CM-Dil Staining and SEC of Plasma as an Approach to Increase Sensitivity of Extracellular Nanovesicles Quantification by Bead-Assisted Flow Cytometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasma Sampling and Processing
2.2. Isolation of ENVs via Differential Ultracentrifugation
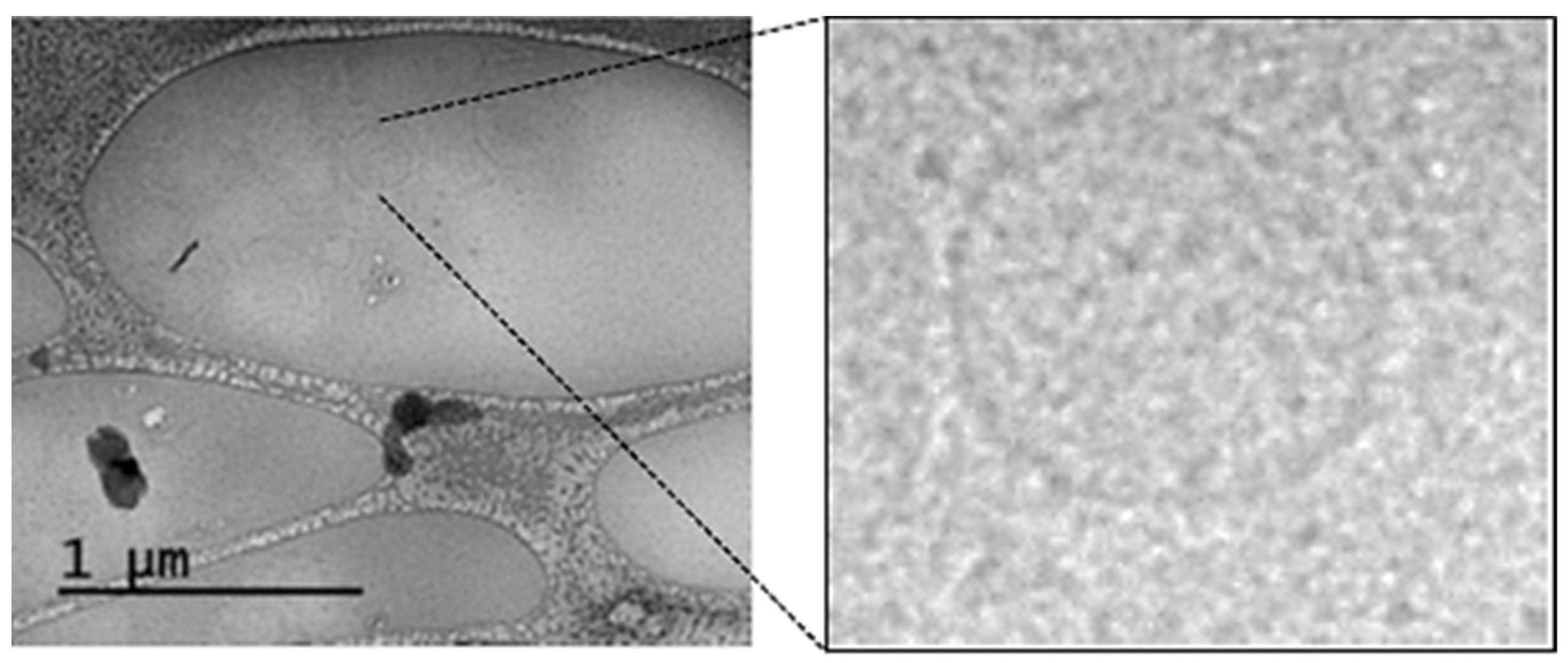
2.3. Transmission Cryo-Electron Microscopy (Cryo-TEM)
2.4. CM-Dil Plasma Labeling and SEC Purification
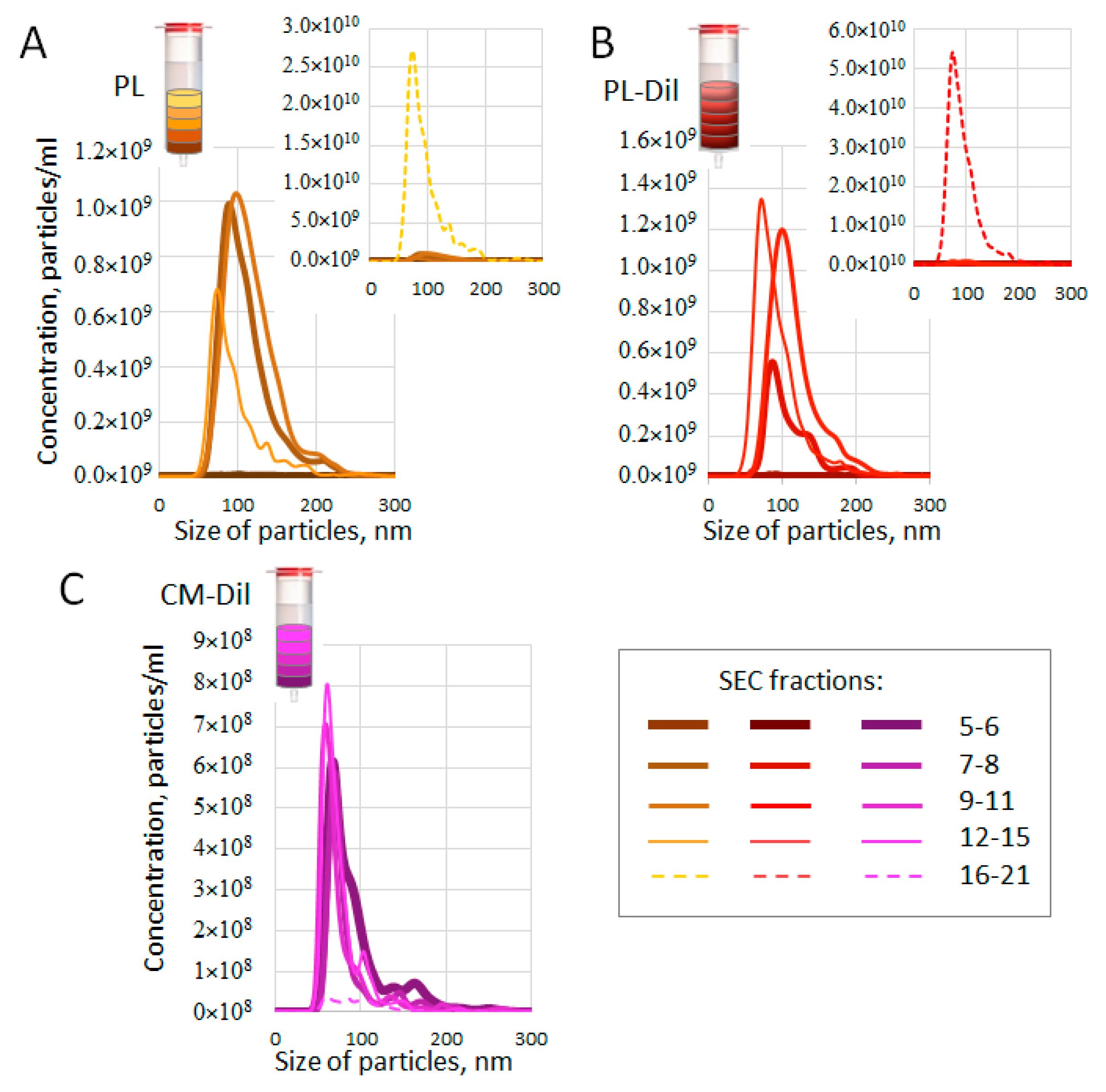
2.5. Nanoparticle Tracking Analysis
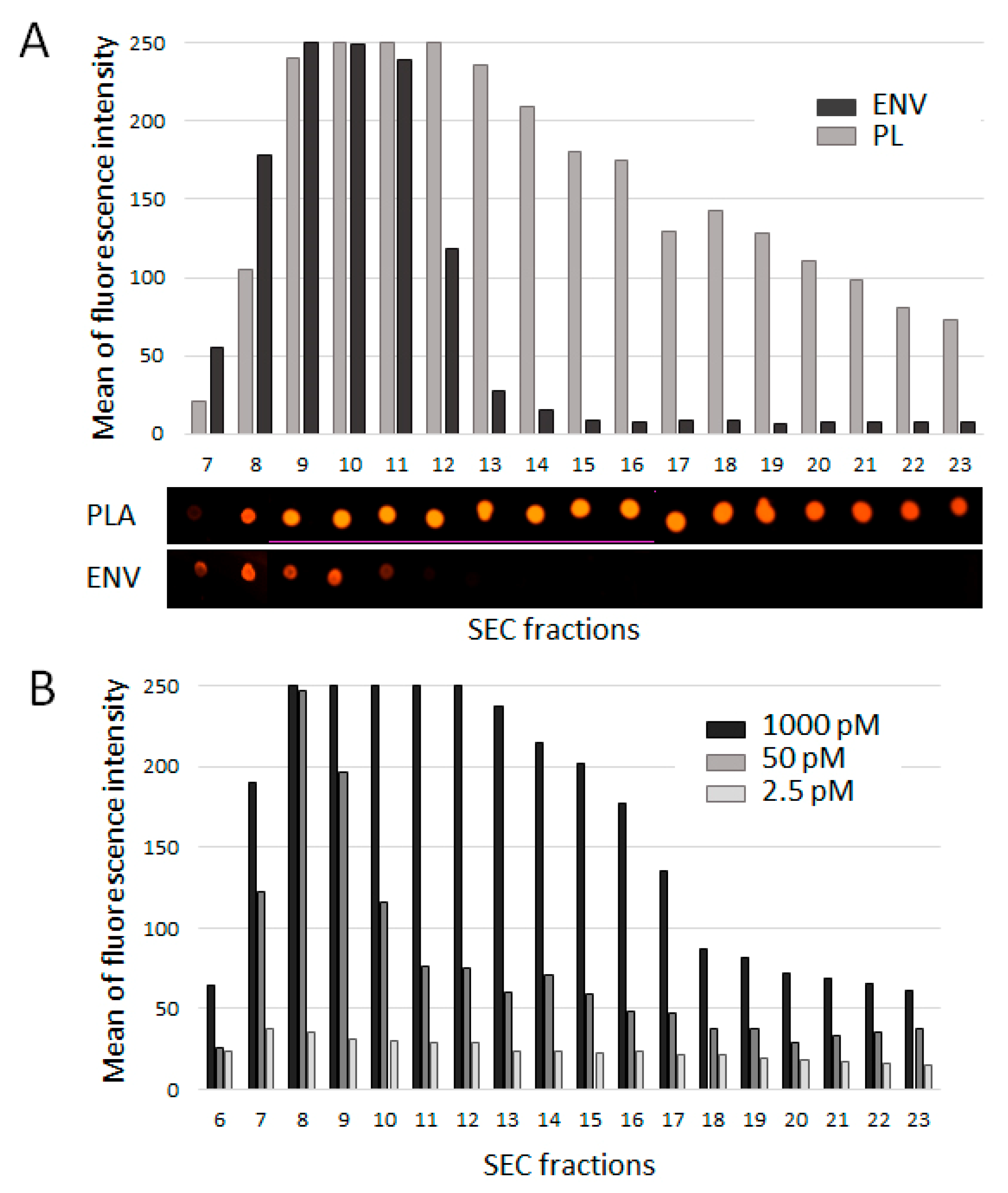
2.6. Fluorescent Intensity Assessment and Dot-Blotting
2.7. Immunobeads Fabrication and On-Beads FCM
3. Results
3.1. Design of Study
3.2. Isolation and Characterization of Plasma ENVs
3.3. Efficacy of Plasma Component Labeling with CM-DIl
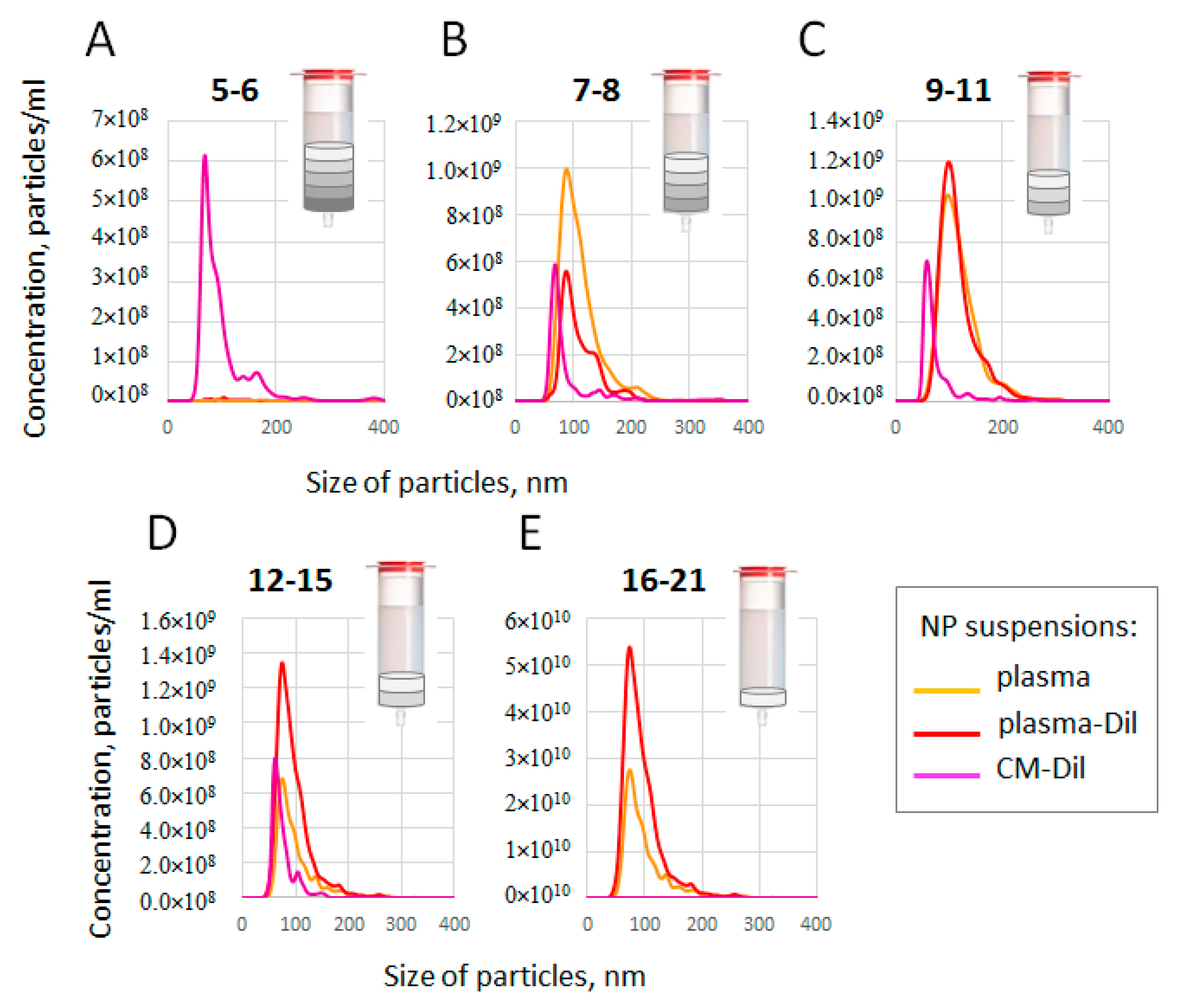
3.4. Size and Concentration of SEC-Fractionated Plasma Particles
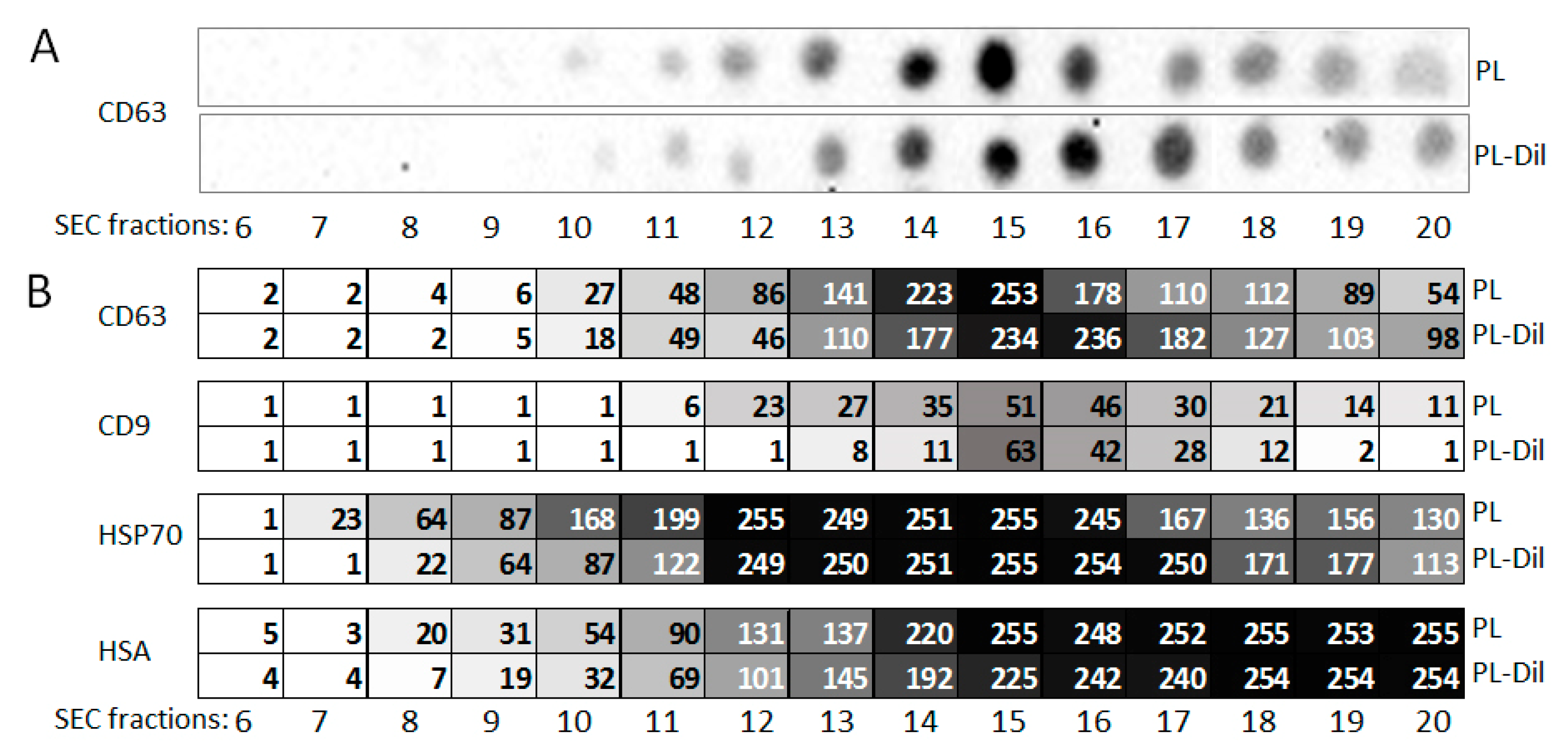
3.5. Protein Content of SEC Fractions of Plasma and CM-Dil-Stained Plasma
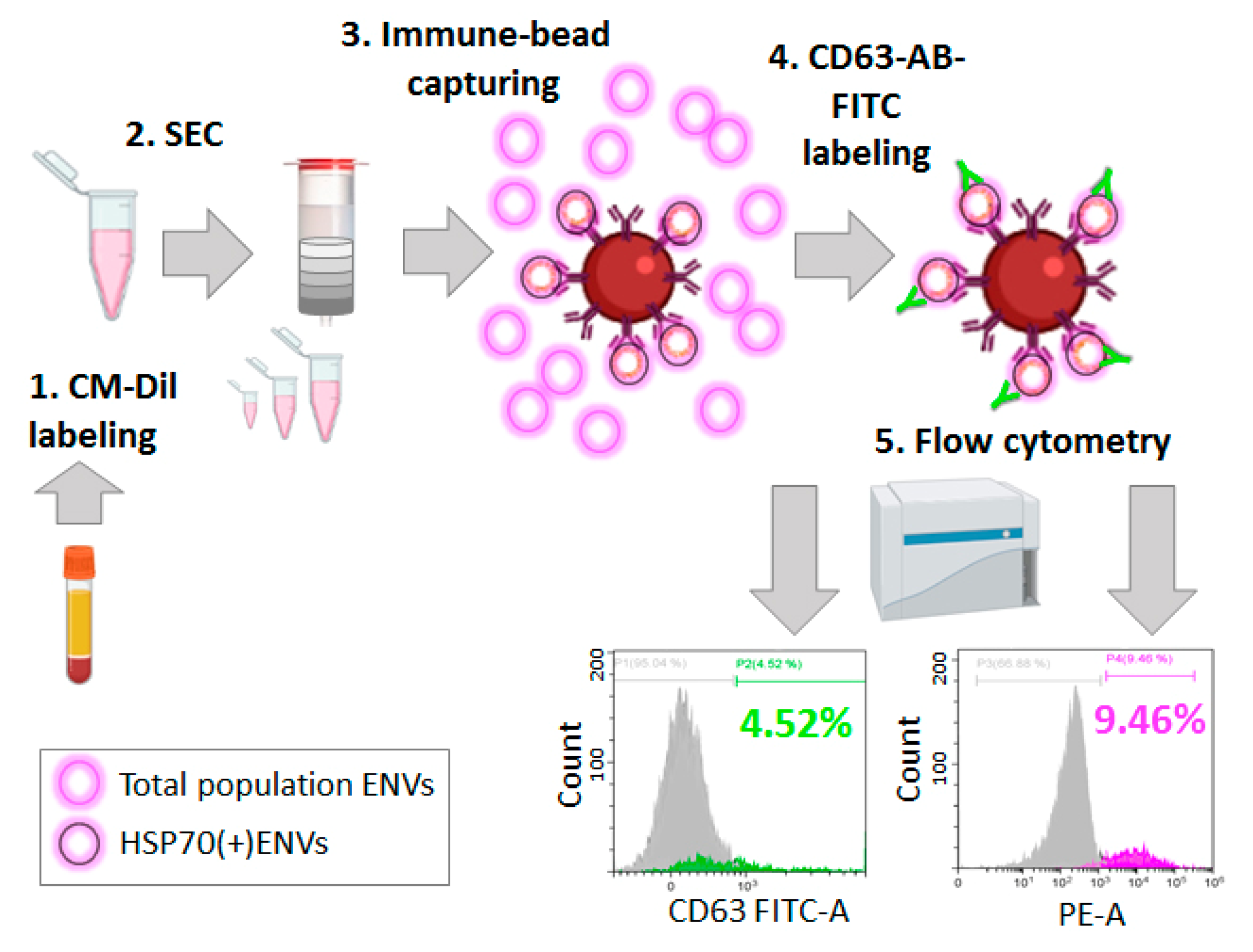
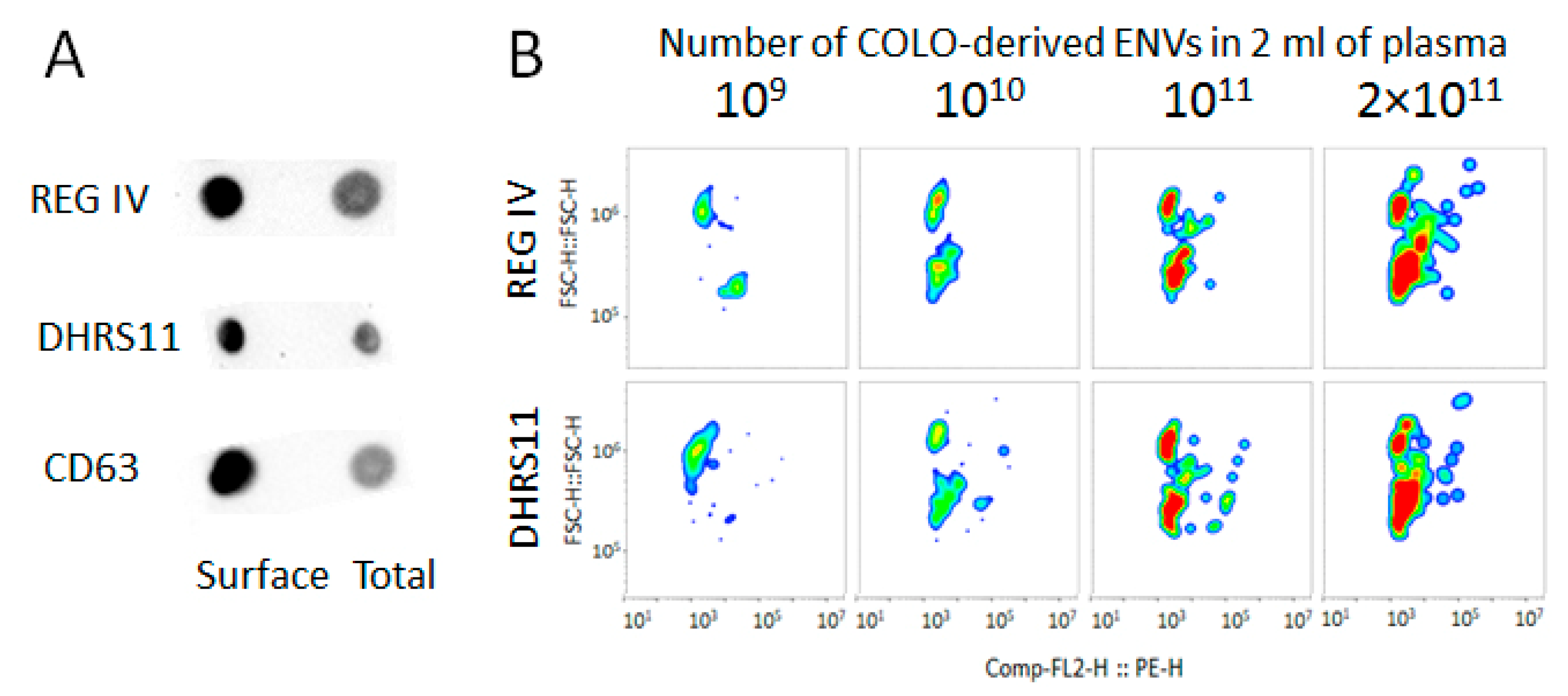
3.6. Immunocapturing and Bead-Assisted FCM
