Assessment of an Anaerobic Membrane Bioreactor (AnMBR) Treating Medium-Strength Synthetic Wastewater under Cyclical Membrane Operation
Abstract
1. Introduction
2. Materials and Methods
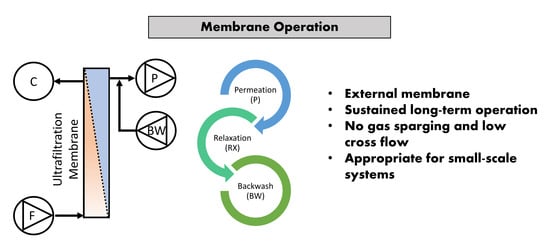
2.1. Experimental Setup and Inoculum
- Qe: daily effluent production, throughput, L/d
- Jnet: net flux, L.m2/h
- Jins: instantaneous set flux, L.m2/h
- Am: total membrane area, m2
- VBW: daily backwash volume, L
- RBW: backwash ratio, %
- tPT: total production cycle duration in a day, h
- tCF: time concentration factor
- tC: one cycle duration, min
- tP: production duration, 30 min
- tRX: relaxation duration, 1 min
- tBW: backwash duration, 15 s
- nC: total cycles per day
2.2. Synthetic Sewage
2.3. COD Mass Balance
2.4. Monitoring Parameters and Analytical Methods
3. Results and Discussion
3.1. Membrane Performance
| References | System Type, Size, and HRT | Influent Characteristics, mg/L | Membrane Characteristics | Initial Flux, LMH | Fouling Rate, kPa/h | Flux Reduction | Fouling Mitigation Methods |
|---|---|---|---|---|---|---|---|
| This study | Anaerobic; 6.2 L; 0.8 days | Synthetic; TSS: 430; COD: 501; TN:28.6; TP:13.6 | 0.03 µm; 0.075 m2; external tubular PVDF | 4.5 | 0.007 | - | CFV: 0.1 m/s; frequent BW; frequent RX |
| Prieto et al., 2013 [10] | Anaerobic; 10 L; 3 days | Synthetic; TSS: 520; TOC: 504; COD: 1260; TN: 54; TP:44 | 0.03 µm; 0.013 m2; external tubular PVDF | 18 | - | 18 LMH to 10–15 LMH in 100 days | gas-lift; CFV: 0.5 m/s; weekly PH and CH cleaning |
| Dolejs et al., 2017 [26] | Anaerobic; 10 L; 30–36 h | Synthetic; TSS: 400; COD: 1000 | 0.03 µm; 0.066 m2; external tubular PVDF | 4.5 | - | 4.5 LMH, no reduction | gas-lift; CFV: 0.1 m/s; frequent BW; CH cleaning on day 19, 42, 89 |
| Pollice et al., 2005 [30] | Aerobic; 20 L; 3.3 h | Domestic and synthetic; COD: 850 | 0.03 µm; 0.5 m2; submerged hollow fibers | 12 | 0.02 | - | air scrubbing; frequent BW and RX; frequent CC |
| Pollice et al., 2005 [30] | UASB; NS *; NS | NS; COD: 10213 | 0.22 µm; external chamber submerged flat-sheet PVDF | 30 | 12 | - | air scrubbing; cleaning but NS |
| Martin-Garcia et al., 2011 [31] | Flocculated Anaerobic; 38 L; 16 h | Domestic; SS: 84; COD: 338; BOD5: 167; ammonia: 35 | 0.03 µm; external tubular PVDF | 11–12 | 24–150 | - | gas-lift; SGD: 0.2–1.2 m3/h |
| Martin-Garcia et al., 2011 [31] | Granulated Anaerobic; 38 L; 16 h | Domestic; SS: 84; COD: 338; BOD5: 167; ammonia: 35 | 0.03 µm; external tubular PVDF | 11–12 | 6–12 | - | gas-lift; SGD: 0.2–1.2 m3/h |
| Martinez et al., 2020 [33] | Anaerobic; 20 L; NS | NS | 0.04 µm; 0.93 m2; submerged hollowfiber PVDF | 15, 20, 25 | 0.009 | - | gas-lift; SGD: 1–1.2 m3/h |
| Martinez et al., 2020 [33] | Anaerobic; 20 L; NS | NS | 0.04 µm; 0.93 m2; external tubular PVDF | 15 | 0.047 | - | CFV: 0.51 m/s; SGD: 0.3–0.4 m3/h |
| Oh et al., 2012 [36] | Aerobic; 1.2 L; 12 h | Synthetic; glucose, 400; yeast extract, 14; bactopeptone, 115; (NH4)2SO4, 104.8; KH2PO4, | NS; 86 cm2; submerged hollowfiber | 18 | 0.047 | - | air scrubbing; quorum quenching with recombinant E. coli |
