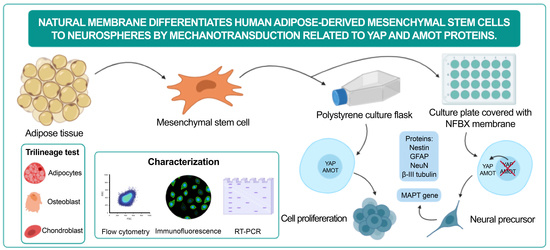
Natural Membrane Differentiates Human Adipose-Derived Mesenchymal Stem Cells to Neurospheres by Mechanotransduction Related to YAP and AMOT Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of ADMSCs
2.2. Characterization of ADMSCs
2.2.1. Flow Cytometry
2.2.2. Trilineage Tests
Adipogenic Differentiation
Osteogenic Differentiation
Chondrogenic Differentiation
2.3. Preparation of the Polyisoprene-Based Membrane
2.4. Production of NPs
2.5. Characterization of NPs
2.5.1. Immunocytochemistry
2.5.2. Qualitative Reverse Transcription–Polymerase Chain Reaction (RT-PCR)
3. Results
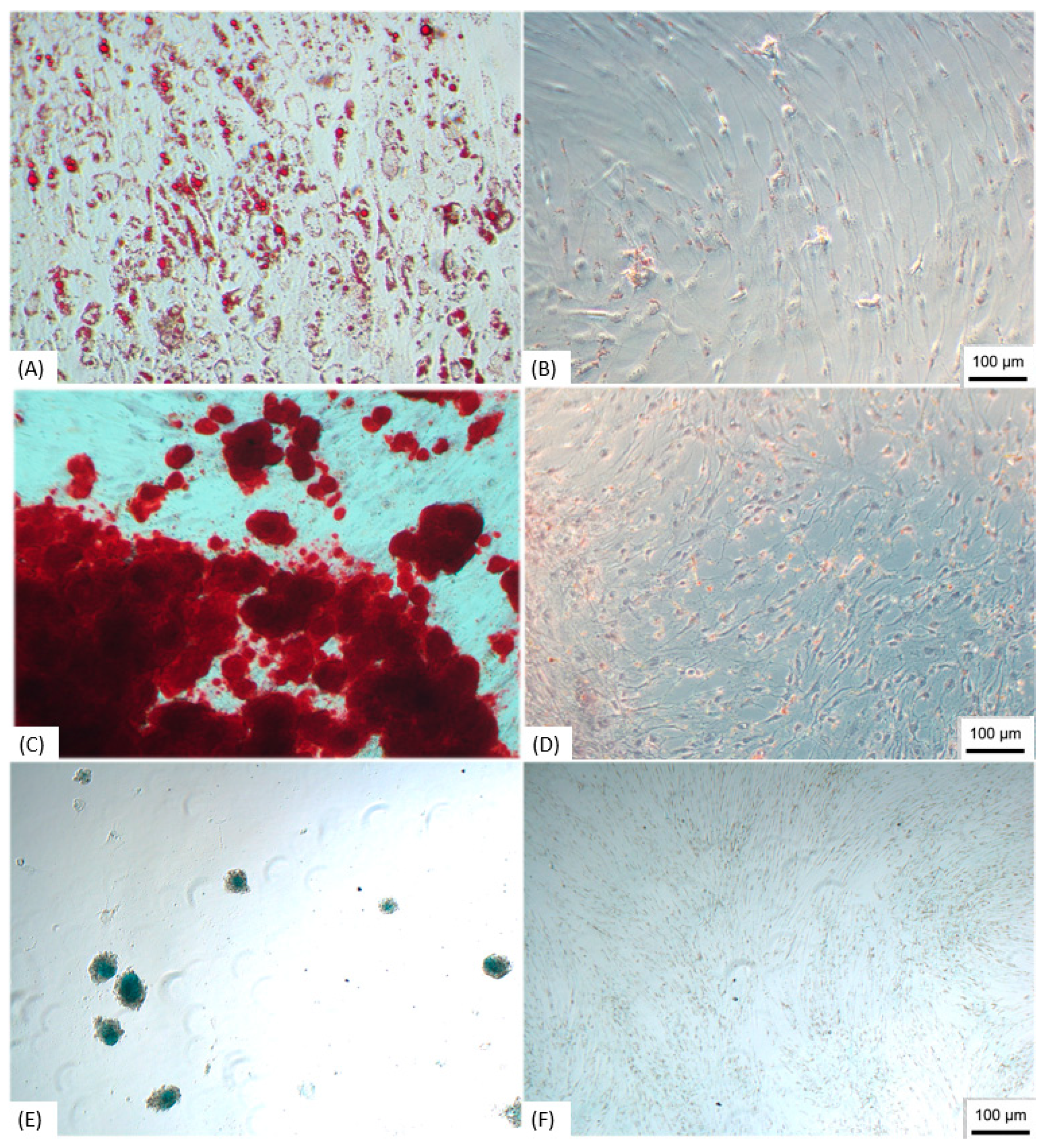
3.1. Characterization of ADMSCs
Trilineage Test
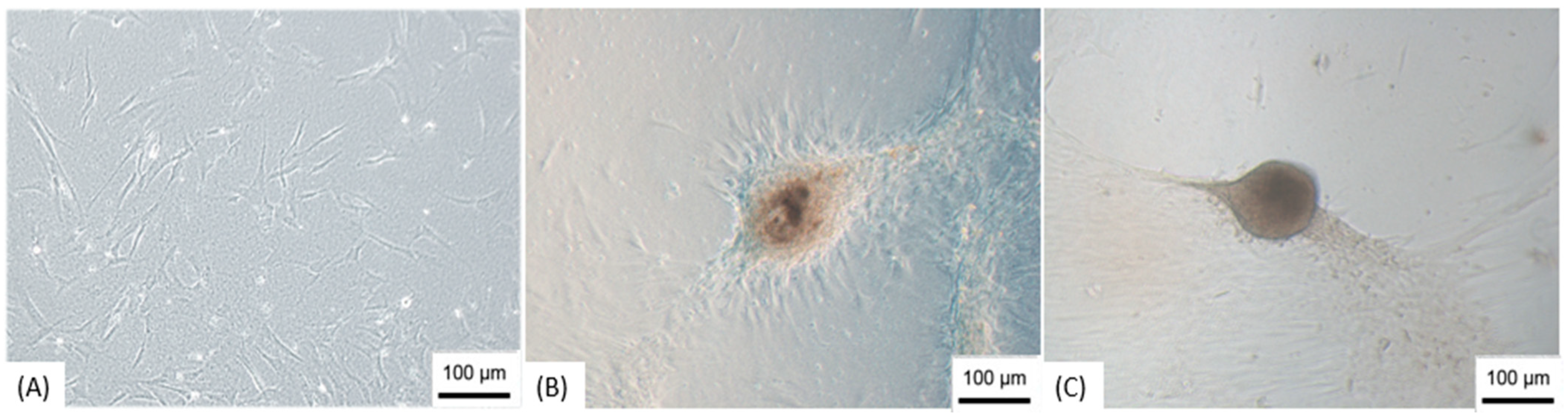
3.2. Production of NPs
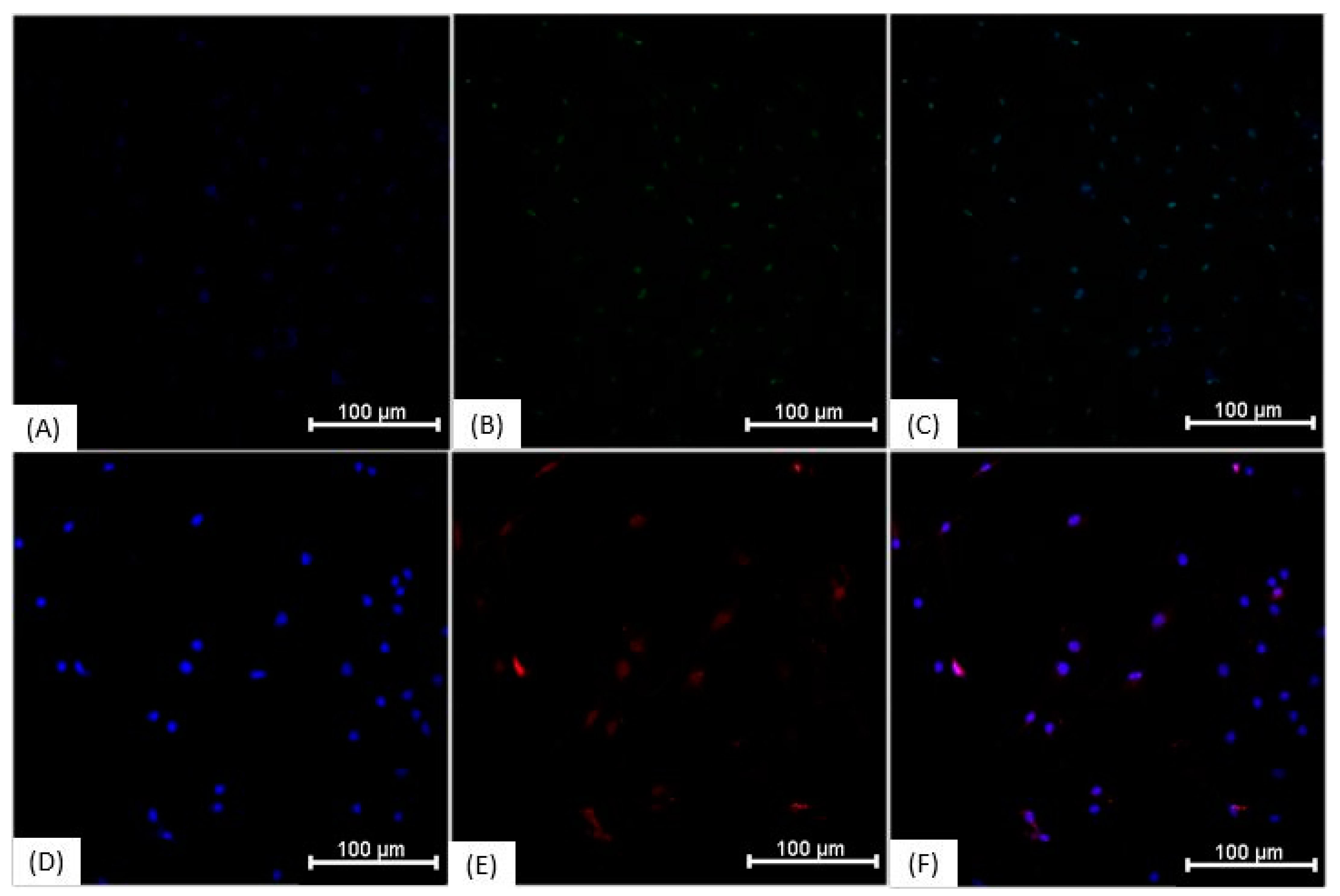
3.3. Immunocytochemistry
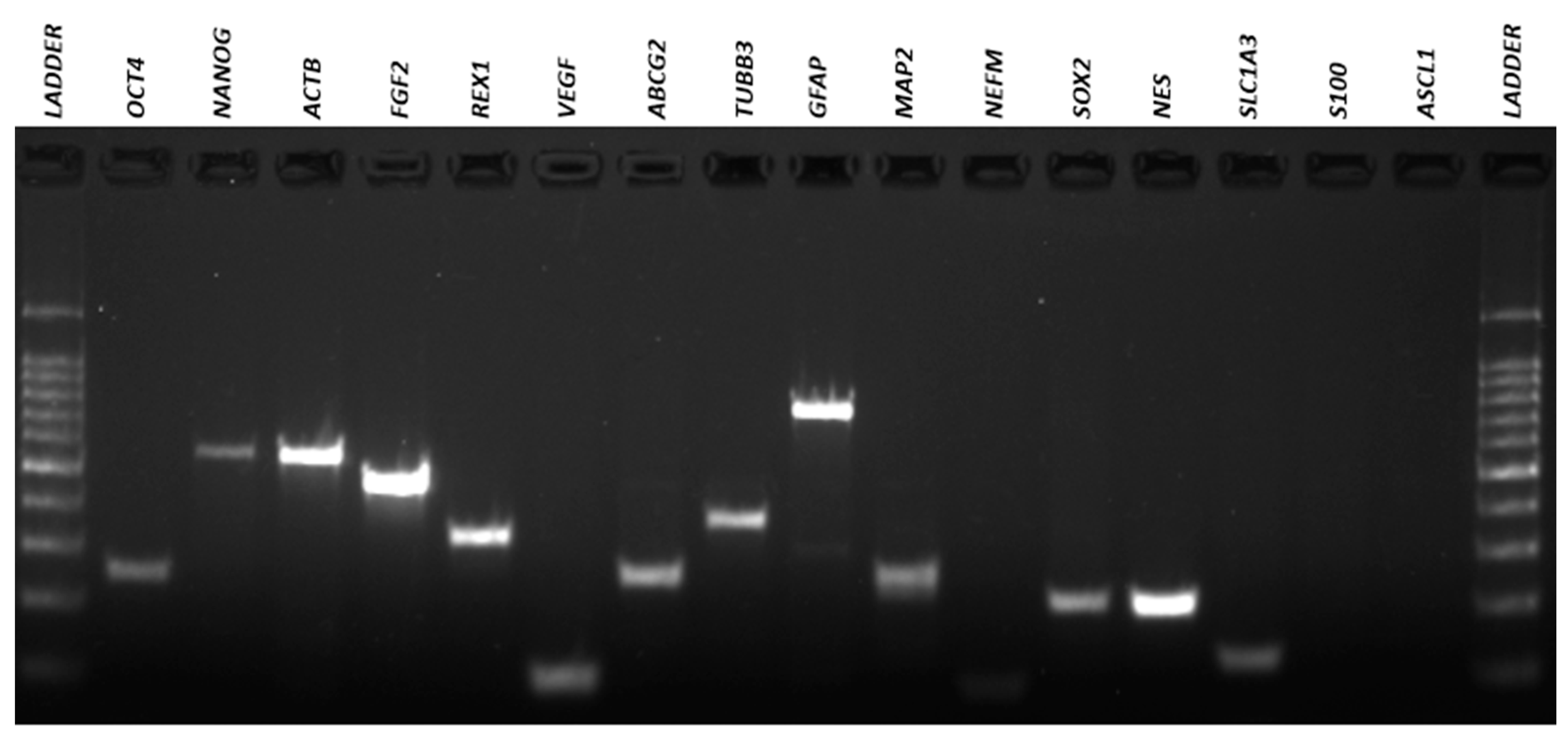
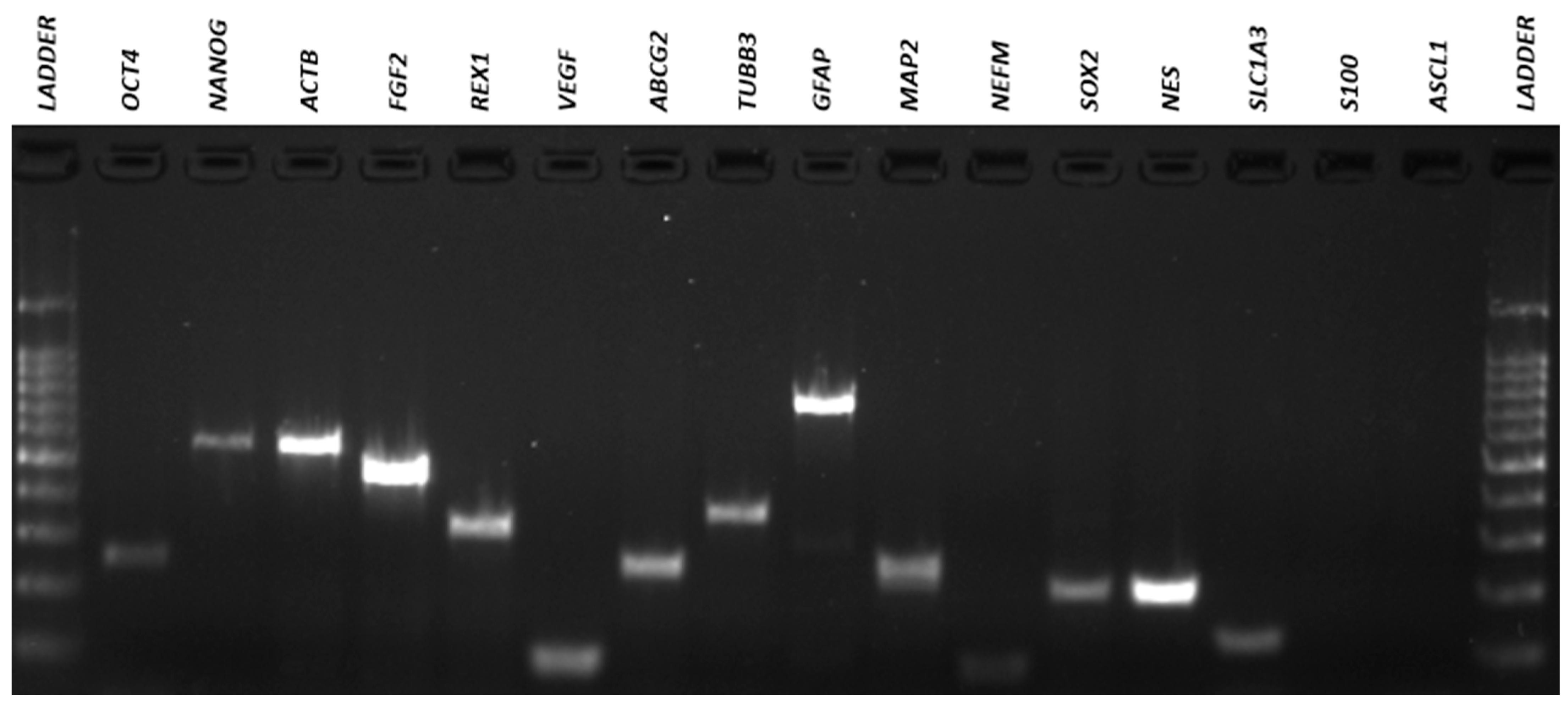
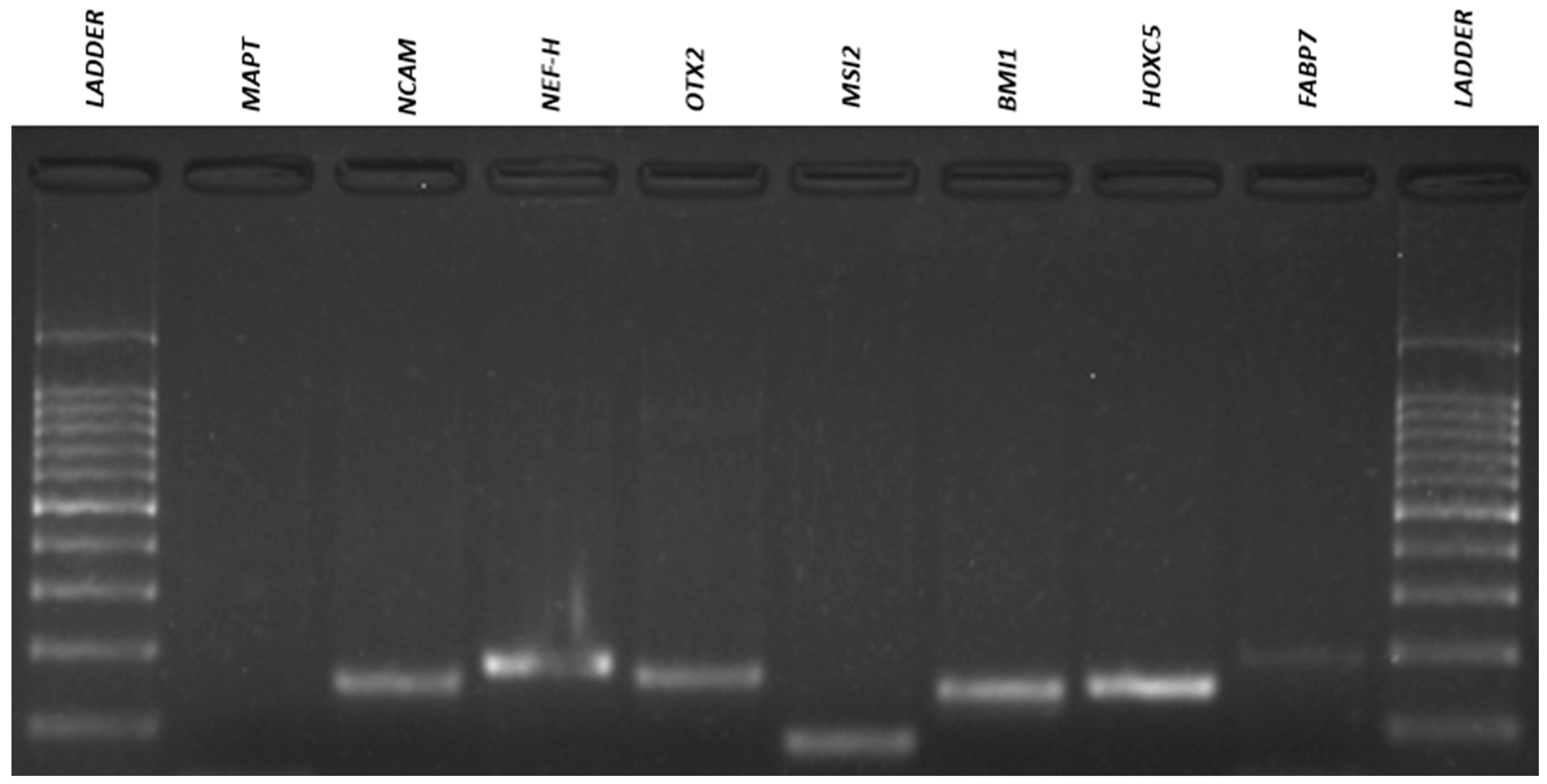
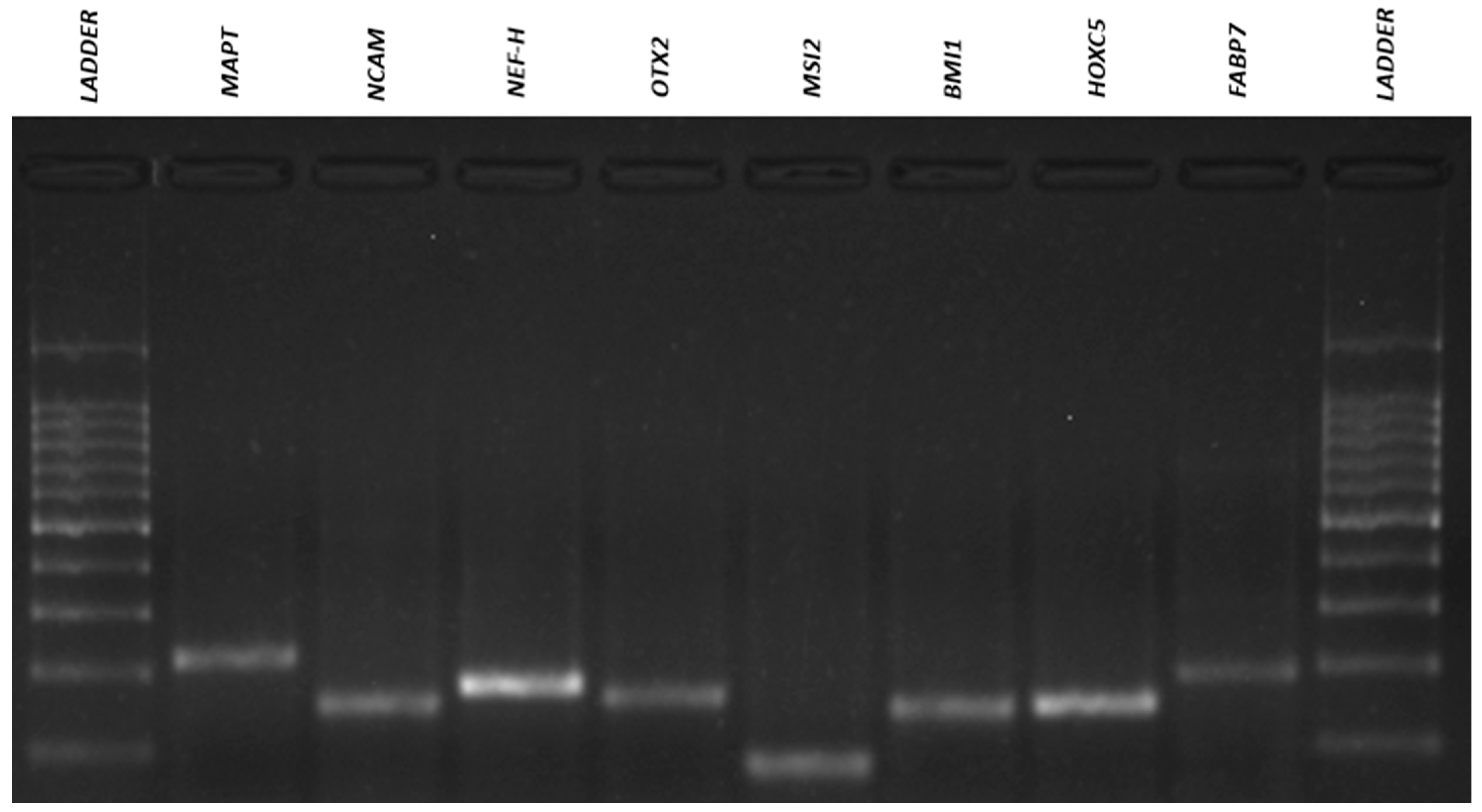
3.4. Qualitative Reverse Transcriptio–-Polymeresae Chain Reaction (RT-PCR)
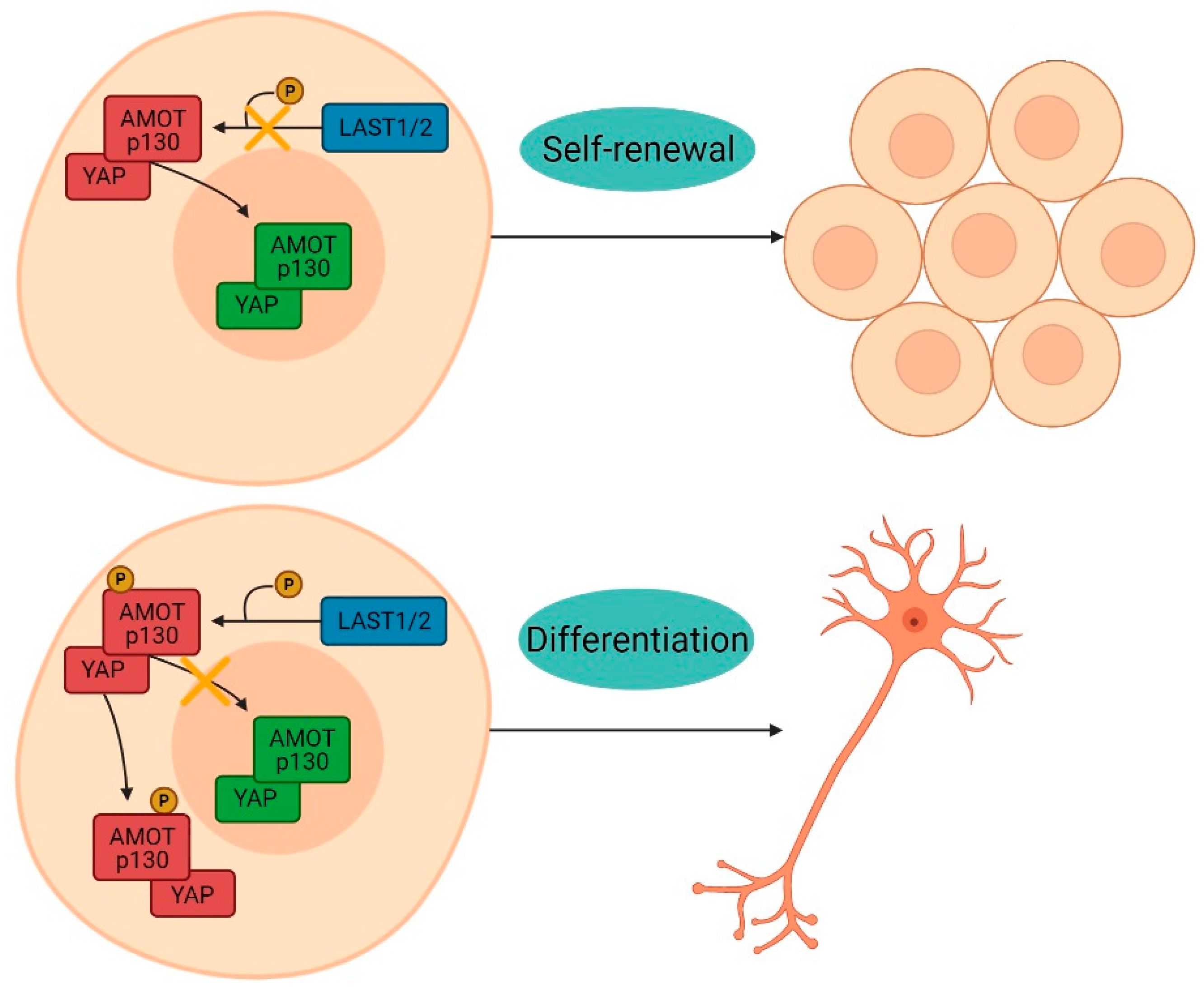
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem Cells: Their Source, Potency and Use in Regenerative Therapies with Focus on Adipose-Derived Stem Cells—A Review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef]
- Arrighi, N. Definition and Classification of Stem Cells. In Stem Cells: Therapeutic Innovations under Control; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–45. ISBN 978-1-78548-254-0. [Google Scholar]
- Paim, Á.; Cardozo, N.S.M.; Tessaro, I.C.; Pranke, P. Relevant Biological Processes for Tissue Development with Stem Cells and Their Mechanistic Modeling: A Review. Math. Biosci. 2018, 301, 147–158. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, K.; Fisk, N.M. Fetal Stem Cells. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 853–875. [Google Scholar] [CrossRef] [PubMed]