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czystowska-Kuzmicz, M.; Whiteside, T.L. The potential role of tumor-derived exosomes in diagnosis, prognosis, and response to therapy in cancer. Expert Opin. Biol. Ther. 2020, 21, 241–258. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed. Res. Int. 2018, 2018, 8545347. Available online: https://www.hindawi.com/journals/bmri/2018/8545347/ (accessed on 30 January 2018). [CrossRef]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; LeClaire, M.; Wohlschlegel, J.; Gimzewski, J. Impact of isolation methods on the biophysical heterogeneity of single extracellular vesicles. Sci. Rep. 2020, 10, 13327. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EV-TRACK Consortium; Van Deun, J.; Mestdagh, P.; Agostinis, P.; Akay, Ö.; Anand, S.; Anckaert, J.; Martinez, Z.A.; Baetens, T.; Beghein, E.; et al. EV-TRACK: Transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods 2017, 14, 228–232. [Google Scholar] [CrossRef]
- Soekmadji, C.; Li, B.; Huang, Y.; Wang, H.; An, T.; Liu, C.; Pan, W.; Chen, J.; Cheung, L.; Falcon-Perez, J.M.; et al. The future of Extracellular Vesicles as Theranostics–an ISEV meeting report. J. Extracell. Vesicles 2020, 9, 1809766. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Van Der Pol, E.; Arkesteijn, G.J.A.; Bremer, M.; Brisson, A.; Coumans, F.; Dignat-George, F.; Duggan, E.; Ghiran, I.; Giebel, B.; et al. MIFlowCyt-EV: A framework for standardized reporting of extracellular vesicle flow cytometry experiments. J. Extracell. Vesicles 2020, 9, 1713526. [Google Scholar] [CrossRef]
- Suárez, H.; Gámez-Valero, A.; Reyes, R.; López-Martín, S.; Rodríguez, M.J.; Carrascosa, J.L.; Cabañas, C.; Borràs, F.E.; Yáñez-Mó, M. A bead-assisted flow cytometry method for the semi-quantitative analysis of Extracellular Vesicles. Sci. Rep. 2017, 7, 11271. [Google Scholar] [CrossRef] [Green Version]
- Morales-Kastresana, A.; Jones, J.C. Flow Cytometric Analysis of Extracellular Vesicles. Methods Mol. Biol. 2017, 1545, 215–225. [Google Scholar]
- Wiklander, O.P.B.; Bostancioglu, R.B.; Welsh, J.A.; Zickler, A.M.; Murke, F.; Corso, G.; Felldin, U.; Hagey, D.; Evertsson, B.; Liang, X.-M.; et al. Systematic Methodological Evaluation of a Multiplex Bead-Based Flow Cytometry Assay for Detection of Extracellular Vesicle Surface Signatures. Front. Immunol. 2018, 9, 1326. [Google Scholar] [CrossRef] [Green Version]