| Jiang et al., 2013 [37] | Aerobic; 3 L; 4.8 h | 500 glucose; 2500 yeast extract; 25 bactopeptone; 250 (NH4)2SO4; 150 K2H2PO4; 150 KH2PO4 | 0.01 µm; 0.07 m2; submerged hollow fibers | 12 | 0.075 | - | air scrubbing; frequent RX; quorum quenching with porcine kidney acylase 1 enzyme |
| Xu et al., 2020 [38] | Anaerobic; 4.5 L; NS | Domestic | NS; NS; external chamber submerged | 7.2 | 0.074 | - | quorum quenching with Acyl-homoserine lactone enzymes |
| Verhuelsdonk et al., 2021 [45] | Aerobic; NS; NS | Brewery WW; COD: 512; BOD5: 124; NH4-N: 45; PO4-P: 9.6 | 0.038 µm; 108 m2; submerged rotating PES | 9.5–11.5 | 0.003 | - | air scrubbing; frequent RX; frequent BW |
3.2. Treatment Performance
3.3. Nutrients and Solids
3.4. Biogas
3.5. NSSS Comparison
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- ISO. ISO 30500:2018—Non-Sewered Sanitation Systems—Prefabricated Integrated Treatment Units—General Safety and Performance Requirements for Design and Testing; International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- McCarty, P.L.; Bae, J.; Kim, J. Domestic wastewater treatment as a net energy producer—Can this be achieved? Environ. Sci. Technol. 2011, 45, 7100–7106. [Google Scholar] [CrossRef]
- Gander, M.A.; Jefferson, B.; Judd, S.J. Membrane bioreactors for use in small wastewater treatment plants: Membrane materials and effluent quality. Water Sci. Technol. 2000, 41, 205–211. [Google Scholar] [CrossRef]
- Judd, S.; Judd, C. The MBR Book: Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2011; ISBN 9780080966823. [Google Scholar]
- Liao, B.-Q.; Kraemer, J.T.; Bagley, D.M. Anaerobic Membrane Bioreactors: Applications and Research Directions. Crit. Rev. Environ. Sci. Technol. 2006, 36, 489–530. [Google Scholar] [CrossRef]
- Gao, D.W.; Zhang, T.; Tang, C.Y.Y.; Wu, W.M.; Wong, C.Y.; Lee, Y.H.; Yeh, D.H.; Criddle, C.S. Membrane fouling in an anaerobic membrane bioreactor: Differences in relative abundance of bacterial species in the membrane foulant layer and in suspension. J. Memb. Sci. 2010, 364, 331–338. [Google Scholar] [CrossRef]
- Uman, A.E.; Usack, J.G.; Lozano, J.L.; Angenent, L.T. Controlled experiment contradicts the apparent benefits of the Fenton reaction during anaerobic digestion at a municipal wastewater treatment plant. Water Sci. Technol. 2018, 78, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Chen, J.; Wang, F.; Ding, L.; Hong, H. Feasibility evaluation of submerged anaerobic membrane bioreactor for municipal secondary wastewater treatment. Desalination 2011, 280, 120–126. [Google Scholar] [CrossRef]
- Pretel, R.; Robles, A.; Ruano, M.V.; Seco, A.; Ferrer, J. Economic and environmental sustainability of submerged anaerobic MBR-based (AnMBR-based) technology as compared to aerobic-based technologies for moderate-/high-loaded urban wastewater treatment. J. Environ. Manag. 2016, 166, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Prieto, A.L.; Futselaar, H.; Lens, P.N.L.; Bair, R.; Yeh, D.H. Development and start up of a gas-lift anaerobic membrane bioreactor (Gl-AnMBR) for conversion of sewage to energy, water and nutrients. J. Memb. Sci. 2013, 441, 158–167. [Google Scholar] [CrossRef]
