Towards Biohybrid Lung Development—Fibronectin-Coating Bestows Hemocompatibility of Gas Exchange Hollow Fiber Membranes by Improving Flow-Resistant Endothelialization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Endothelial Cell Isolation and Characterization
2.2. Hollow Fiber Membrane Preparation, Coating Assessment and Endothelialization
2.3. Qualitative and Quantitative Analysis of the Endothelial Monolayer on HFM
2.3.1. Cell Counting of Adherent ECs on HFM
2.3.2. Cell Tracking Dye and Cell Viability Staining
2.3.3. Immunofluorescence Detection of Endothelial-Specific Cell Junction Protein VE-Cadherin
2.3.4. Visualization of Extracellular Matrix Protein Collagen Type-IV
2.4. Gene Expression Analysis by Realtime qRT-PCR
2.5. Functional Leukocyte Adhesion Assay
2.5.1. Qualitative Analysis of Adherent Leukocytes by Fluorescence Microscopy
2.5.2. Qualitative Analysis by Scanning Electron Microscopy
2.6. Functional Thrombocyte Adhesion Assay
2.6.1. Qualitative Analysis by Fluorescence Microscopy
2.6.2. Qualitative Analysis by Scanning Electron Microscopy
2.6.3. Quantitative Analysis by Sudan Black B Staining
2.7. Flow Exposure of Endothelialized HFM
2.8. Statistics
3. Results
3.1. Effective FN Coating of HFM Facilitates the Formation of a Viable and Confluent Endothelial Monolayer
3.2. EC Monolayer on FN Coated HFM Shows Physiological and Hemocomatible Behavior
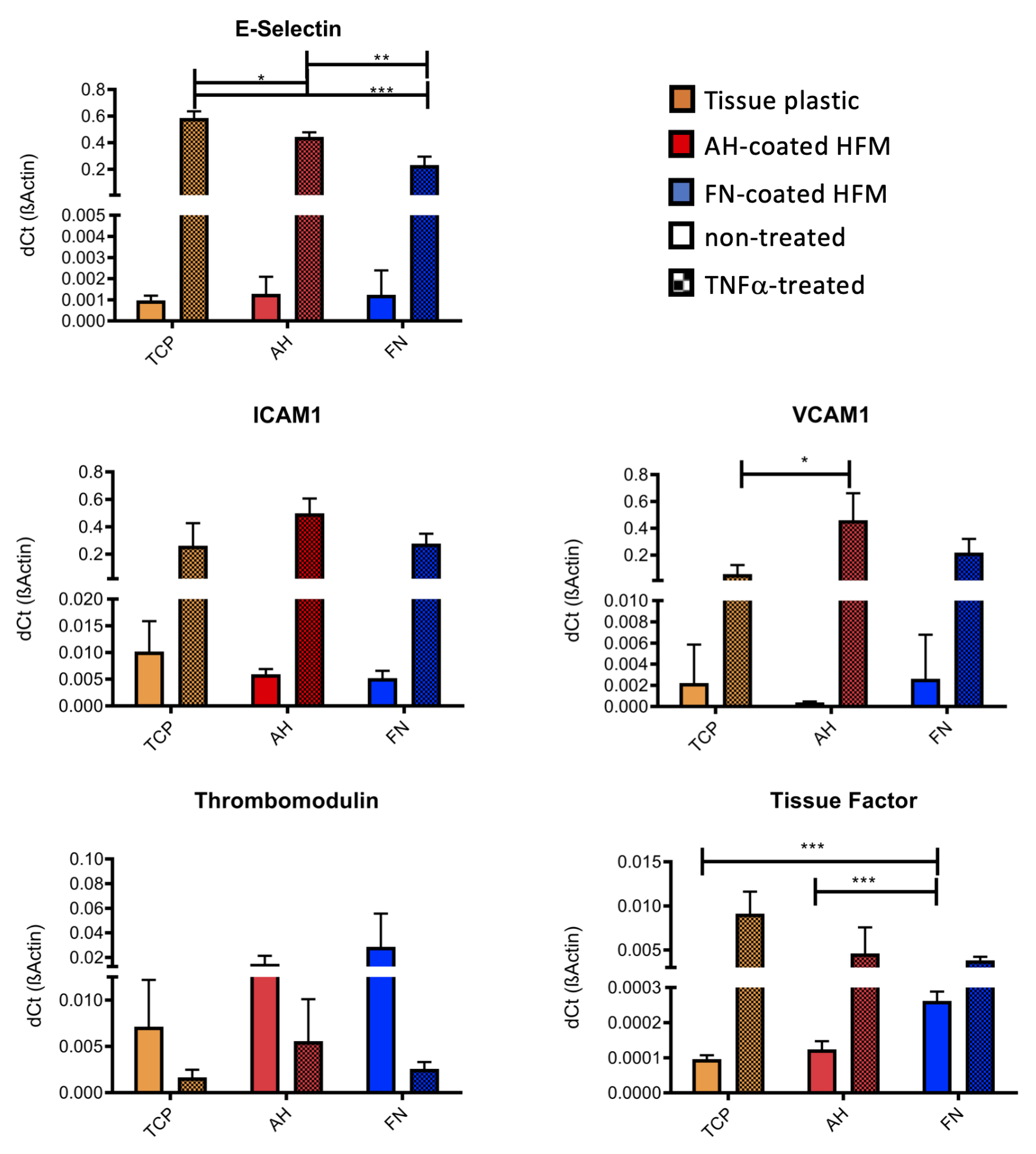
3.2.1. qRT-PCR Confirms Anti-Thrombogenic and Non-Inflammatory Endothelial Status
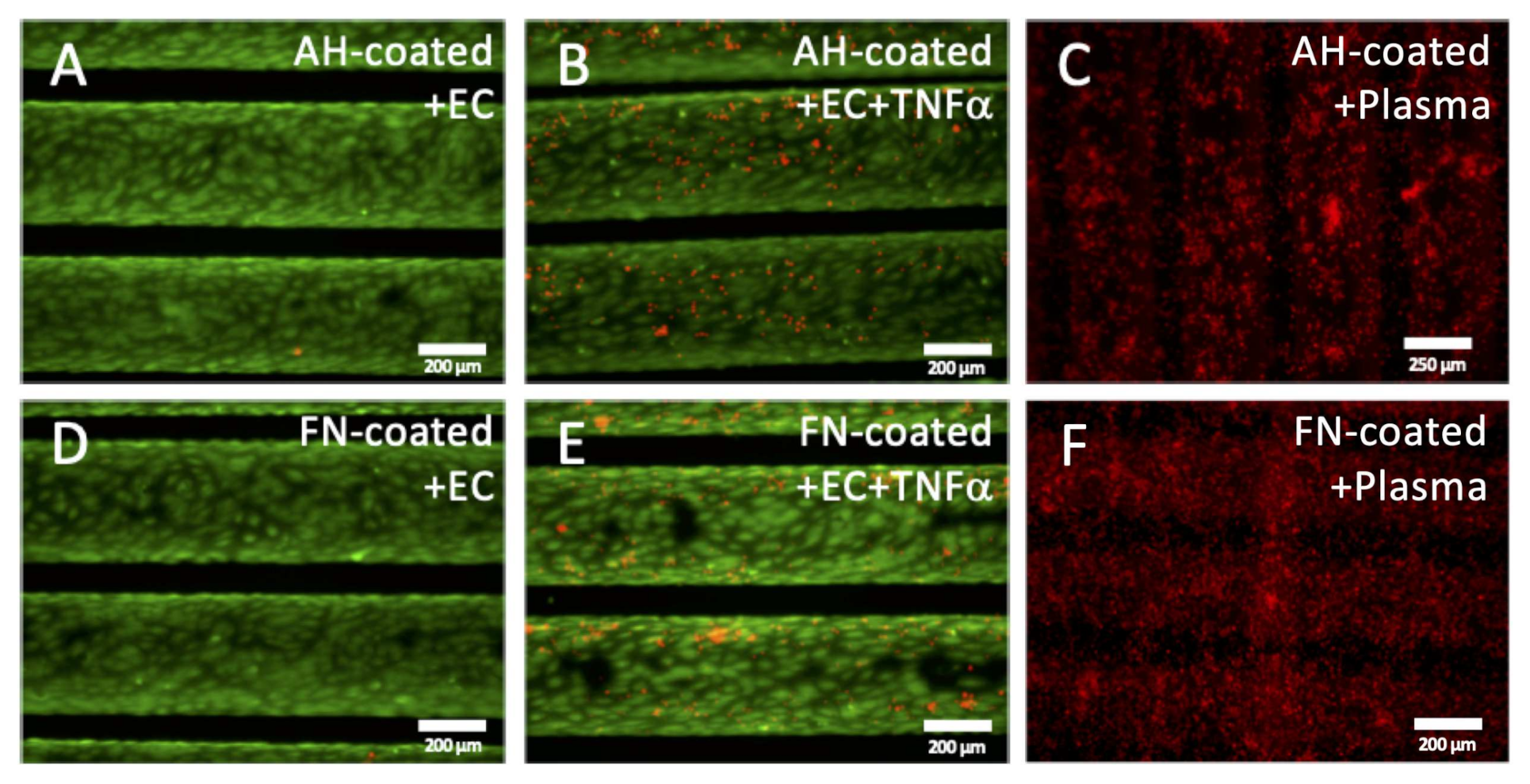
3.2.2. Functional Leukocyte Adhesion Assay Approved Non-Inflammatory Endothelial Status
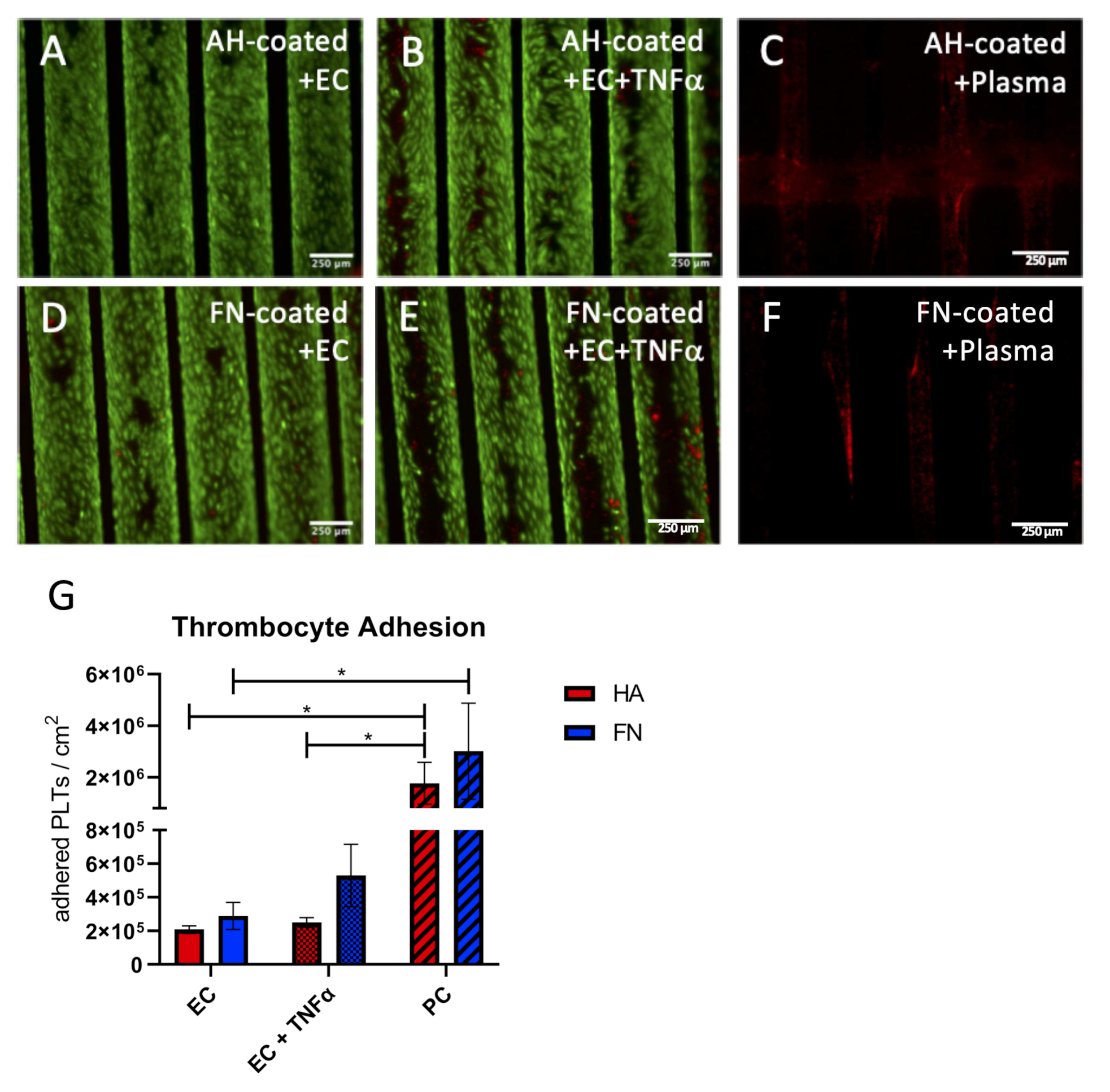
3.2.3. Functional Thrombocyte Adhesion Assay Approves Anti-Thrombogenic Endothelial Status
3.3. Endothelial Monolayer on FN Coated HFM Physiologically Respond and Resist to Clinically Relevant Flow Conditions
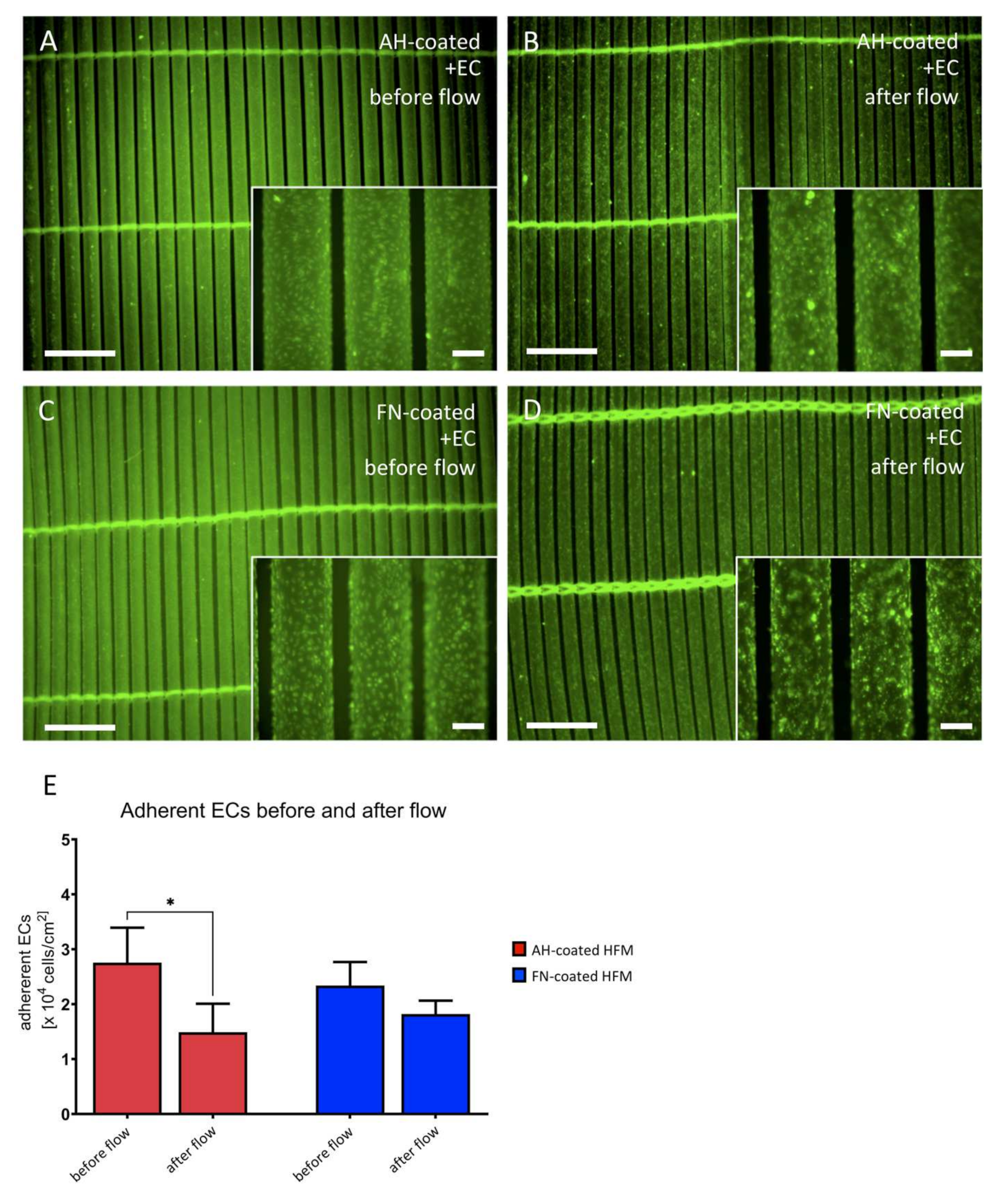
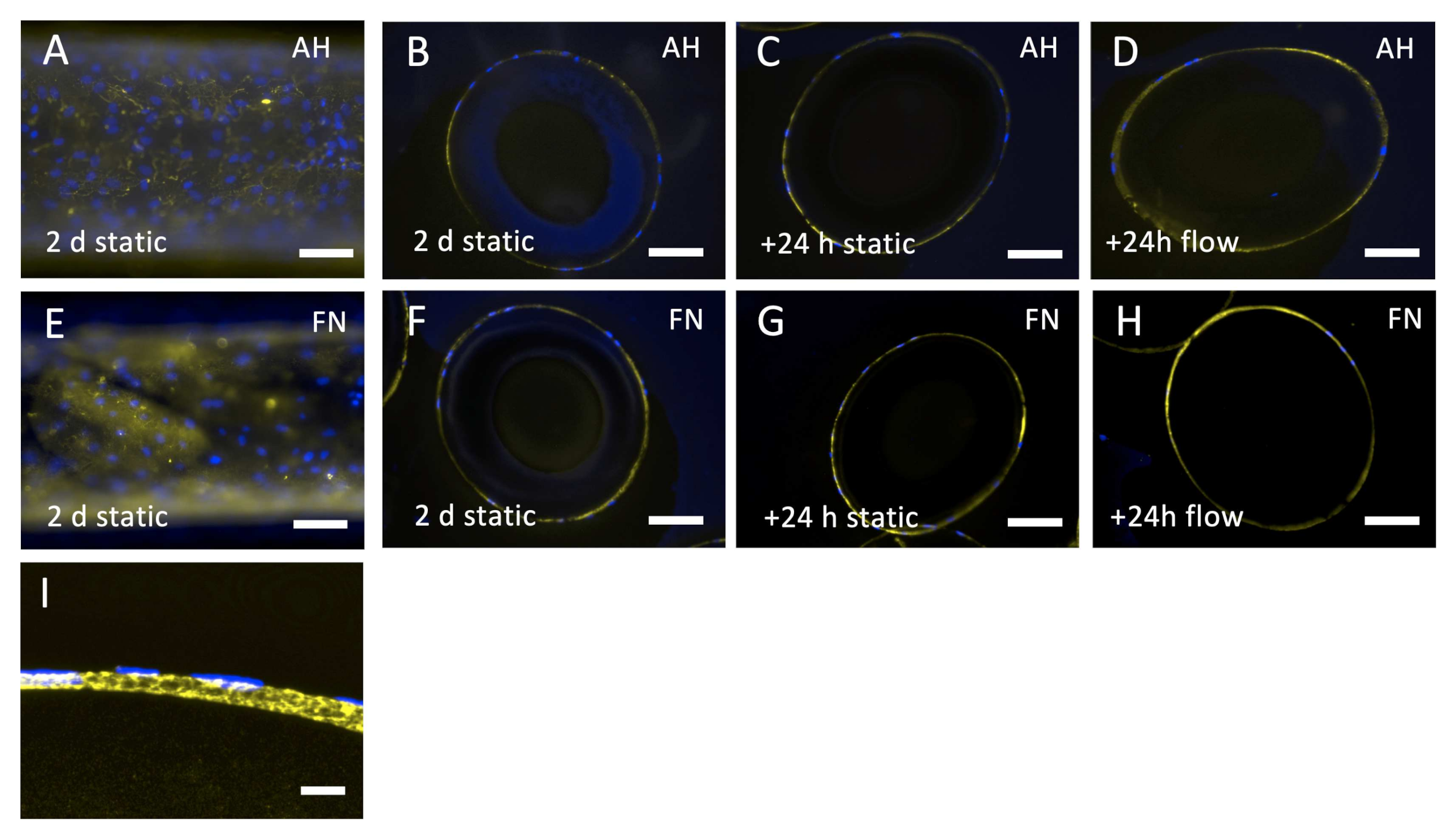
3.3.1. Endothelial Monolayer Resists the Applied Flow Conditions for 24 h under Stable Arterial Conditions
3.3.2. Endothelial Monolayer Preserves Non-Activated Status and Expresses Extracellular Matrix Proteins
