Separation of H2O/CO2 Mixtures by MFI Membranes: Experiment and Monte Carlo Study
Abstract
:1. Introduction
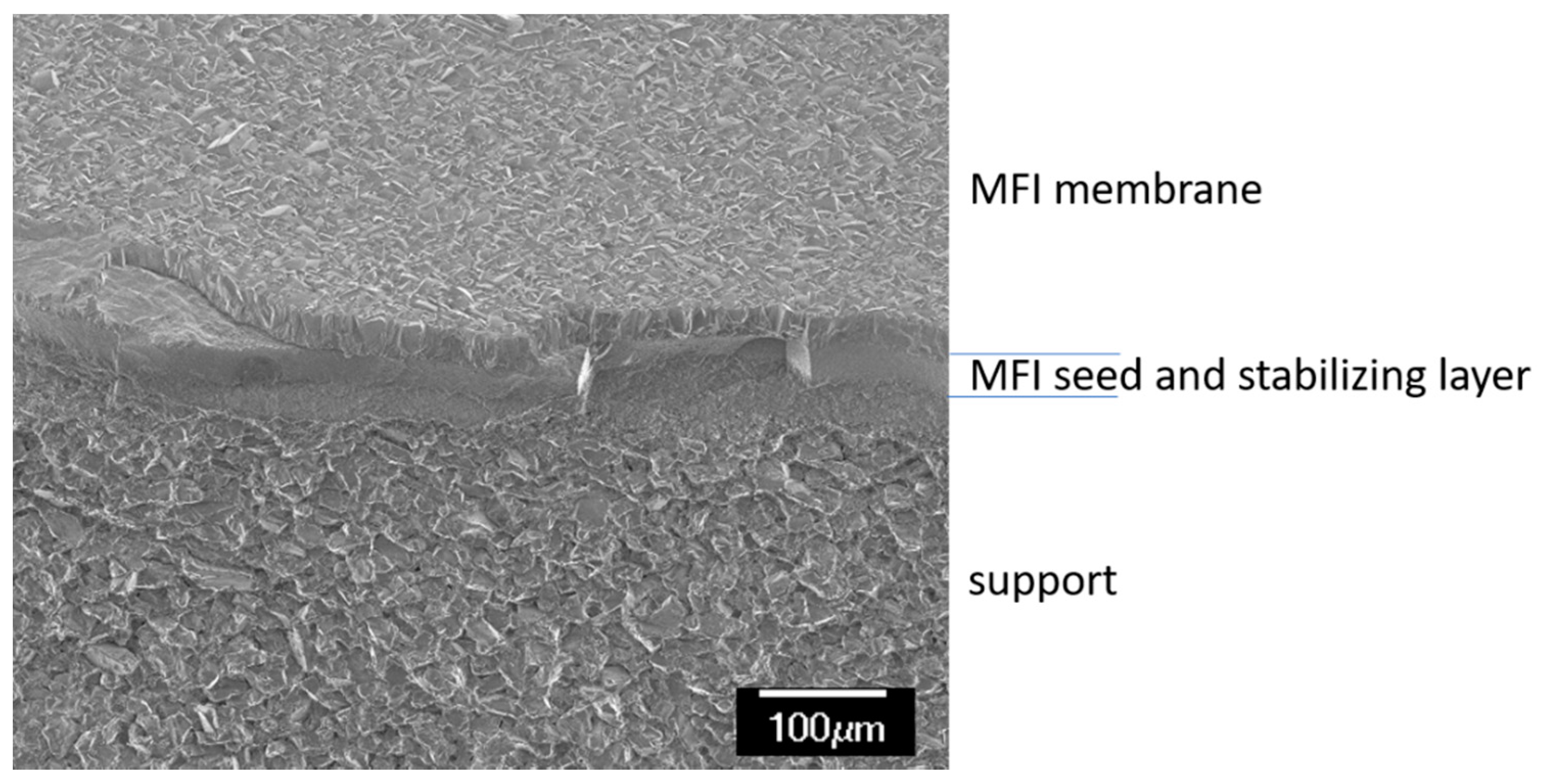
2. Materials and Methods
3. Results
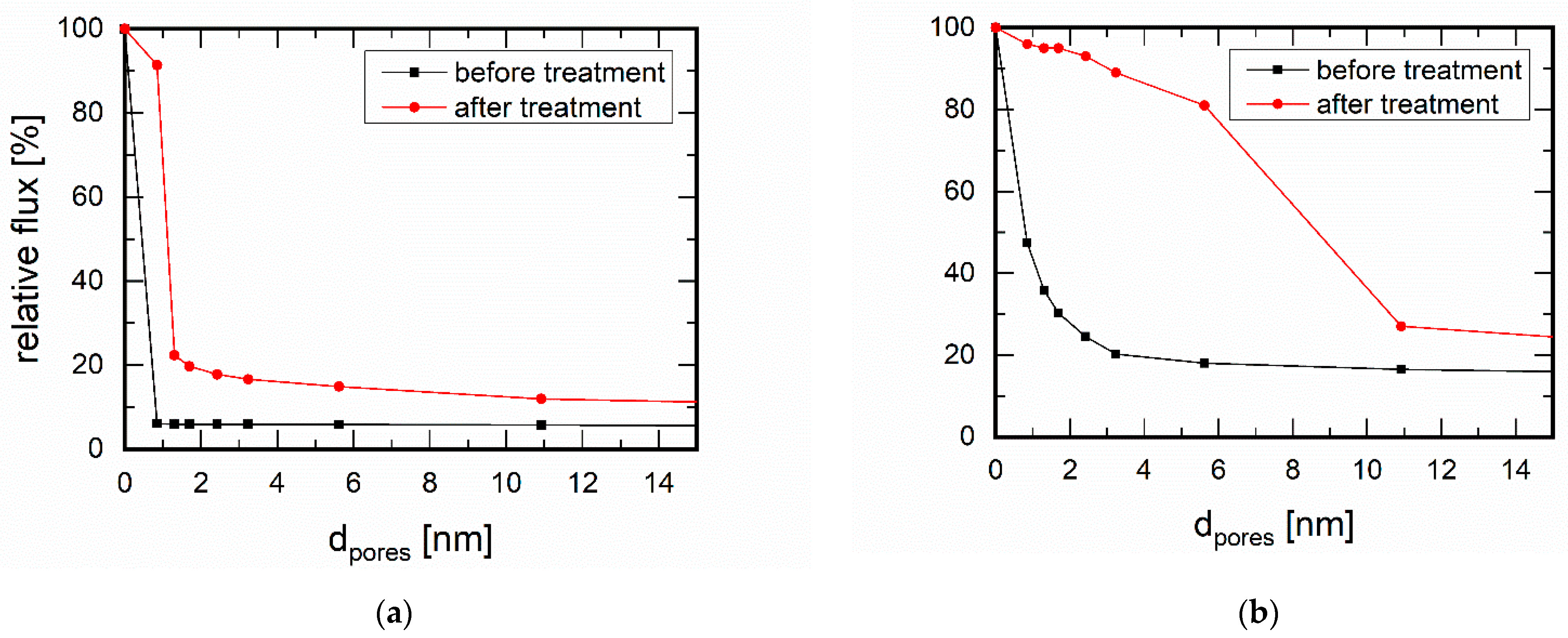
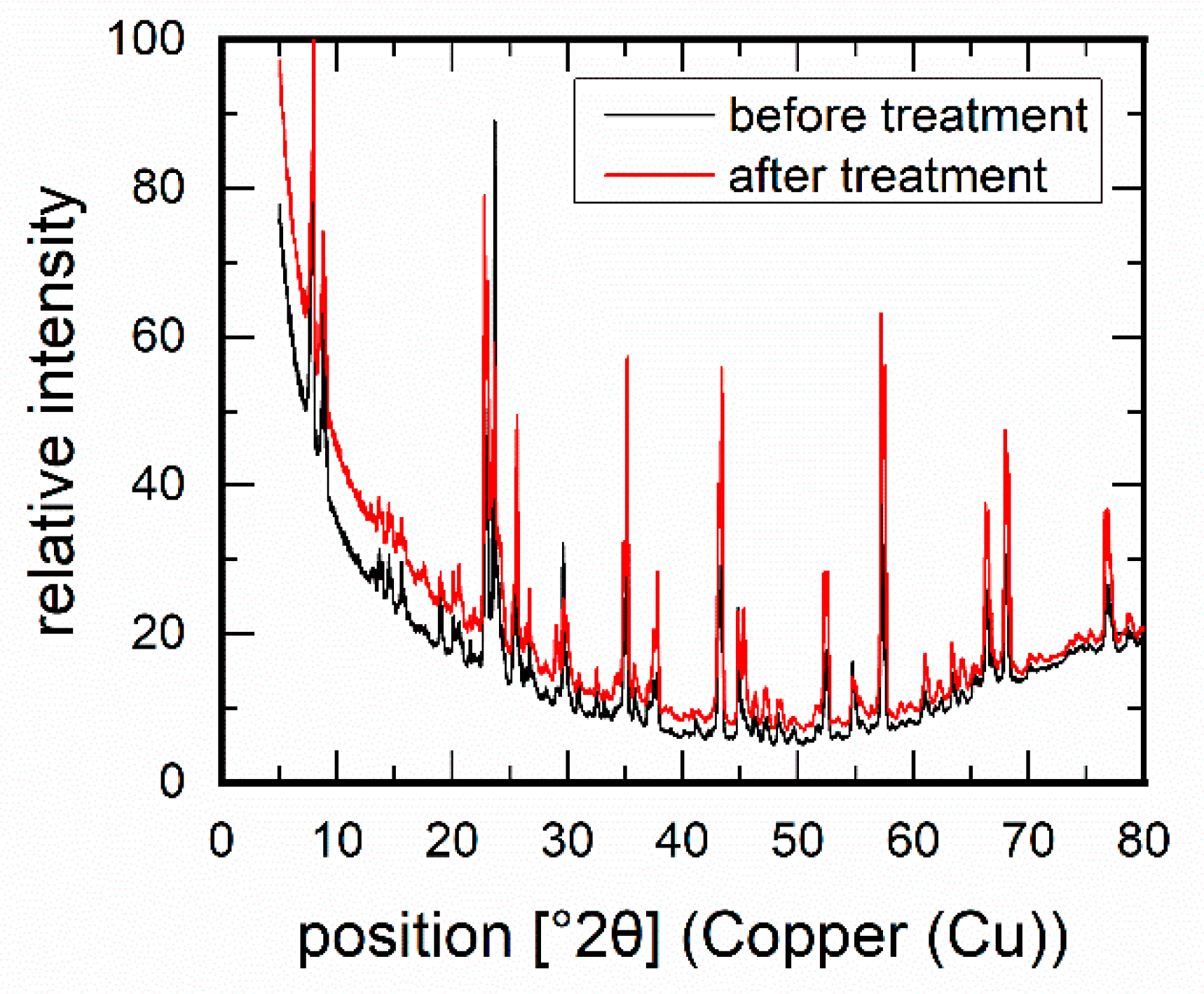
3.1. Membrane Stability in the Presence of Amines
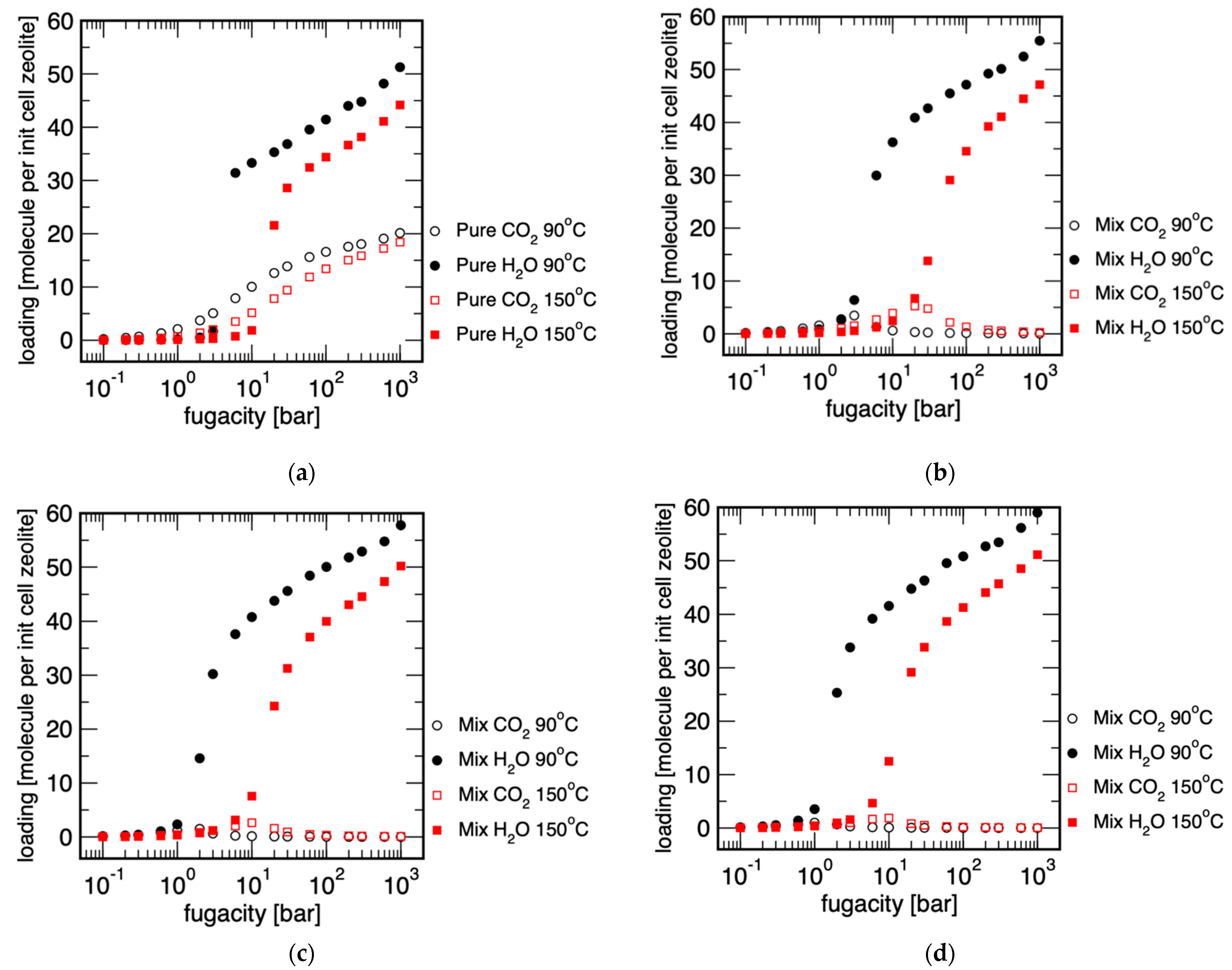
3.2. Determination of Possible Separation Conditions via Monte Carlo Simulation
3.3. Separation of CO2/H2O Gas Streams at MFI Membranes
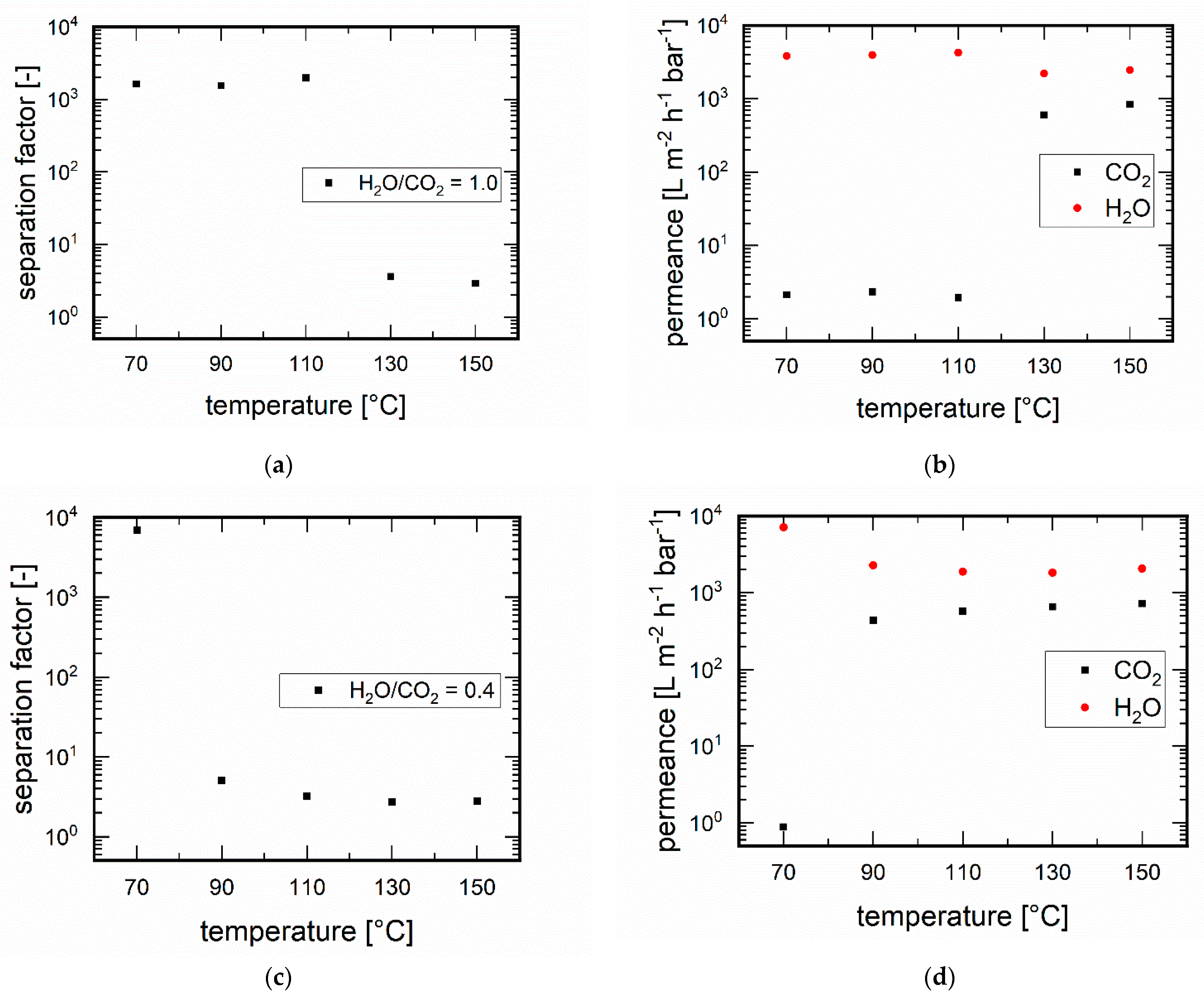
3.3.1. Influence of Temperature
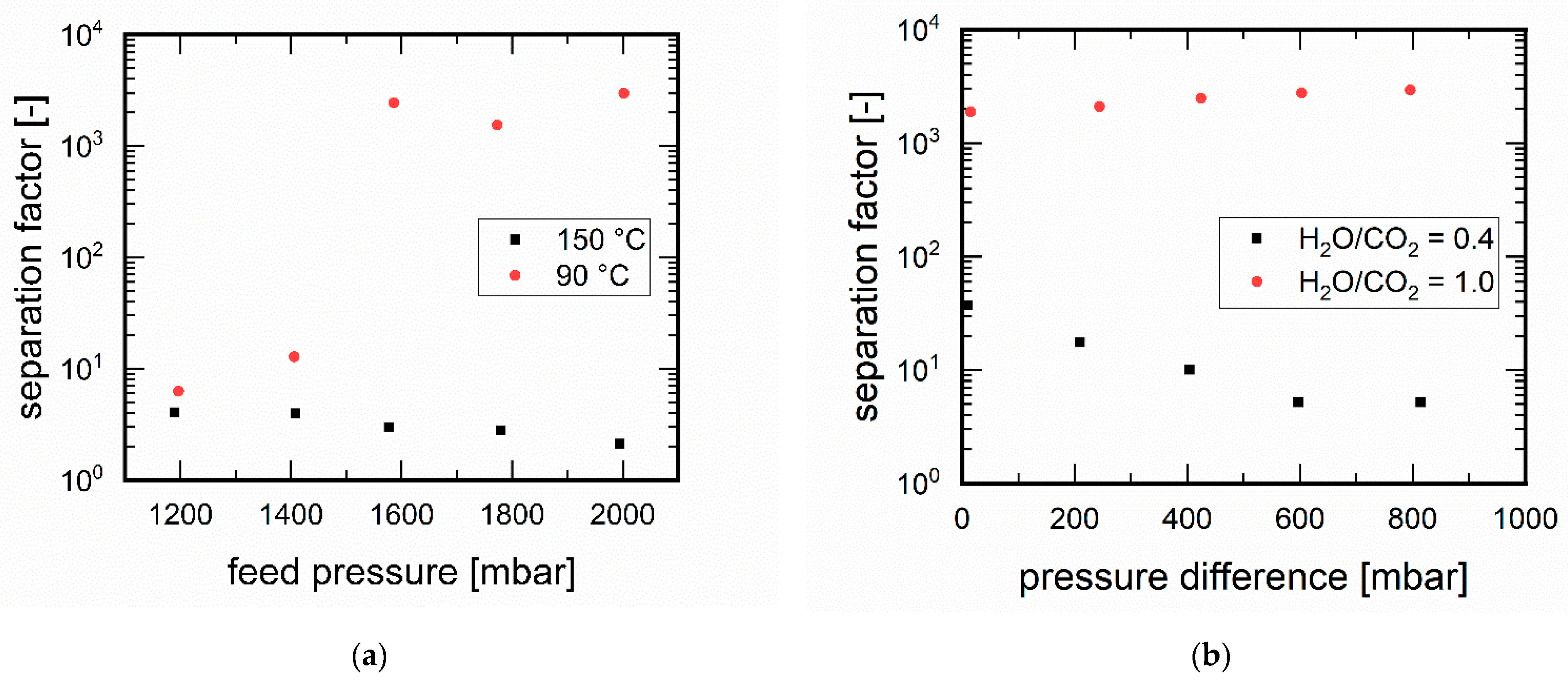
3.3.2. Influence of Pressure
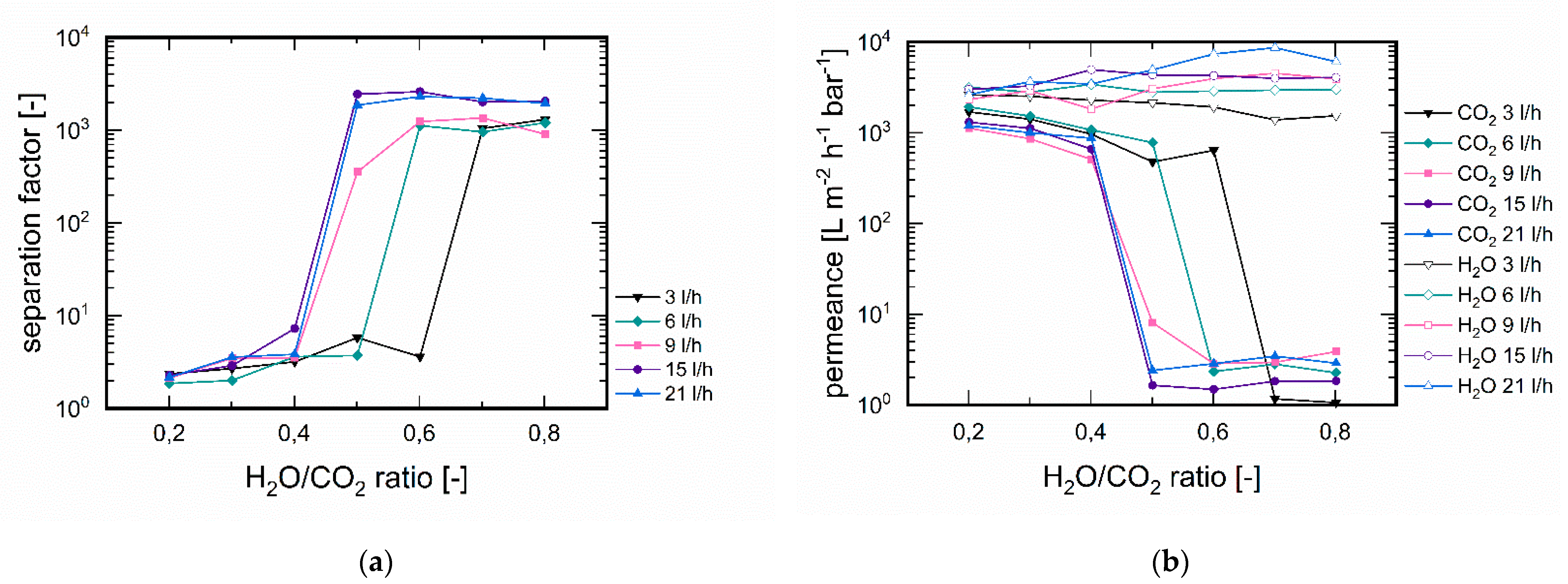
3.3.3. Influence of Feed Flux
3.3.4. Separation Performance of Amine-Treated MFI Membranes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chowdhury, F.A.; Yamada, H.; Higashii, T.; Goto, K.; Onoda, M. CO2 Capture by Tertiary Amine Absorbents: A Performance Comparison Study. Ind. Eng. Chem. Res. 2013, 52, 8323–8331. [Google Scholar] [CrossRef]
- Dutcher, B.; Fan, M.; Russell, A.G. Amine-Based CO2 Capture Technology Development from the Beginning of 2013—A Review. ACS Appl. Mater. Interf. 2015, 7, 2137–2148. [Google Scholar] [CrossRef] [PubMed]
- Ochedi, F.O.; Yu, J.; Yu, H.; Liu, Y.; Hussain, A. Carbon dioxide capture using liquid absorption methods: A review. Environ. Chem. Lett. 2021, 19, 77–109. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Rochelle, G.T. 3–Conventional Amine Scrubbing for CO2 Capture. In Absorption-Based Post-Combustion Capture of Carbon Dioxide; Feron, P.H.M., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 35–67. [Google Scholar]