- Bateman, M.; Strong, A.; Gimble, J.; Bunnell, B. Concise Review: Using Fat to Fight Disease: A Systematic Review of Non-Homologous Adipose-Derived Stromal/Stem Cell Therapies. Stem Cells 2018, 36, 1311–1328. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.; McKee, C.; Bakshi, S.; Walker, K.; Hakman, E.; Halassy, S.; Svinarich, D.; Dodds, R.; Govind, C.K.; Chaudhry, G.R. Mesenchymal Stem Cells: Cell Therapy and Regeneration Potential. J. Tissue Eng. Regen. Med. 2019, 13, 1738–1755. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef]
- De Carvalho, K.A.T.; Steinhoff, G.; Chachques, J.C. Mesenchymal Stem Cell Therapy in Nonhematopoietic Diseases. Stem Cells Int. 2015, 2015, 676903. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Wang, X.; Sun, C.; Kang, Y.; Xu, J.; Wang, X.; Hui, Y. Adipose-Derived Stem Cells: Sources, Potency, and Implications for Regenerative Therapies. Biomed. Pharmacother. 2019, 114, 108765. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Jeong, H.-S. Histone Deacetylase Inhibition-Mediated Neuronal Differentiation via the Wnt Signaling Pathway in Human Adipose Tissue-Derived Mesenchymal Stem Cells. Neurosci. Lett. 2018, 668, 24–30. [Google Scholar] [CrossRef]
- Mahmoudian-Sani, M.-R.; Mehri-Ghahfarrokhi, A.; Hashemzadeh-Chaleshtori, M.; Saidijam, M.; Jami, M.-S. Comparison of Three Types of Mesenchymal Stem Cells (Bone Marrow, Adipose Tissue, and Umbilical Cord-Derived) as Potential Sources for Inner Ear Regeneration. Int. Tinnitus J. 2017, 21, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Mortada, I.; Mortada, R. Epigenetic Changes in Mesenchymal Stem Cells Differentiation. Eur. J. Med. Genet. 2018, 61, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Khilan, A.A.; Al-Maslamani, N.A.; Horn, H.F. Cell Stretchers and the LINC Complex in Mechanotransduction. Arch. Biochem. Biophys. 2021, 702, 108829. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, C.P. Mechanotransduction: Overview. In Encyclopedia of Bone Biology; Zaidi, M., Ed.; Academic Press: Oxford, UK, 2020; p. 217. ISBN 978-0-12-814082-6. [Google Scholar]