3.3.3. Immunofluorescence Stainings Indicate Extracellular Matrix Protein Collagen Type-IV Synthesis by Endothelial Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AH | albumin/heparin; |
| BHL | biohybrid lung |
| DMSO | dimethyl sulfoxide |
| EC | endothelial cell |
| ECMO | extracorporeal membrane oxygenation |
| EGM-2 | endothelial growth medium-2 |
| ELD | endstage lung disease; |
| FN | fibronectin; |
| HFM | hollow fiber membranes; |
| LTx | lung transplantation |
| NC | negative control |
| PC | positive control |
| PDMS | polydimethylsiloxane |
| PMP | polymethylpentene |
| SBB | Sudan black B |
| SEM | scanning electron microscopy |
| TCP | tissue culture plastic |
| TNFα | tumor necrosis factor alpha |
References
- Leard, L.E.; Holm, A.M.; Valapour, M.; Glanville, A.R.; Attawar, S.; Aversa, M.; Campos, S.V.; Christon, L.M.; Cypel, M.; Dellgren, G.; et al. Consensus Document for the Selection of Lung Transplant Candidates: An Update from the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2021, 40, 1349–1379. [Google Scholar] [CrossRef]
- Biscotti, M.; Gannon, W.D.; Agerstrand, C.; Abrams, D.; Sonett, J.; Brodie, D.; Bacchetta, M. Awake Extracorporeal Membrane Oxygenation as Bridge to Lung Transplantation: A 9-Year Experience. Ann. Thorac. Surg. 2017, 104, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-Associated Thrombosis: Roles of Coagulation Factors, Complement, Platelets and Leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Kostousov, V.; Teruya, J. Bleeding and Thrombotic Complications in the Use of Extracorporeal Membrane Oxygenation. Semin. Thromb. Hemost. 2017, 44, 020–029. [Google Scholar] [CrossRef] [PubMed]
- Lubnow, M.; Schäfer, A.; Philipp, A.; Foltan, M.; Enger, T.B.; Lunz, D.; Bein, T.; Haneya, A.; Schmid, C.; Riegger, G.; et al. Technical Complications during Veno-Venous Extracorporeal Membrane Oxygenation and Their Relevance Predicting a System-Exchange—Retrospective Analysis of 265 Cases. PLoS ONE 2014, 9, e112316. [Google Scholar]
- He, T.; He, J.; Wang, Z.; Cui, Z. Modification Strategies to Improve the Membrane Hemocompatibility in Extracorporeal Membrane Oxygenator (ECMO). Adv. Compos. Hybrid Mater. 2021, 4, 847–864. [Google Scholar] [CrossRef] [PubMed]