- Puxty, G.; Rowland, R.; Allport, A.; Yang, Q.; Bown, M.; Burns, R.; Maeder, M.; Attalla, M. Carbon Dioxide Postcombustion Capture: A Novel Screening Study of the Carbon Dioxide Absorption Performance of 76 Amines. Environ. Sci. Technol. 2009, 43, 6427–6433. [Google Scholar] [CrossRef]
- Mukesh, C.; Khokarale, S.G.; Virtanen, P.; Mikkola, J.-P. Rapid desorption of CO2 from deep eutectic solvents based on polyamines at lower temperatures: An alternative technology with industrial potential. Sustain. Energy Fuels 2019, 3, 2125–2134. [Google Scholar] [CrossRef]
- de Vos, R.M.; Maier, W.F.; Verweij, H. Hydrophobic silica membranes for gas separation. J. Membr. Sci. 1999, 158, 277–288. [Google Scholar] [CrossRef]
- Pakizeh, M.; Omidkhah, M.R.; Zarringhalam, A. Synthesis and characterization of new silica membranes using template–sol–gel technology. Int. J. Hydrogen Energy 2007, 32, 1825–1836. [Google Scholar] [CrossRef]
- Assa, F. Synthesis and Performance of Nanostructure Templated Silica Membranes Surface-modified by Two Different Procedures. Chem. Biochem. Eng. Quart. 2015, 29, 417–427. [Google Scholar] [CrossRef]
- Fan, S.; Liu, J.; Zhang, F.; Zhou, S.; Sun, F. Fabrication of zeolite MFI membranes supported by α-Al2O3 hollow ceramic fibers for CO2 separation. J. Mater. Res. 2013, 28, 1870–1876. [Google Scholar] [CrossRef]
- Sublet, J.; Pera-Titus, M.; Guilhaume, N.; Farrusseng, D.; Schrive, L.; Chanaud, P.; Siret, B.; Durécu, S. Technico-economical assessment of MFI-type zeolite membranes for CO2 capture from postcombustion flue gases. AIChE J. 2012, 58, 3183–3194. [Google Scholar] [CrossRef]