- Safi-Stibler, S.; Gabory, A. Epigenetics and the Developmental Origins of Health and Disease: Parental Environment Signalling to the Epigenome, Critical Time Windows and Sculpting the Adult Phenotype. Semin. Cell Dev. Biol. 2020, 97, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Rausch, V.; Hansen, C.G. The Hippo Pathway, YAP/TAZ, and the Plasma Membrane. Trends Cell Biol. 2020, 30, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Moleirinho, S.; Hoxha, S.; Mandati, V.; Curtale, G.; Troutman, S.; Ehmer, U.; Kissil, J.L. Regulation of Localization and Function of the Transcriptional Co-Activator YAP by Angiomotin. eLife 2017, 6, e23966. [Google Scholar] [CrossRef]
- Park, J.A.; Kwon, Y.-G. Hippo-YAP/TAZ Signaling in Angiogenesis. BMB Rep. 2018, 51, 157–162. [Google Scholar] [CrossRef]
- Lee, S.; Stanton, A.E.; Tong, X.; Yang, F. Hydrogels with Enhanced Protein Conjugation Efficiency Reveal Stiffness-Induced YAP Localization in Stem Cells Depends on Biochemical Cues. Biomaterials 2019, 202, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, Q.; Liu, Y.; Chu, Z.; Yu, L.; Hou, Y.; Kang, H.; Wei, Q.; Zhao, W.; Spatz, J.P.; et al. Controllable Ligand Spacing Stimulates Cellular Mechanotransduction and Promotes Stem Cell Osteogenic Differentiation on Soft Hydrogels. Biomaterials 2021, 268, 120543. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S. Role of YAP/TAZ in Cell-Matrix Adhesion-Mediated Signalling and Mechanotransduction. Exp. Cell Res. 2016, 343, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Zaltsman, Y.; Masuko, S.; Bensen, J.J.; Kiessling, L.L. Angiomotin Regulates YAP Localization during Neural Differentiation of Human Pluripotent Stem Cells. Stem Cell Rep. 2019, 12, 869–877. [Google Scholar] [CrossRef]
