390/membranes11120910
Chicago/Turabian StyleMalatjie, Kgolofelo I., Bhekani S. Mbuli, Richard M. Moutloali, and Catherine J. Ngila. 2021. "An In Situ Incorporation of Acrylic Acid and ZnO Nanoparticles into Polyamide Thin Film Composite Membranes for Their Effect on Membrane pH Responsive Behavior" Membranes 11, no. 12: 910. https://doi.org/10.3390/membranes11120910
APA StyleMalatjie, K. I., Mbuli, B. S., Moutloali, R. M., & Ngila, C. J. (2021). An In Situ Incorporation of Acrylic Acid and ZnO Nanoparticles into Polyamide Thin Film Composite Membranes for Their Effect on Membrane pH Responsive Behavior. Membranes, 11(12), 910. https://doi.org/10.3390/membranes11120910