- Yang, S.; Cao, Z.; Arvanitis, A.; Sun, X.; Xu, Z.; Dong, J. DDR-type zeolite membrane synthesis, modification and gas permeation studies. J. Membr. Sci. 2016, 505, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Junaidi, M.U.M.; Leo, C.P.; Ahmad, A.L.; Ahmad, N.A. Fluorocarbon functionalized SAPO-34 zeolite incorporated in asymmetric mixed matrix membranes for carbon dioxide separation in wet gases. Microporous Mesoporous Mat. 2015, 206, 23–33. [Google Scholar] [CrossRef]
- Wang, J.; Hao, Z.; Wohlrab, S. Continuous CO2 esterification to diethyl carbonate (DEC) at atmospheric pressure: Application of porous membranes for in situ H2O removal. Green Chem. 2017, 19, 3595–3600. [Google Scholar] [CrossRef]
- Wohlrab, S.; Meyer, T.; Stöhr, M.; Hecker, C.; Lubenau, U.; Oßmann, A. On the performance of customized MFI membranes for the separation of n-butane from methane. J. Membr. Sci. 2011, 369, 96–104. [Google Scholar] [CrossRef]
- Bolto, B.; Hoang, M.; Xie, Z. A review of water recovery by vapour permeation through membranes. Water Res. 2012, 46, 259–266. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M. Zeolite membranes–Recent developments and progress. Microporous Mesoporous Mat. 2008, 115, 215–233. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.; Su, X.; Cao, S.; Liu, Y.; Gao, D.; An, L. Experimental study of water recovery from flue gas using hollow micro–nano porous ceramic composite membranes. J. Ind. Eng. Chem. 2018, 57, 349–355. [Google Scholar] [CrossRef]
- Kim, J.F.; Park, A.; Kim, S.-J.; Lee, P.; Cho, Y.; Park, H.; Nam, S.; Park, Y. Harnessing Clean Water from Power Plant Emissions Using Membrane Condenser Technology. ACS Sustain. Chem. Eng. 2018, 6, 6425–6433. [Google Scholar] [CrossRef]
- Gao, D.; Li, Z.; Zhang, H.; Zhang, J.; Chen, H.; Fu, H. Moisture recovery from gas-fired boiler exhaust using membrane module array. J. Clean. Prod. 2019, 231, 1110–1121. [Google Scholar] [CrossRef]