- Kang, P.H.; Schaffer, D.V.; Kumar, S. Angiomotin Links ROCK and YAP Signaling in Mechanosensitive Differentiation of Neural Stem Cells. Mol. Biol. Cell 2020, 31, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Musah, S.; Wrighton, P.J.; Zaltsman, Y.; Zhong, X.; Zorn, S.; Parlato, M.B.; Hsiao, C.; Palecek, S.P.; Chang, Q.; Murphy, W.L.; et al. Substratum-Induced Differentiation of Human Pluripotent Stem Cells Reveals the Coactivator YAP Is a Potent Regulator of Neuronal Specification. Proc. Natl. Acad. Sci. USA 2014, 111, 13805–13810. [Google Scholar] [CrossRef] [PubMed]
- Irioda, A.C.; Cassilha, R.; Zocche, L.; Francisco, J.C.; Cunha, R.C.; Ferreira, P.E.; Guarita-Souza, L.C.; Ferreira, R.J.; Mogharbel, B.F.; Garikipati, V.N.S.; et al. Human Adipose-Derived Mesenchymal Stem Cells Cryopreservation and Thawing Decrease A4-Integrin Expression. Stem Cells Int. 2016, 2016, e2562718. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-Derived Stem Cells: Isolation, Expansion and Differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef]
- Carvalho, K.A.T.; Simeoni, R.B.; Guarita-Souza, L.C.; Francisco, J.C.; Abdelwahid, E.; Myiague, N.I.; Chachques, J.C.; Rivetti, L.A.; Oliveira, L.; Malvezzi, M.; et al. Angiogenesis without Functional Outcome after Mononuclear Stem Cell Transplant in a Doxorubicin-Induced Dilated Myocardiopathy Murine Model. Int. J. Artif. Organs 2008, 31, 431–438. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Stricker, P.E.F.; de Souza Dobuchak, D.; Irioda, A.C.; Mogharbel, B.F.; Franco, C.R.C.; de Souza Almeida Leite, J.R.; de Araújo, A.R.; Borges, F.A.; Herculano, R.D.; de Oliveira Graeff, C.F.; et al. Human Mesenchymal Stem Cells Seeded on the Natural Membrane to Neurospheres for Cholinergic-like Neurons. Membranes 2021, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Trzaska, K.A.; Rameshwar, P. Dopaminergic Neuronal Differentiation Protocol for Human Mesenchymal Stem Cells. Methods Mol. Biol. 2011, 698, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The Development of Fibroblast Colonies in Monolayer Cultures of Guinea-Pig Bone Marrow and Spleen Cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal Stem Cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Miana, V.V.; González, E.A.P. Adipose Tissue Stem Cells in Regenerative Medicine. Ecancermedicalscience 2018, 12, 822. [Google Scholar] [CrossRef] [PubMed]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal Cells from the Adipose Tissue-Derived Stromal Vascular Fraction and Culture Expanded Adipose Tissue-Derived Stromal/Stem Cells: A Joint Statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Stanton, A.E.; Tong, X.; Yang, F. Extracellular Matrix Type Modulates Mechanotransduction of Stem Cells. Acta Biomater. 2019, 96, 310–320. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. The Impact of Extracellular Matrix Viscoelasticity on Cellular Behavior. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Kassianidou, E.; Kalita, J.; Lim, R.Y.H. The Role of Nucleocytoplasmic Transport in Mechanotransduction. Exp. Cell Res. 2019, 377, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, I.; McCollum, D. Control of Cellular Responses to Mechanical Cues through YAP/TAZ Regulation. J. Biol. Chem. 2019, 294, 17693–17706. [Google Scholar] [CrossRef]
- Chen, T.-J.; Wu, C.-C.; Tang, M.-J.; Huang, J.-S.; Su, F.-C. Complexity of the Tensegrity Structure for Dynamic Energy and Force Distribution of Cytoskeleton during Cell Spreading. PLoS ONE 2010, 5, e14392. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.A.; Mullins, R.D. Cell Mechanics and the Cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kumar, A.; Makhija, E.; Shivashankar, G.V. The Regulation of Dynamic Mechanical Coupling between Actin Cytoskeleton and Nucleus by Matrix Geometry. Biomaterials 2014, 35, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Shiu, J.-Y.; Aires, L.; Lin, Z.; Vogel, V. Nanopillar Force Measurements Reveal Actin-Cap-Mediated YAP Mechanotransduction. Nat. Cell Biol. 2018, 20, 262–271. [Google Scholar] [CrossRef]
- Chan, S.W.; Lim, C.J.; Chong, Y.F.; Pobbati, A.V.; Huang, C.; Hong, W. Hippo Pathway-Independent Restriction of TAZ and YAP by Angiomotin. J. Biol. Chem. 2011, 286, 7018–7026. [Google Scholar] [CrossRef] [PubMed]