- Calabria, J.L. Wastewater Nutrient Recovery Using Anaerobic Membrane Bioreactor (AnMBR) Permeate for Hydroponic Fertigation; University of South Florida: Tampa, FL, USA, 2014. [Google Scholar]
- Song, K.G.; Kim, Y.; Ahn, K.H. Effect of coagulant addition on membrane fouling and nutrient removal in a submerged membrane bioreactor. Desalination 2008, 221, 467–474. [Google Scholar] [CrossRef]
- Gong, H.; Jin, Z.; Xu, H.; Yuan, Q.; Zuo, J.; Wu, J.; Wang, K. Enhanced membrane-based pre-concentration improves wastewater organic matter recovery: Pilot-scale performance and membrane fouling. J. Clean. Prod. 2019, 206, 307–314. [Google Scholar] [CrossRef]
- Wu, J.; Chen, F.; Huang, X.; Geng, W.; Wen, X. Using inorganic coagulants to control membrane fouling in a submerged membrane bioreactor. Desalination 2006, 197, 124–136. [Google Scholar] [CrossRef]
- Maaz, M.; Yasin, M.; Aslam, M.; Kumar, G.; Atabani, A.E.; Idrees, M.; Anjum, F.; Jamil, F.; Ahmad, R.; Khan, A.L.; et al. Anaerobic membrane bioreactors for wastewater treatment: Novel configurations, fouling control and energy considerations. Bioresour. Technol. 2019, 283, 358–372. [Google Scholar] [CrossRef]
- Wu, B.; Kim, J. Anaerobic Membrane Bioreactors for Nonpotable Water Reuse and Energy Recovery. J. Environ. Eng. 2020, 146, 03119002. [Google Scholar] [CrossRef]
- Martin, I.; Pidou, M.; Soares, A.; Judd, S.; Jefferson, B. Modelling the energy demands of aerobic and anaerobic membrane bioreactors for wastewater treatment. Environ. Technol. 2011, 32, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Field, R.W.; Wu, D.; Howell, J.A.; Gupta, B.B. Critical flux concept for microfiltration fouling. J. Memb. Sci. 1995, 100, 259–272. [Google Scholar] [CrossRef]
- Prieto, A.L.; Criddle, C.S.; Yeh, D.H. Complex organic particulate artificial sewage (COPAS) as surrogate wastewater in anaerobic assays. Environ. Sci. Water Res. Technol. 2019, 5, 1661–1671. [Google Scholar] [CrossRef]
- Raunkjær, K.; Hvitved-Jacobsen, T.; Nielsen, P.H. Measurement of pools of protein, carbohydrate and lipid in domestic wastewater. Water Res. 1994, 28, 251–262. [Google Scholar] [CrossRef]
- Sötemann, S.W.; van Rensburg, P.; Ristow, N.E.; Wentzel, M.C.; Loewenthal, R.E.; Ekama, G.A. Integrated chemical/physical and biological processes modelling Part 2—Anaerobic digestion of sewage sludges. Water SA 2006, 31, 545–568. [Google Scholar] [CrossRef][Green Version]
- Rittmann, B.E.; McCarty, P.L. Environmental Biotechnology: Principles and Applications; Current Opinion in Biotechnology; McGraw Hill: New York City, NY, USA, 2001. [Google Scholar]
- Tchobanoglous, G.; Stensel, H.D.; Ryujiro Tsuchihashi, F.B. Wastewater Engineering, Treatment and Resource Recovery, 5th ed.; Metcalf & Eddy: Boston, MA, USA, 2014. [Google Scholar]
- Henze, M.; Harremoes, P.; la Jansen, J.C.; Arvin, E. Wastewater Treatment: Biological and Chemical Processes; Springer: New York City, NY, USA, 1996; Volume 33. [Google Scholar]
- Eaton, A.D.; Baird, R.B.; Rice, E.W. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Dolejs, P.; Ozcan, O.; Bair, R.; Ariunbaatar, J.; Bartacek, J.; Lens, P.N.L.; Yeh, D.H. Effect of psychrophilic temperature shocks on a gas-lift anaerobic membrane bioreactor (Gl-AnMBR) treating synthetic domestic wastewater. J. Water Process. Eng. 2017, 16, 108–114. [Google Scholar] [CrossRef]