- Biran, R.; Pond, D. Heparin Coatings for Improving Blood Compatibility of Medical Devices. Adv. Drug Deliv. Rev. 2017, 112, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, F.; Rogers, J.G. Left Ventricular Assist Device Therapy in Advanced Heart Failure: Patient Selection and Outcomes. Eur. J. Heart Fail. 2017, 19, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, B.H.; Azimzadeh, A.M.; Schroeder, C.; Buddensick, T.; Zhang, T.; Laaris, A.; Cochrane, M.; Schuurman, H.; Sachs, D.H.; Allan, J.S.; et al. Absence of Gal Epitope Prolongs Survival of Swine Lungs in an Ex Vivo Model of Hyperacute Rejection. Xenotransplantation 2011, 18, 94–107. [Google Scholar] [CrossRef]
- Nichols, J.E.; Francesca, S.L.; Niles, J.A.; Vega, S.P.; Argueta, L.B.; Frank, L.; Christiani, D.C.; Pyles, R.B.; Himes, B.E.; Zhang, R.; et al. Production and Transplantation of Bioengineered Lung into a Large-Animal Model. Sci. Transl. Med. 2018, 10, eaao3926. [Google Scholar] [CrossRef] [Green Version]
- Gilpin, S.E.; Ott, H.C. Using Nature’s Platform to Engineer Bio-Artificial Lungs. Ann. Am. Thorac. Soc. 2015, 12, S45–S49. [Google Scholar] [CrossRef] [PubMed]
- Dabaghi, M.; Saraei, N.; Fusch, G.; Rochow, N.; Brash, J.L.; Fusch, C.; Selvaganapathy, P.R. An Ultra-Thin Highly Flexible Microfluidic Device for Blood Oxygenation. Lab Chip 2018, 18, 3780–3789. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular Networks and Functional Intravascular Topologies within Biocompatible Hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef]
- McGuigan, A.P.; Sefton, M.V. The Influence of Biomaterials on Endothelial Cell Thrombogenicity. Biomaterials 2007, 28, 2547–2571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiegmann, B.; Figueiredo, C.; Gras, C.; Pflaum, M.; Schmeckebier, S.; Korossis, S.; Haverich, A.; Blasczyk, R. Prevention of Rejection of Allogeneic Endothelial Cells in a Biohybrid Lung by Silencing HLA-Class I Expression. Biomaterials 2014, 35, 8123–8133. [Google Scholar] [CrossRef]