- Kim, J.F.; Drioli, E. Transport Membrane Condenser Heat Exchangers to Break the Water-Energy Nexus—A Critical Review. Membranes 2021, 11, 12. [Google Scholar] [CrossRef]
- Lin, H.; Thompson, S.M.; Serbanescu-Martin, A.; Wijmans, J.G.; Amo, K.D.; Lokhandwala, K.A.; Merkel, T.C. Dehydration of natural gas using membranes. Part I: Composite membranes. J. Membr. Sci. 2012, 413–414, 70–81. [Google Scholar] [CrossRef]
- Li, G.M.; Feng, C.; Li, J.F.; Liu, J.Z.; Wu, Y.L. Water vapor permeation and compressed air dehydration performances of modified polyimide membrane. Sep. Purif. Technol. 2008, 60, 330–334. [Google Scholar] [CrossRef]
- Zagho, M.M.; Hassan, M.K.; Khraisheh, M.; Al-Maadeed, M.A.A.; Nazarenko, S. A review on recent advances in CO2 separation using zeolite and zeolite-like materials as adsorbents and fillers in mixed matrix membranes (MMMs). Chem. Eng. J. Adv. 2021, 6, 100091. [Google Scholar] [CrossRef]
- Dubbeldam, D.; Calero, S.; Ellis, D.E.; Snurr, R.Q. RASPA: Molecular simulation software for adsorption and diffusion in flexible nanoporous materials. Mol. Simul. 2016, 42, 81–101. [Google Scholar] [CrossRef] [Green Version]
- Vlugt, T.J.H.; Martin, M.G.; Smit, B.; Siepmann, J.I.; Krishna, R. Improving the efficiency of the configurational-bias Monte Carlo algorithm. Mol. Phys. 1998, 94, 727–733. [Google Scholar] [CrossRef]
- Ewald, P.P. Die Berechnung optischer und elektrostatischer Gitterpotentiale. Ann. Phys. 1921, 369, 253–287. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.G.; Yung, K.H. Carbon Dioxide’s Liquid-Vapor Coexistence Curve And Critical Properties as Predicted by a Simple Molecular Model. J. Phys. Chem. 1995, 99, 12021–12024. [Google Scholar] [CrossRef]