- Rabuni, M.F.; Nik Sulaiman, N.M.; Aroua, M.K.; Yern Chee, C.; Awanis Hashim, N. Impact of in situ physical and chemical cleaning on PVDF membrane properties and performances. Chem. Eng. Sci. 2015, 122, 426–435. [Google Scholar] [CrossRef]
- Zsirai, T.; Buzatu, P.; Aerts, P.; Judd, S. Efficacy of relaxation, backflushing, chemical cleaning and clogging removal for an immersed hollow fibre membrane bioreactor. Water Res. 2012, 46, 4499–4507. [Google Scholar] [CrossRef] [PubMed]
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Memb. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Pollice, A.; Brookes, A.; Jefferson, B.; Judd, S. Sub-critical flux fouling in membrane bioreactors—A review of recent literature. Desalination 2005, 174, 221–230. [Google Scholar] [CrossRef]
- Martin-Garcia, I.; Monsalvo, V.; Pidou, M.; Le-Clech, P.; Judd, S.J.; McAdam, E.J.; Jefferson, B. Impact of membrane configuration on fouling in anaerobic membrane bioreactors. J. Membr. Sci. 2011, 382, 41–49. [Google Scholar] [CrossRef]
- Navaratna, D.; Jegatheesan, V. Implications of short and long term critical flux experiments for laboratory-scale MBR operations. Bioresour. Technol. 2011, 102, 5361–5369. [Google Scholar] [CrossRef]
- Martínez, R.; Ruiz, M.O.; Ramos, C.; Cámara, J.M.; Diez, V. Comparison of external and submerged membranes used in anaerobic membrane bioreactors: Fouling related issues and biological activity. Biochem. Eng. J. 2020, 159, 107558. [Google Scholar] [CrossRef]
- Shrout, J.D.; Nerenberg, R. Monitoring bacterial twitter: Does quorum sensing determine the behavior of water and wastewater treatment biofilms? Environ. Sci. Technol. 2012, 46, 1995–2005. [Google Scholar] [CrossRef]
- Liu, J.; Eng, C.Y.; Ho, J.S.; Chong, T.H.; Wang, L.; Zhang, P.; Zhou, Y. Quorum quenching in anaerobic membrane bioreactor for fouling control. Water Res. 2019, 156, 159–167. [Google Scholar] [CrossRef]
- Oh, H.S.; Yeon, K.M.; Yang, C.S.; Kim, S.R.; Lee, C.H.; Park, S.Y.; Han, J.Y.; Lee, J.K. Control of membrane biofouling in MBR for wastewater treatment by quorum quenching bacteria encapsulated in microporous membrane. Environ. Sci. Technol. 2012, 46, 4877–4884. [Google Scholar] [CrossRef]
- Jiang, W.; Xia, S.; Liang, J.; Zhang, Z.; Hermanowicz, S.W. Effect of quorum quenching on the reactor performance, biofouling and biomass characteristics in membrane bioreactors. Water Res. 2013, 47, 187–196. [Google Scholar] [CrossRef]
- Xu, B.; Albert Ng, T.C.; Huang, S.; Shi, X.; Ng, H.Y. Feasibility of isolated novel facultative quorum quenching consortiums for fouling control in an AnMBR. Water Res. 2020, 169, 115251. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.R.; Su, Y.C.; Huang, C.; Lee, H.C. Effect of sludge characteristics on membrane fouling in membrane bioreactors. J. Memb. Sci. 2010, 349, 287–294. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Ohandja, D.G.; Yang, F.; Wong, F.S.; Chua, H.C. Impact of filamentous bacteria on properties of activated sludge and membrane-fouling rate in a submerged MBR. Sep. Purif. Technol. 2008, 59, 238–243. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, H.; Yang, F.; Li, Y.; Xiao, J.; Zhang, X. Effect of filamentous bacteria on membrane fouling in submerged membrane bioreactor. J. Memb. Sci. 2006, 272, 161–168. [Google Scholar] [CrossRef]