- Pflaum, M.; Dahlmann, J.; Engels, L.; Naghilouy-Hidaji, H.; Adam, D.; Zöllner, J.; Otto, A.; Schmeckebier, S.; Martin, U.; Haverich, A.; et al. Towards Biohybrid Lung: Induced Pluripotent Stem Cell Derived Endothelial Cells as Clinically Relevant Cell Source for Biologization. Micromachines 2021, 12, 981. [Google Scholar] [CrossRef]
- Pflaum, M.; Kühn-Kauffeldt, M.; Schmeckebier, S.; Dipresa, D.; Chauhan, K.; Wiegmann, B.; Haug, R.J.; Schein, J.; Haverich, A.; Korossis, S. Endothelialization and Characterization of Titanium Dioxide-Coated Gas-Exchange Membranes for Application in the Bioartificial Lung. Acta Biomater. 2017, 50, 510–521. [Google Scholar] [CrossRef]
- Hess, C.; Wiegmann, B.; Maurer, A.N.; Fischer, P.; Möller, L.; Martin, U.; Hilfiker, A.; Haverich, A.; Fischer, S. Reduced Thrombocyte Adhesion to Endothelialized Poly 4-Methyl-1-Pentene Gas Exchange Membranes—A First Step Toward Bioartificial Lung Development. Tissue Eng. Part A 2010, 16, 3043–3053. [Google Scholar] [CrossRef]
- Zwirner, U.; Höffler, K.; Pflaum, M.; Korossis, S.; Haverich, A.; Wiegmann, B. Identifying an Optimal Seeding Protocol and Endothelial Cell Substrate for Biohybrid Lung Development. J. Tissue Eng. Regen. Med. 2018, 12, 2319–2330. [Google Scholar] [CrossRef]
- Wiegmann, B.; von Seggern, H.; Höffler, K.; Korossis, S.; Dipresa, D.; Pflaum, M.; Schmeckebier, S.; Seume, J.; Haverich, A. Developing a Biohybrid Lung—Sufficient Endothelialization of Poly-4-Methly-1-Pentene Gas Exchange Hollow-Fiber Membranes. J. Mech. Behav. Biomed. Mater. 2016, 60, 301–311. [Google Scholar] [CrossRef]
- Möller, L.; Hess, C.; Paleček, J.; Su, Y.; Haverich, A.; Kirschning, A.; Dräger, G. Towards a Biocompatible Artificial Lung: Covalent Functionalization of Poly(4-Methylpent-1-Ene) (TPX) with CRGD Pentapeptide. Beilstein J. Org. Chem. 2013, 9, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Schlie, S.; Gruene, M.; Dittmar, H.; Chichkov, B.N. Dynamics of Cell Attachment: Adhesion Time and Force. Tissue Eng. Part C Methods 2012, 18, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Maubant, S.; Saint-Dizier, D.; Boutillon, M.; Perron-Sierra, F.; Casara, P.J.; Hickman, J.A.; Tucker, G.C.; Obberghen-Schilling, E.V. Blockade of Avβ3 and Avβ5 Integrins by RGD Mimetics Induces Anoikis and Not Integrin-Mediated Death in Human