- Ernkvist, M.; Birot, O.; Sinha, I.; Veitonmaki, N.; Nyström, S.; Aase, K.; Holmgren, L. Differential Roles of P80- and P130-Angiomotin in the Switch between Migration and Stabilization of Endothelial Cells. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2008, 1783, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Borkowska, P.; Fila-Danilow, A.; Paul-Samojedny, M.; Kowalczyk, M.; Hart, J.; Ryszawy, J.; Kowalski, J. Differentiation of Adult Rat Mesenchymal Stem Cells to GABAergic, Dopaminergic and Cholinergic Neurons. Pharmacol. Rep. 2015, 67, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-T.; Liu, Z.-L.; Yao, X.-Q.; Yang, Z.-J.; Xu, R.-X. Neural Differentiation Ability of Mesenchymal Stromal Cells from Bone Marrow and Adipose Tissue: A Comparative Study. Cytotherapy 2012, 14, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Jahan-Abad, A.J.; Morteza-Zadeh, P.; Sahab Negah, S.; Gorji, A. Curcumin Attenuates Harmful Effects of Arsenic on Neural Stem/Progenitor Cells. Avicenna J. Phytomed. 2017, 7, 376–388. [Google Scholar]
- Peng, C.; Lu, L.; Li, Y.; Hu, J. Neurospheres Induced from Human Adipose-Derived Stem Cells as a New Source of Neural Progenitor Cells. Cell Transplant. 2019, 28, 66S–75S. [Google Scholar] [CrossRef]
- Foudah, D.; Monfrini, M.; Donzelli, E.; Niada, S.; Brini, A.T.; Orciani, M.; Tredici, G.; Miloso, M. Expression of Neural Markers by Undifferentiated Mesenchymal-like Stem Cells from Different Sources. J. Immunol. Res. 2014, 2014, 987678. [Google Scholar] [CrossRef] [PubMed]
- Caillet-Boudin, M.-L.; Buée, L.; Sergeant, N.; Lefebvre, B. Regulation of Human MAPT Gene Expression. Mol. Neurodegener. 2015, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects. Front. Aging Neurosci. 2019, 11, 204. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, C.; Liu, F.; Lin, H.; Yang, X.; Zhang, Z. Comparison of the Neuronal Differentiation Abilities of Bone Marrow-derived and Adipose Tissue-derived Mesenchymal Stem Cells. Mol. Med. Rep. 2017, 16, 3877–3886. [Google Scholar] [CrossRef] [PubMed][Green Version]


