Nanocomposite Membranes Based on Fluoropolymers for Electrochemical Energy Sources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Transport Properties
2.4. Mechanical Properties
3. Results and Discussion
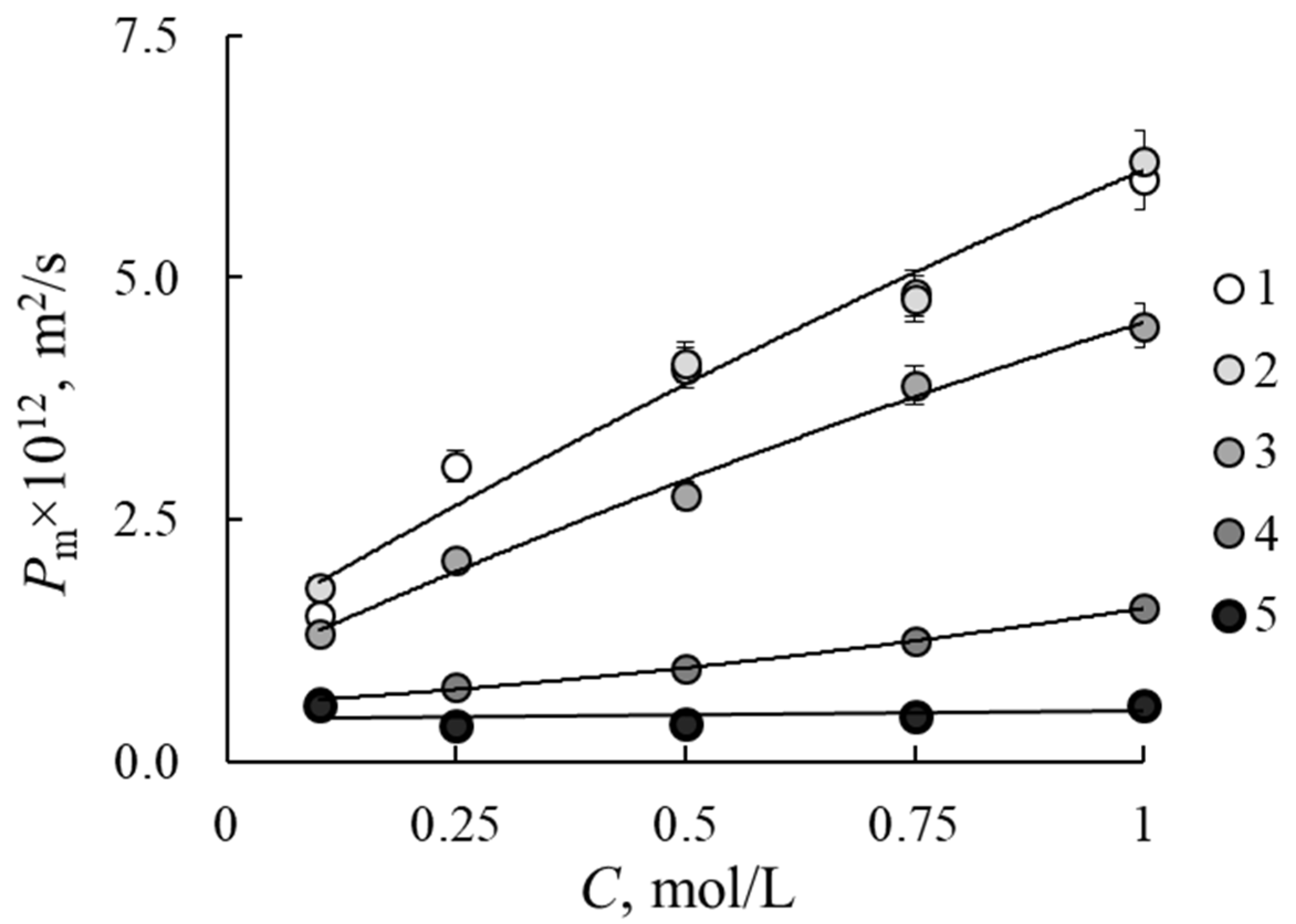