Endothelial Cells. Blood 2006, 108, 3035–3044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornelissen, C.G.; Dietrich, M.; Gromann, K.; Frese, J.; Krueger, S.; Sachweh, J.S.; Jockenhoevel, S. Fibronectin Coating of Oxygenator Membranes Enhances Endothelial Cell Attachment. Biomed. Eng. Online 2013, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Gulbins, H.; Pritisanac, A.; Petzold, R.; Goldemund, A.; Doser, M.; Dauner, M.; Meiser, B.; Reichart, B.; Daebritz, S. A Low-Flow Adaptation Phase Improves Shear-Stress Resistance of Artificially Seeded Endothelial Cells. Thorac. Cardiovasc. Surg. 2005, 53, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gao, X.; Pan, R.; Lu, D.; Dai, Y. A Simple Adhesion Assay for Studying Interactions between Platelets and Endothelial Cells in Vitro. Cytotechnology 2010, 62, 17–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaoing, L.R.; Post, A.D.; Lin, A.Y.; Tseng, H.; Moake, J.L.; Grande-Allen, K.J. Laminin Peptide-Immobilized Hydrogels Modulate Valve Endothelial Cell Hemostatic Regulation. PLoS ONE 2015, 10, e0130749. [Google Scholar] [CrossRef] [Green Version]
- Schumer, E.; Höffler, K.; Kuehn, C.; Slaughter, M.; Haverich, A.; Wiegmann, B. In-Vitro Evaluation of Limitations and Possibilities for the Future Use of Intracorporeal Gas Exchangers Placed in the Upper Lobe Position. J. Artif. Organs 2018, 21, 68–75. [Google Scholar] [CrossRef]
- Anastasiou, G.; Gialeraki, A.; Merkouri, E.; Politou, M.; Travlou, A. Thrombomodulin as a Regulator of the Anticoagulant Pathway. Blood Coagul. Fibrinol. 2012, 23, 1–10. [Google Scholar] [CrossRef]
- Kanie, K.; Narita, Y.; Zhao, Y.; Kuwabara, F.; Satake, M.; Honda, S.; Kaneko, H.; Yoshioka, T.; Okochi, M.; Honda, H.; et al. Collagen Type IV-specific Tripeptides for Selective Adhesion of Endothelial and Smooth Muscle Cells. Biotechnol. Bioeng. 2012, 109, 1808–1816. [Google Scholar] [CrossRef]