- Theodoraki, M.; Hong, C.; Donnenberg, V.S.; Donnenberg, A.D.; Whiteside, T.L. Evaluation of Exosome Proteins by on-Bead Flow Cytometry. Cytom. Part A 2020, 99, 372–381. [Google Scholar] [CrossRef]
- Campos-Silva, C.; Suárez, H.; Jara-Acevedo, R.; Linares-Espinós, E.; Martinez-Piñeiro, L.; Yáñez-Mó, M.; Valés-Gómez, M. High sensitivity detection of extracellular vesicles immune-captured from urine by conventional flow cytometry. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Dominkuš, P.; Stenovec, M.; Sitar, S.; Lasič, E.; Zorec, R.; Plemenitaš, A.; Žagar, E.; Kreft, M.; Lenassi, M. PKH26 labeling of extracellular vesicles: Characterization and cellular internalization of contaminating PKH26 nanoparticles. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1350–1361. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.; Gulvin, S.M.; Flax, J.; Gaborski, T.R. Systematic Evaluation of PKH Labelling on Extracellular Vesicle Size by Nanoparticle Tracking Analysis. Sci. Rep. 2020, 10, 9533. [Google Scholar] [CrossRef] [PubMed]
- De Rond, L.; Van Der Pol, E.; Hau, C.M.; Varga, Z.; Sturk, A.; Van Leeuwen, T.G.; Nieuwland, R.; Coumans, F.A. Comparison of generic fluorescent markers for detection of extracellular vesicles by flow cytometry. Clin. Chem. 2018, 64, 680–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maia, J.; Batista, S.; Couto, N.; Gregório, A.C.; Bodo, C.; Elzanowska, J.; Moraes, M.C.S.; Costa-Silva, B. Employing Flow Cytometry to Extracellular Vesicles Sample Microvolume Analysis and Quality Control. Front. Cell Dev. Biol. 2020, 8, 593750. [Google Scholar] [CrossRef] [PubMed]
- Nazarova, I.V.; Nikiforova, N.S.; Sidina, E.I.; Slyusarenko, M.A.; Kotova, Z.S.; Semiglazova, T.Y.; Sluzhev, M.A.; Semiglazov, V.V.; Gogolev, A.B.; Artemyeva, A.S.; et al. Colorectal cancer diagnostics via detection of tissue-specific extracellular nano-vesicles. Koloproktologia 2020, 19, 32–56. Available online: https://www.ruproctology.com/jour/article/view/1607/1612 (accessed on 30 November 2020). [CrossRef]