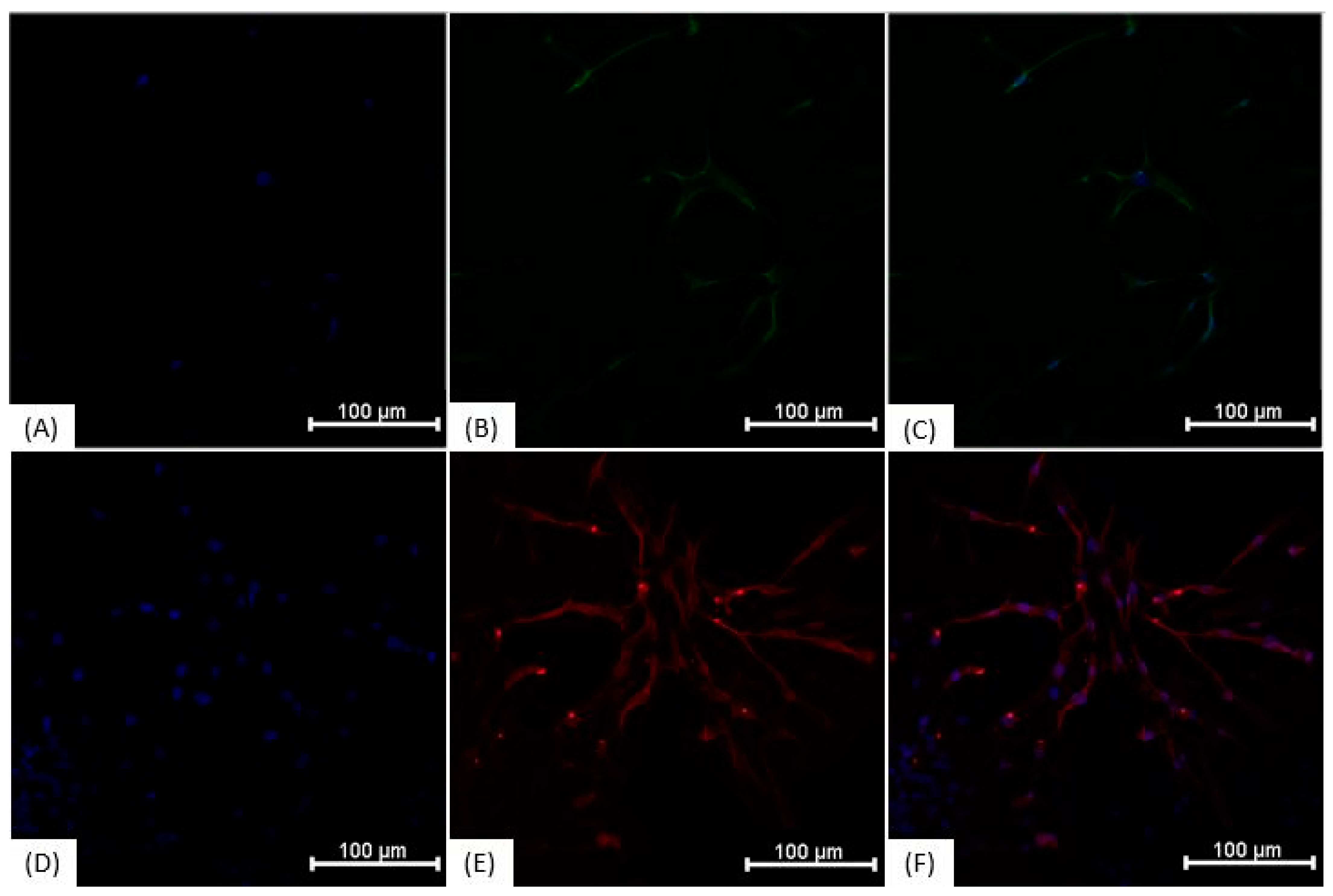

| Antigen | Clone | Fluorochrome |
|---|---|---|
| CD13 | SJ1D1 | PE |
| CD34 | 581 | PE-CY7 |
| CD45 | 2D1 | APC-CY7 |
| CD73 | AD2 | APC |
| CD90 | 5E10 | FITC |
| CD105 | 266 | PE |
| HLA-DR | L243 | FITC |
| HLA-ABC | G46-2.6 | APC |
| Antibody | Dilution | Code |
|---|---|---|
| Nestin | 1:300 | S1409 |
| 𝛽-III tubulin | 4 µg/mL | T2200 |
| GFAP | 1:300 | G3893 |
| NeuN | 1:50 | SAB4300883 |
| YAP1 | 20 µg/mL | WH0010413M1 |
| AMOT | 2 µg/mL | HPA067853 |
| Anti-rabbit secondary antibody with FITC | 10 μg/mL | F7512 |
| Anti-mouse secondary antibody with Cy5 | 10 μg/mL | A10524 |
| Gene | Forward (5′-3′) | Reverse (5′-3′) | AT (°C) 1 | MW (bp) 2 |
|---|---|---|---|---|
| OCT4 | AGCCCTCATTTCACCAGGCC | CCCCCACAGAACTCATACGG | 62 | 292 |
| NANOG | TCCAGGATTTTAACGTTCTGCT | TTCTTGCATCTGCTGGAGGC | 60 | 578 |
| ACTB | CTGGGACGACATGGAGAAAA | AAGGAAGGCTGGAAGAGTGC | 56 | 564 |
| FGF2 | TGCTGGTGATGGGAGTTGTA | CCTCCAAGTAGCAGCCAAAG | 60 | 482 |
| REX1 | CTGAAGAAACGGGCAAAGAC | GAACATTCAAGGGAGCTTGC | 60 | 344 |
| VEGF | AGCCTTGTTCAGAGCGGAGAA | TAACTCAAGCTGCCTCGCCTT | 60 | 107 |
| ABCG2 | GTCTAAGCAGGGACGAACAATC | GGCTCTATGATCTCTGTGGCTT | 62 | 270 |
| TUBB3 | GGAGATCGTGCACATCCAGG | CAGGCAGTCGCAGTTTTCAC | 62 | 385 |
| SOX2 | ACACCAATCCCATCCACACT | GCAAACTTCCTGCAAAGCTC | 62 | 224 |
| MAPT | GGCTACACCATGCACCAAGA | CCTTCTGGGATCTCCGTGTG | 62 | 218 |
| NCAM | CCTGAAGCCCGAAACAAC | TTTCCATCCTCTCCCATCT | 58 | 150 |
| OTX2 | CTCTGAACCTGTCCACCC | AGCAAGTCCATACCCGAA | 60 | 163 |
| NEF-H | GACATTGCCTCCTACCAG | AAGCCAATCCGACACTCT | 58 | 179 |
| GFAP | CTCACCAAATTCCACCCGCA | ACCGCACACAGTACCTGAAG | 60 | 769 |
| MAP2 | GCTAAATCGTAAGTGAGGGCTG | TGGCTCTCTGGCTCTCTAGC | 60 | 241 |
| NEFM | ACATCGAGAGCGCCACAA | GACGAGCCATTTCCCACTTTG | 60 | 98 |
| SLC1A3 | GGCGGCGCTAGATAGTAAGG | TTCCTTTGTGCCCTTCCCAG | 62 | 136 |