- Hess, C.; Schwenke, A.; Wagener, P.; Franzka, S.; Sajti, C.L.; Pflaum, M.; Wiegmann, B.; Haverich, A.; Barcikowski, S. Dose-Dependent Surface Endothelialization and Biocompatibility of Polyurethane Noble Metal Nanocomposites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 102, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Linder, E.; Stenman, S.; Lehto, V.-P.; Vaheri, A. Distribution of Fibronectin in Human Tissues and Relationship to Other Connective Tissue Components. Ann. N. Y. Acad. Sci. 1978, 312, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.E.; Senger, D.R. Endothelial Extracellular Matrix. Circ. Res. 2005, 97, 1093–1107. [Google Scholar] [CrossRef] [Green Version]
- Pierschbacher, M.D.; Ruoslahti, E. Cell Attachment Activity of Fibronectin Can Be Duplicated by Small Synthetic Fragments of the Molecule. Nature 1984, 309, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef]
- Abbi, S.; Guan, J.L. Focal Adhesion Kinase: Protein Interactions and Cellular Functions. Histol. Histopathol. 2002, 17, 1163–1171. [Google Scholar] [PubMed]
- Burridge, K.; Chrzanowska-Wodnicka, M. Focal Adhesions, Contractility, and Signaling. Annu. Rev. Cell Dev. Biol. 1996, 12, 463–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daum, R.; Visser, D.; Wild, C.; Kutuzova, L.; Schneider, M.; Lorenz, G.; Weiss, M.; Hinderer, S.; Stock, U.A.; Seifert, M.; et al. Fibronectin Adsorption on Electrospun Synthetic Vascular Grafts Attracts Endothelial Progenitor Cells and Promotes Endothelialization in Dynamic In Vitro Culture. Cells 2020, 9, 778. [Google Scholar] [CrossRef] [Green Version]
- Budd, J.S.; Allen, K.E.; Bell, P.R.F.; James, R.F.L. The Effect of Varying Fibronectin Concentration on the Attachment of Endothelial Cells to Polytetrafluoroethylene Vascular Grafts. J. Vasc. Surg. 1990, 12, 126–130. [Google Scholar] [CrossRef] [Green Version]
- Aoki, J.; Serruys, P.W.; van Beusekom, H.; Ong, A.T.L.; McFadden, E.P.; Sianos, G.; van der Giessen, W.J.; Regar, E.; de Feyter, P.J.; Davis, H.R.; et al. Endothelial Progenitor Cell Capture by Stents Coated With Antibody Against CD34 The HEALING-FIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth-First In Man) Registry. J. Am. Coll. Cardiol. 2005, 45, 1574–1579. [Google Scholar] [CrossRef] [Green Version]
- Napp, L.C.; Kühn, C.; Hoeper, M.M.; Vogel-Claussen, J.; Haverich, A.; Schäfer, A.; Bauersachs, J. Cannulation Strategies for Percutaneous Extracorporeal Membrane Oxygenation in Adults. Clin. Res. Cardiol. 2016, 105, 283–296. [Google Scholar] [CrossRef] [Green Version]