- Rick, S.W. A reoptimization of the five-site water potential (TIP5P) for use with Ewald sums. J. Chem. Phys. 2004, 120, 6085–6093. [Google Scholar] [CrossRef] [Green Version]
- Mahoney, M.W.; Jorgensen, W.L. A five-site model for liquid water and the reproduction of the density anomaly by rigid, nonpolarizable potential functions. J. Chem. Phys. 2000, 112, 8910–8922. [Google Scholar] [CrossRef]
- Jorabchi, M.N.; Ludwig, R.; Paschek, D. Quasi-Universal Solubility Behavior of Light Gases in Imidazolium-Based Ionic Liquids with Varying Anions: A Molecular Dynamics Simulation Study. J. Phys. Chem. B 2021, 125, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- Koros, W.J.; Ma, Y.H.; Shimidzu, T. Terminology for Membranes and membrane processes (IUPAC recommendations 1996). Pure Appl. Chem. 1996, 68, 1479–1489. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M.; Kölsch, P. Zeolite Membranes: From the Laboratory Scale to Technical Applications. Adsorption 2005, 11, 215–227. [Google Scholar] [CrossRef]
- Hedlund, J.; Korelskiy, D.; Sandström, L.; Lindmark, J. Permporometry analysis of zeolite membranes. J. Membr. Sci. 2009, 345, 276–287. [Google Scholar] [CrossRef]
- van Koningsveld, H.; Jansen, J.C. Single crystal structure analysis of zeolite H-ZSM-5 loaded with naphthalene. Microporous Mater. 1996, 6, 159–167. [Google Scholar] [CrossRef]
- Dragomirova, R.; Stohr, M.; Hecker, C.; Lubenau, U.; Paschek, D.; Wohlrab, S. Desorption-controlled separation of natural gas alkanes by zeolite membranes. RSC Adv. 2014, 4, 59831–59834. [Google Scholar] [CrossRef] [Green Version]