- Morales-Kastresana, A.; Telford, B.; Musich, T.A.; McKinnon, K.; Clayborne, C.; Braig, Z.; Rosner, A.; Demberg, T.; Watson, D.C.; Karpova, T.; et al. Labeling extracellular vesicles for nanoscale flow cytometry. Sci. Rep. 2017, 7, 1–10. [Google Scholar]
- Chuo, S.T.-Y.; Chien, J.C.-Y.; Lai, C.P.-K. Imaging extracellular vesicles: Current and emerging methods. J. Biomed. Sci. 2018, 25, 91. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zong, S.; Wang, Z.; Lu, J.; Zhu, D.; Zhang, Y.; Cui, Y. Imaging and Intracellular Tracking of Cancer-Derived Exosomes Using Single-Molecule Localization-Based Super-Resolution Microscope. ACS Appl. Mater. Interfaces 2016, 8, 25825–25833. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.D.; Mitchell, A.J.; Searles, C.D. An accurate, precise method for general labeling of extracellular vesicles. MethodsX 2015, 2, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Ender, F.; Zamzow, P.; von Bubnoff, N.; Gieseler, F. Detection and quantification of extracellular vesicles via FACS: Membrane labeling matters! Int. J. Mol. Sci. 2020, 21, 291. [Google Scholar] [CrossRef] [Green Version]