- Duni, A.; Liakopoulos, V.; Koutlas, V.; Pappas, C.; Mitsis, M.; Dounousi, E. The Endothelial Glycocalyx as a Target of Ischemia and Reperfusion Injury in Kidney Transplantation—Where Have We Gone So Far? Int. J. Mol. Sci. 2021, 22, 2157. [Google Scholar] [CrossRef] [PubMed]
- Raffaeli, G.; Ghirardello, S.; Passera, S.; Mosca, F.; Cavallaro, G. Oxidative Stress and Neonatal Respiratory Extracorporeal Membrane Oxygenation. Front. Physiol. 2018, 9, 1739. [Google Scholar] [CrossRef] [PubMed]
- Pahakis, M.Y.; Kosky, J.R.; Dull, R.O.; Tarbell, J.M. The Role of Endothelial Glycocalyx Components in Mechanotransduction of Fluid Shear Stress. Biochem. Biophys. Res. Commun. 2007, 355, 228–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebong, E.E.; Macaluso, F.P.; Spray, D.C.; Tarbell, J.M. Imaging the Endothelial Glycocalyx In Vitro by Rapid Freezing/Freeze Substitution Transmission Electron Microscopy. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1908–1915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potter, D.R.; Damiano, E.R. The Hydrodynamically Relevant Endothelial Cell Glycocalyx Observed In Vivo Is Absent In Vitro. Circ. Res. 2008, 102, 770–776. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Kostidis, S.; Tiemeier, G.L.; Sol, W.M.P.J.; de Vries, M.R.; Giera, M.; Carmeliet, P.; van den Berg, B.M.; Rabelink, T.J. Shear Stress Regulation of Endothelial Glycocalyx Structure Is Determined by Glucobiosynthesis. Arterioscler. Thromb. Vasc. Biol. 2019, 40, 350–364. [Google Scholar] [CrossRef]
- Zeng, Y. Endothelial Glycocalyx as a Critical Signalling Platform Integrating the Extracellular Haemodynamic Forces and Chemical Signalling. J. Cell. Mol. Med. 2017, 21, 1457–1462. [Google Scholar] [CrossRef]
- Kraus, X.; Pflaum, M.; Thoms, S.; Jonczyk, R.; Witt, M.; Scheper, T.; Blume, C. A Pre-Conditioning Protocol of Peripheral Blood Derived Endothelial Colony Forming Cells for Endothelialization of Tissue Engineered Constructs. Microvasc. Res. 2021, 134, 104107. [Google Scholar] [CrossRef]