- Desbiens, N.; Boutin, A.; Demachy, I. Water Condensation in Hydrophobic Silicalite-1 Zeolite: A Molecular Simulation Study. J. Phys. Chem. B 2005, 109, 24071–24076. [Google Scholar] [CrossRef]
- Ektefa, F.; Javadian, S.; Rahmati, M. Computational comparison of the efficiency of nanoporous zeolite frameworks for separation of phenol from water. J. Taiwan Inst. Chem. Eng. 2018, 88, 104–113. [Google Scholar] [CrossRef]
- Lu, L.; Shao, Q.; Huang, L.; Lu, X. Simulation of adsorption and separation of ethanol–water mixture with zeolite and carbon nanotube. Fluid Phase Equilib. 2007, 261, 191–198. [Google Scholar] [CrossRef]
- van den Broeke, L.J.P.; Bakker, W.J.W.; Kapteijn, F.; Moulijn, J.A. Transport and separation properties of a silicalite-1 membrane—I. Operating conditions. Chem. Eng. Sci. 1999, 54, 245–258. [Google Scholar] [CrossRef]
- Arruebo, M.; Coronas, J.; Menéndez, M.; Santamaría, J. Separation of hydrocarbons from natural gas using silicalite membranes. Sep. Purif. Technol. 2001, 25, 275–286. [Google Scholar] [CrossRef]
- Gump, C.J.; Lin, X.; Falconer, J.L.; Noble, R.D. Experimental configuration and adsorption effects on the permeation of C4 isomers through ZSM-5 zeolite membranes. J. Membr. Sci. 2000, 173, 35–52. [Google Scholar] [CrossRef]
- van de Graaf, J.M.; Kapteijn, F.; Moulijn, J.A. Methodological and operational aspects of permeation measurements on silicalite-1 membranes. J. Membr. Sci. 1998, 144, 87–104. [Google Scholar] [CrossRef]
- Wang, D.; Bao, A.; Kunc, W.; Liss, W. Coal power plant flue gas waste heat and water recovery. Appl. Energy 2012, 91, 341–348. [Google Scholar] [CrossRef]
- Khalilpour, R.; Mumford, K.; Zhai, H.; Abbas, A.; Stevens, G.; Rubin, E.S. Membrane-based carbon capture from flue gas: A review. J. Clean. Prod. 2015, 103, 286–300. [Google Scholar] [CrossRef]