- Kalimuthu, K.; Kwon, W.Y.; Park, K.S. A simple approach for rapid and cost-effective quantification of extracellular vesicles using a fluorescence polarization technique. J. Biol. Eng. 2019, 13, 31. [Google Scholar] [CrossRef] [PubMed]
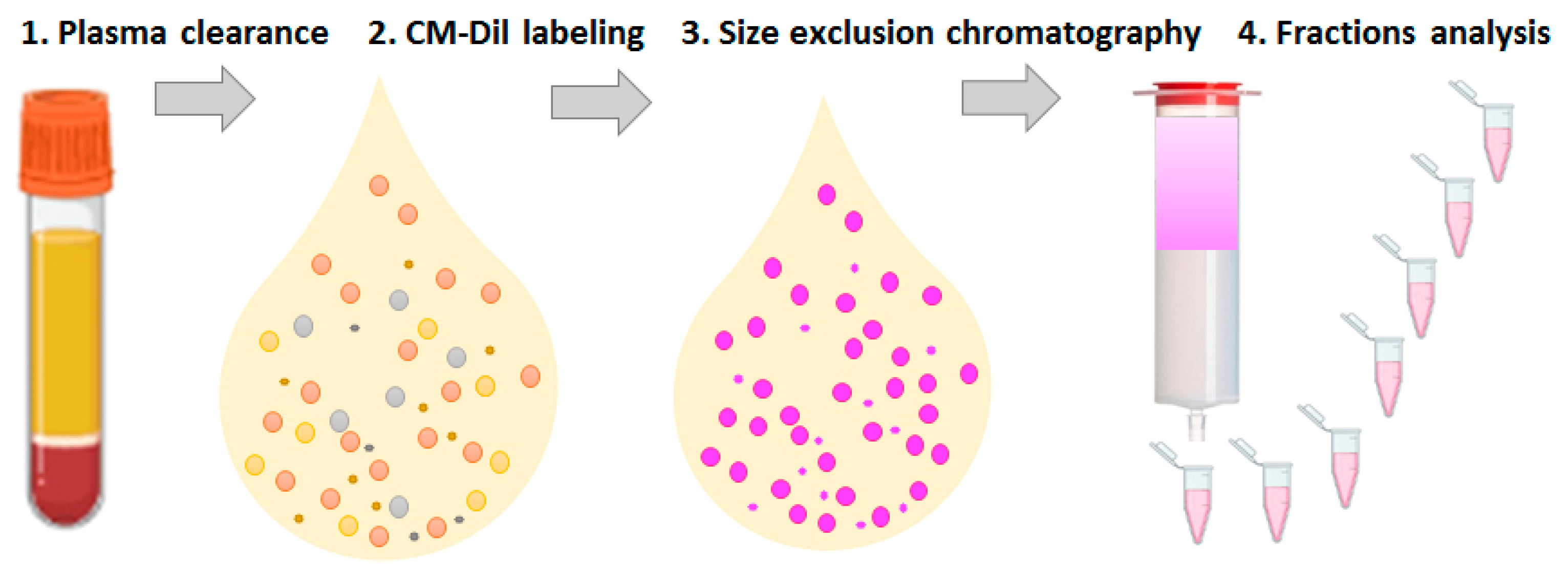
| Objectives | Methods/Readout | Analyzed Samples * | |||
|---|---|---|---|---|---|
| ENV/ CMdil | PPP/ CMdil | PPP - | - CMdil | ||
| 1. Efficacy of labeling (SEC fractions 6–23) | 1. Fluo-dot/fluorescent intensity assessment | + | + | ||
| 2. Nanoparticles’ characteristics (SEC fraction 5–21) | 2. NTA/particle size and concentration. | + | + | + | |
| 3. Dot blotting/specific protein content (CD9, CD63, HSP70, albumin) | + | + | |||
| 3. Evaluation of on-bead FCM sensitivity (SEC fractions 9–11) | 4. On-bead FCM of samples labeled by two alternative approaches/fluorescent intensity assessment | + | |||
| 5. On-bead FCM of samples containing different amount of ENVs of interest/fluorescent intensity assessment | + | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikiforova, N.; Chumachenko, M.; Nazarova, I.; Zabegina, L.; Slyusarenko, M.; Sidina, E.; Malek, A. CM-Dil Staining and SEC of Plasma as an Approach to Increase Sensitivity of Extracellular Nanovesicles Quantification by Bead-Assisted Flow Cytometry. Membranes 2021, 11, 526. https://doi.org/10.3390/membranes11070526
Nikiforova N, Chumachenko M, Nazarova I, Zabegina L, Slyusarenko M, Sidina E, Malek A. CM-Dil Staining and SEC of Plasma as an Approach to Increase Sensitivity of Extracellular Nanovesicles Quantification by Bead-Assisted Flow Cytometry. Membranes. 2021; 11(7):526. https://doi.org/10.3390/membranes11070526
Chicago/Turabian StyleNikiforova, Nadezhda, Maria Chumachenko, Inga Nazarova, Lidia Zabegina, Maria Slyusarenko, Elena Sidina, and Anastasia Malek. 2021. "CM-Dil Staining and SEC of Plasma as an Approach to Increase Sensitivity of Extracellular Nanovesicles Quantification by Bead-Assisted Flow Cytometry" Membranes 11, no. 7: 526. https://doi.org/10.3390/membranes11070526
APA StyleNikiforova, N., Chumachenko, M., Nazarova, I., Zabegina, L., Slyusarenko, M., Sidina, E., & Malek, A. (2021). CM-Dil Staining and SEC of Plasma as an Approach to Increase Sensitivity of Extracellular Nanovesicles Quantification by Bead-Assisted Flow Cytometry. Membranes, 11(7), 526. https://doi.org/10.3390/membranes11070526