- Dekker, R.J.; van Soest, S.; Fontijn, R.D.; Salamanca, S.; de Groot, P.G.; VanBavel, E.; Pannekoek, H.; Horrevoets, A.J.G. Prolonged Fluid Shear Stress Induces a Distinct Set of Endothelial Cell Genes, Most Specifically Lung Krüppel-like Factor (KLF2). Blood 2002, 100, 1689–1698. [Google Scholar] [CrossRef]
- Meyns, B.; Vercaemst, L.; Vandezande, E.; Bollen, H.; Vlasselaers, D. Plasma Leakage of Oxygenators in ECMO Depends on the Type of Oxygenator and on Patient Variables. Int. J. Artif. Organs 2005, 28, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Yamane, T.; Mitsumata, M.; Yamaguchi, N.; Nakazawa, T.; Mochizuki, K.; Kondo, T.; Kawasaki, T.; Murata, S.; Yoshida, Y.; Katoh, R. Laminar High Shear Stress Up-Regulates Type IV Collagen Synthesis and down-Regulates MMP-2 Secretion in Endothelium. A Quantitative Analysis. Cell Tissue Res. 2010, 340, 471–479. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pflaum, M.; Jurmann, S.; Katsirntaki, K.; Mälzer, M.; Haverich, A.; Wiegmann, B. Towards Biohybrid Lung Development—Fibronectin-Coating Bestows Hemocompatibility of Gas Exchange Hollow Fiber Membranes by Improving Flow-Resistant Endothelialization. Membranes 2022, 12, 35. https://doi.org/10.3390/membranes12010035
Pflaum M, Jurmann S, Katsirntaki K, Mälzer M, Haverich A, Wiegmann B. Towards Biohybrid Lung Development—Fibronectin-Coating Bestows Hemocompatibility of Gas Exchange Hollow Fiber Membranes by Improving Flow-Resistant Endothelialization. Membranes. 2022; 12(1):35. https://doi.org/10.3390/membranes12010035
Chicago/Turabian StylePflaum, Michael, Sophie Jurmann, Katherina Katsirntaki, Marisa Mälzer, Axel Haverich, and Bettina Wiegmann. 2022. "Towards Biohybrid Lung Development—Fibronectin-Coating Bestows Hemocompatibility of Gas Exchange Hollow Fiber Membranes by Improving Flow-Resistant Endothelialization" Membranes 12, no. 1: 35. https://doi.org/10.3390/membranes12010035
APA StylePflaum, M., Jurmann, S., Katsirntaki, K., Mälzer, M., Haverich, A., & Wiegmann, B. (2022). Towards Biohybrid Lung Development—Fibronectin-Coating Bestows Hemocompatibility of Gas Exchange Hollow Fiber Membranes by Improving Flow-Resistant Endothelialization. Membranes, 12(1), 35. https://doi.org/10.3390/membranes12010035