| S100 | GGAGACAAGCACAAGCTGAA | CTTGCATGACCGTCTCTGTT | 58 | 261 |
| ASCL1 | CGAACTGATGCGCTGCAAA | TGACCAACTTGACGCGGTT | 58 | 122 |
| HOXC5 | TCAAAGAGTCACAAATCACCC | ATCCATAGTTCCCACAAGTT | 58 | 149 |
| FABP7 | GTGGTCATCAGGACTCTCAGC | CGAACAGCAACCACATCACC | 62 | 233 |
| MSI2 | CCCCAAAGTTGCATTTCCTCG | TTCGCAGATAACCCGCCTAC | 60 | 84 |
| BMI1 | CGCTTGGCTCGCATTC | AGCTCAGTGATCTTGATTCTCGTTG | 62 | 148 |
| NES | AACAGCGACGGAGGTCTCTA | TTCTCTTGTCCCGCAGACTT | 58 | 220 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, N.B.; Irioda, A.C.; Stricker, P.E.F.; Mogharbel, B.F.; da Rosa, N.N.; Dziedzic, D.S.M.; de Carvalho, K.A.T. Natural Membrane Differentiates Human Adipose-Derived Mesenchymal Stem Cells to Neurospheres by Mechanotransduction Related to YAP and AMOT Proteins. Membranes 2021, 11, 687. https://doi.org/10.3390/membranes11090687
de Oliveira NB, Irioda AC, Stricker PEF, Mogharbel BF, da Rosa NN, Dziedzic DSM, de Carvalho KAT. Natural Membrane Differentiates Human Adipose-Derived Mesenchymal Stem Cells to Neurospheres by Mechanotransduction Related to YAP and AMOT Proteins. Membranes. 2021; 11(9):687. https://doi.org/10.3390/membranes11090687
Chicago/Turabian Stylede Oliveira, Nathalia Barth, Ana Carolina Irioda, Priscila Elias Ferreira Stricker, Bassam Felipe Mogharbel, Nádia Nascimento da Rosa, Dilcele Silva Moreira Dziedzic, and Katherine Athayde Teixeira de Carvalho. 2021. "Natural Membrane Differentiates Human Adipose-Derived Mesenchymal Stem Cells to Neurospheres by Mechanotransduction Related to YAP and AMOT Proteins" Membranes 11, no. 9: 687. https://doi.org/10.3390/membranes11090687
APA Stylede Oliveira, N. B., Irioda, A. C., Stricker, P. E. F., Mogharbel, B. F., da Rosa, N. N., Dziedzic, D. S. M., & de Carvalho, K. A. T. (2021). Natural Membrane Differentiates Human Adipose-Derived Mesenchymal Stem Cells to Neurospheres by Mechanotransduction Related to YAP and AMOT Proteins. Membranes, 11(9), 687. https://doi.org/10.3390/membranes11090687