- Hao, L.; Liss, S.N.; Liao, B.Q. Influence of COD: N ratio on sludge properties and their role in membrane fouling of a submerged membrane bioreactor. Water Res. 2016, 89, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Banti, D.C.; Karayannakidis, P.D.; Samaras, P.; Mitrakas, M.G. An innovative bioreactor set-up that reduces membrane fouling by adjusting the filamentous bacterial population. J. Memb. Sci. 2017, 542, 430–438. [Google Scholar] [CrossRef]
- Zhou, Z.; Tao, Y.; Zhang, S.; Xiao, Y.; Meng, F.; Stuckey, D.C. Size-dependent microbial diversity of sub-visible particles in a submerged anaerobic membrane bioreactor (SAnMBR): Implications for membrane fouling. Water Res. 2019, 159, 20–29. [Google Scholar] [CrossRef]
- Verhuelsdonk, M.; Glas, K.; Parlar, H. Long-Term Operation of a Pilot-Scale Membrane Bioreactor Treating Brewery Wastewater: Relaxation as a Method for Detection of Membrane Fouling. J. Environ. Eng. 2021, 147, 04021005. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Z.; Stuckey, D.C.; Meng, F. Micro-particles-A Neglected but Critical Cause of Different Membrane Fouling between Aerobic and Anaerobic Membrane Bioreactors. ACS Sustain. Chem. Eng. 2020, 8. [Google Scholar] [CrossRef]
- Fan, G.; Su, Z.; Lin, R.; Lin, X.; Xu, R.; Chen, W. Influence of membrane materials and operational modes on the performance of ultrafiltration modules for drinking water treatment. Int. J. Polym. Sci. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Jeon, S.; Rajabzadeh, S.; Okamura, R.; Ishigami, T.; Hasegawa, S.; Kato, N.; Matsuyama, H. The effect of membrane material and surface pore size on the fouling properties of submerged membranes. Water 2016, 8, 602. [Google Scholar] [CrossRef]
- Anjum, F.; Khan, I.M.; Kim, J.; Aslam, M.; Blandin, G.; Heran, M.; Lesage, G. Trends and progress in AnMBR for domestic wastewater treatment and their impacts on process efficiency and membrane fouling. Environ. Technol. Innov. 2021, 21, 101204. [Google Scholar] [CrossRef]
- Skouteris, G.; Hermosilla, D.; López, P.; Negro, C.; Blanco, Á. Anaerobic membrane bioreactors for wastewater treatment: A review. Chem. Eng. J. 2012, 198–199, 138–148. [Google Scholar] [CrossRef]
- Giménez, J.B.; Robles, A.; Carretero, L.; Durán, F.; Ruano, M.V.; Gatti, M.N.; Ribes, J.; Ferrer, J.; Seco, A. Experimental study of the anaerobic urban wastewater treatment in a submerged hollow-fibre membrane bioreactor at pilot scale. Bioresour. Technol. 2011, 102, 8799–8806. [Google Scholar] [CrossRef] [PubMed]
- Choo, K.H.; Lee, C.H. Membrane fouling mechanisms in the membrane-coupled anaerobic bioreactor. Water Res. 1996, 30, 1771–1780. [Google Scholar] [CrossRef]
- Ho, J.; Sung, S. Anaerobic Membrane Bioreactor Treatment of Synthetic Municipal Wastewater at Ambient Temperature. Water Environ. Res. 2009, 81, 922–928. [Google Scholar] [CrossRef]