- Li, W.; Choi, S.; Drese, J.H.; Hornbostel, M.; Krishnan, G.; Eisenberger, P.M.; Jones, C.W. Steam-Stripping for Regeneration of Supported Amine-Based CO2 Adsorbents. ChemSusChem 2010, 3, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Gebald, C.; Wurzbacher, J.A.; Tingaut, P.; Zimmermann, T.; Steinfeld, A. Amine-Based Nanofibrillated Cellulose As Adsorbent for CO2 Capture from Air. Environ. Sci. Technol. 2011, 45, 9101–9108. [Google Scholar] [CrossRef]
- Sujan, A.R.; Pang, S.H.; Zhu, G.; Jones, C.W.; Lively, R.P. Direct CO2 Capture from Air using Poly(ethylenimine)-Loaded Polymer/Silica Fiber Sorbents. ACS Sustain. Chem. Eng. 2019, 7, 5264–5273. [Google Scholar] [CrossRef]
- Sehaqui, H.; Gálvez, M.E.; Becatinni, V.; cheng Ng, Y.; Steinfeld, A.; Zimmermann, T.; Tingaut, P. Fast and Reversible Direct CO2 Capture from Air onto All-Polymer Nanofibrillated Cellulose—Polyethylenimine Foams. Environ. Sci. Technol. 2015, 49, 3167–3174. [Google Scholar] [CrossRef]
- Deng, X.; Yang, W.; Li, S.; Liang, H.; Shi, Z.; Qiao, Z. Large-Scale Screening and Machine Learning to Predict the Computation-Ready, Experimental Metal-Organic Frameworks for CO2 Capture from Air. Appl. Sci. 2020, 10, 569. [Google Scholar] [CrossRef] [Green Version]
- Didas, S.A.; Choi, S.; Chaikittisilp, W.; Jones, C.W. Amine–Oxide Hybrid Materials for CO2 Capture from Ambient Air. Acc. Chem. Res. 2015, 48, 2680–2687. [Google Scholar] [CrossRef] [PubMed]