- Robles, Á.; Durán, F.; Giménez, J.B.; Jiménez, E.; Ribes, J.; Serralta, J.; Seco, A.; Ferrer, J.; Rogalla, F. Anaerobic membrane bioreactors (AnMBR) treating urban wastewater in mild climates. Bioresour. Technol. 2020, 314, 123763. [Google Scholar] [CrossRef]
- Galib, M.; Elbeshbishy, E.; Reid, R.; Hussain, A.; Lee, H.S. Energy-positive food wastewater treatment using an anaerobic membrane bioreactor (AnMBR). J. Environ. Manag. 2016, 182, 477–485. [Google Scholar] [CrossRef]
- Crone, B.C.; Garland, J.L.; Sorial, G.A.; Vane, L.M. Significance of dissolved methane in effluents of anaerobically treated low strength wastewater and potential for recovery as an energy product: A review. Water Res. 2016, 104, 520–531. [Google Scholar] [CrossRef] [PubMed]





| AVG | n | |
|---|---|---|
| Temperature, C | 34.6 ± 1 | - |
| Trans membrane pressure (TMP), bar | 0.25 ± 0.11 | 4872 |
| Daily permeate production, mL | 7647 ± 1238 | 200 |
| Biogas production, L/day | 0.75 ± 0.44 | 150 |
| Net flux (Jnet), LMH | 4.3 ± 0.7 | - |
| Specific flux, LMH/bar | 20.9 ± 10.7 | - |
| pH | 6.72 ± 0.3 | 75 |
| Total solids, mg/L | 9742 ± 3876 | 27 |
| Volatile solids, mg/L | 6253 ± 2707 | 27 |
| Total suspended solids, mg/L | 8284 ± 2479 | 27 |
| Total volatile suspended solids, mg/L | 6165 ± 2385 | 27 |
| Total chemical oxygen demand removal efficiency, % | 85.8 ± 8.9 | 27 |
| Permeate Quality (first 50 days excluded) | ||
| Total chemical oxygen demand, mg/L | 63.9 ± 31.1 | 20 |
| Total organic carbon, mg/L | 11.3 ± 10.6 | 20 |
| Ammonia, mg/L | 18.8 ± 3.7 | 20 |
| Total phosphorous, mg/L | 6.9 ± 2.0 | 20 |
| Total nitrogen, mg/L | 22.7 ± 5.1 | 20 |
| Turbidity, NTU | 0.6 ± 0.2 | 20 |
| Parameters | Influent | Effluent | % Reduction | ISO 30500 (CAT A/B) ** |
|---|---|---|---|---|
| TSS | 198.4 ± 12.5 | ND *** | 100 | 10/30 |
| tCOD (mg/L) | 501 ± 43 | 63.9 ± 31.1 | 87.2 ± 6.2 | 50/150 |
| pH | 6.65 ± 0.2 | 6.72 ± 0.3 | - | 6–9 |
| TN (mg/L) | 28.6 ± 2.9 | 22.7 ± 5.1 | 22.6 ± 13.7 | 70% reduction |
| TP (mg/L) | 13.6 ± 3.6 | 6.9 ± 2.0 | 49.3 ± 14.7 | 80% reduction |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uman, A.E.; Bair, R.A.; Yeh, D.H. Assessment of an Anaerobic Membrane Bioreactor (AnMBR) Treating Medium-Strength Synthetic Wastewater under Cyclical Membrane Operation. Membranes 2021, 11, 415. https://doi.org/10.3390/membranes11060415
Uman AE, Bair RA, Yeh DH. Assessment of an Anaerobic Membrane Bioreactor (AnMBR) Treating Medium-Strength Synthetic Wastewater under Cyclical Membrane Operation. Membranes. 2021; 11(6):415. https://doi.org/10.3390/membranes11060415
Chicago/Turabian StyleUman, Ahmet E., Robert A. Bair, and Daniel H. Yeh. 2021. "Assessment of an Anaerobic Membrane Bioreactor (AnMBR) Treating Medium-Strength Synthetic Wastewater under Cyclical Membrane Operation" Membranes 11, no. 6: 415. https://doi.org/10.3390/membranes11060415
APA StyleUman, A. E., Bair, R. A., & Yeh, D. H. (2021). Assessment of an Anaerobic Membrane Bioreactor (AnMBR) Treating Medium-Strength Synthetic Wastewater under Cyclical Membrane Operation. Membranes, 11(6), 415. https://doi.org/10.3390/membranes11060415