- Wijesiri, R.P.; Knowles, G.P.; Yeasmin, H.; Hoadley, A.F.A.; Chaffee, A.L. CO2 Capture from Air Using Pelletized Polyethylenimine Impregnated MCF Silica. Ind. Eng. Chem. Res. 2019, 58, 3293–3303. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Eisenberger, P.M.; Jones, C.W. Application of Amine-Tethered Solid Sorbents for Direct CO2 Capture from the Ambient Air. Environ. Sci. Technol. 2011, 45, 2420–2427. [Google Scholar] [CrossRef] [PubMed]


| m/u | q/e | 𝞼/Å | ||
|---|---|---|---|---|
| O_CO2 | 15.9994 | −0.3256 | 80.507 | 3.033 |
| C_CO2 | 12.0 | 0.6512 | 28.129 | 2.76 |
| O_H2O | 15.9994 | 0.0 | 89.633 | 3.097 |
| H_H2O | 1.008 | 0.241 | - | - |
| V_H2O | 0.0 | −0.241 | - | - |
| H2O/CO2 Ratio | Change of the Separation Factor (–) | CO2 Permeance (L⋅m−2⋅h−1⋅bar−1) | H2O Permeance (m−2⋅h−1⋅bar−1) |
|---|---|---|---|
| 0.4 | 2.37 | 6936 | 12893 |
| ↓ | ↓ | ↓ | |
| 3.43 | 2630 | 7981 | |
| 0.6 | 217 | 26.5 | 6604 |
| ↓ | ↓ | ↓ | |
| 401 | 16.4 | 7417 | |
| 1.0 | 726 | 9.0 | 7429 |
| ↓ | ↓ | ↓ | |
| 505 | 14.8 | 8584 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wotzka, A.; Jorabchi, M.N.; Wohlrab, S. Separation of H2O/CO2 Mixtures by MFI Membranes: Experiment and Monte Carlo Study. Membranes 2021, 11, 439. https://doi.org/10.3390/membranes11060439
Wotzka A, Jorabchi MN, Wohlrab S. Separation of H2O/CO2 Mixtures by MFI Membranes: Experiment and Monte Carlo Study. Membranes. 2021; 11(6):439. https://doi.org/10.3390/membranes11060439
Chicago/Turabian StyleWotzka, Alexander, Majid Namayandeh Jorabchi, and Sebastian Wohlrab. 2021. "Separation of H2O/CO2 Mixtures by MFI Membranes: Experiment and Monte Carlo Study" Membranes 11, no. 6: 439. https://doi.org/10.3390/membranes11060439
APA StyleWotzka, A., Jorabchi, M. N., & Wohlrab, S. (2021). Separation of H2O/CO2 Mixtures by MFI Membranes: Experiment and Monte Carlo Study. Membranes, 11(6), 439. https://doi.org/10.3390/membranes11060439