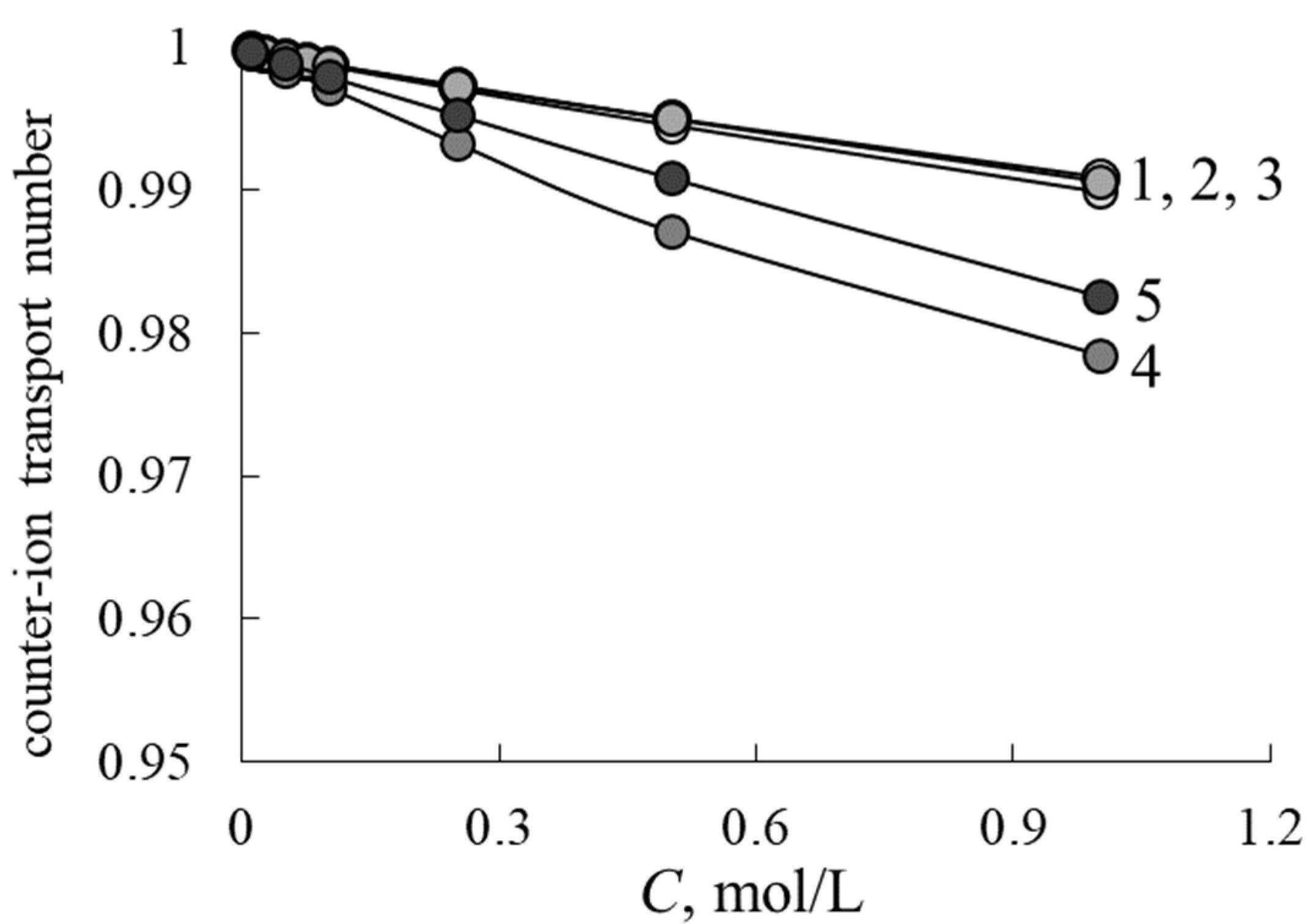
3.1. Diffusion Permeability
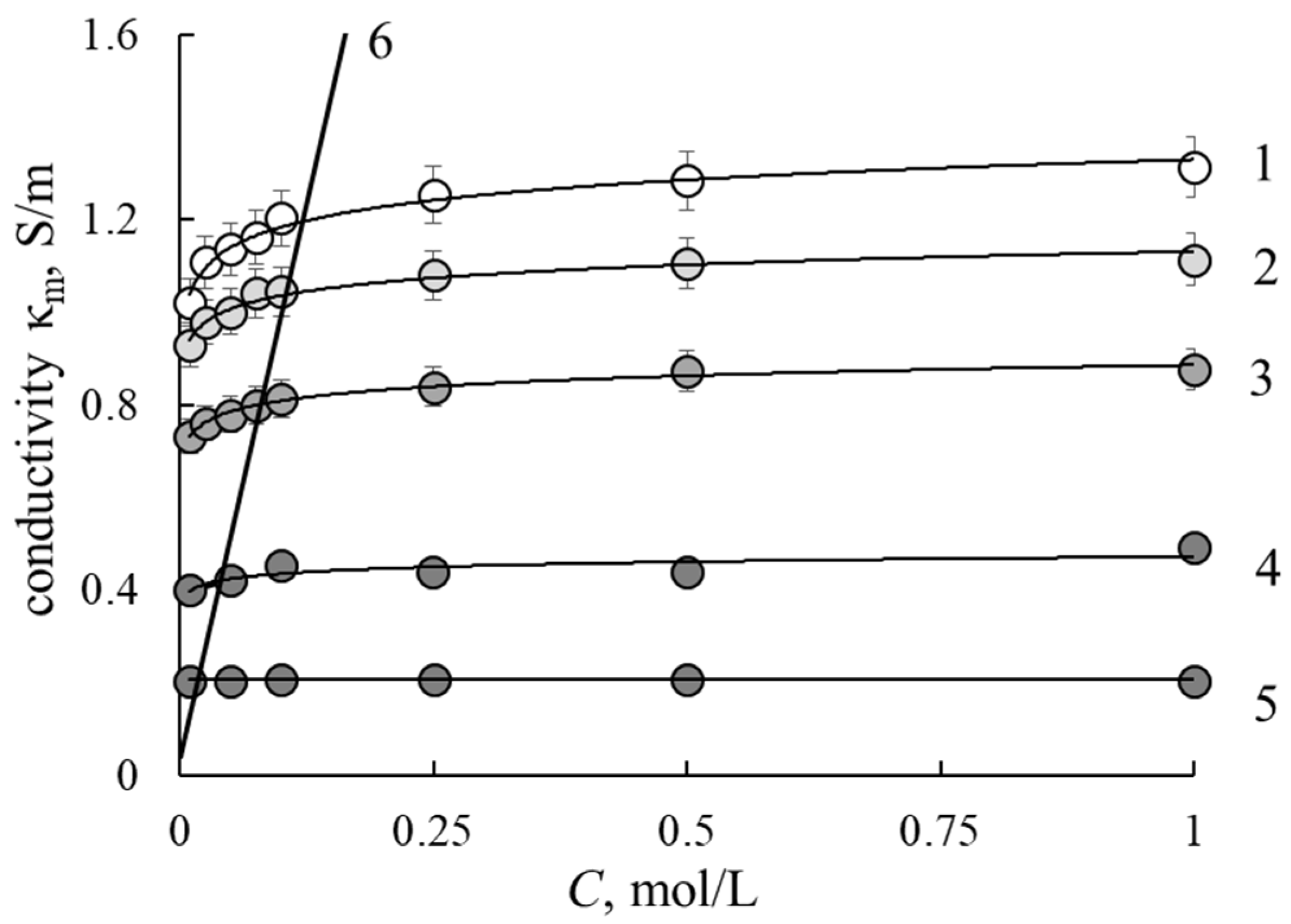
3.2. Conductivity
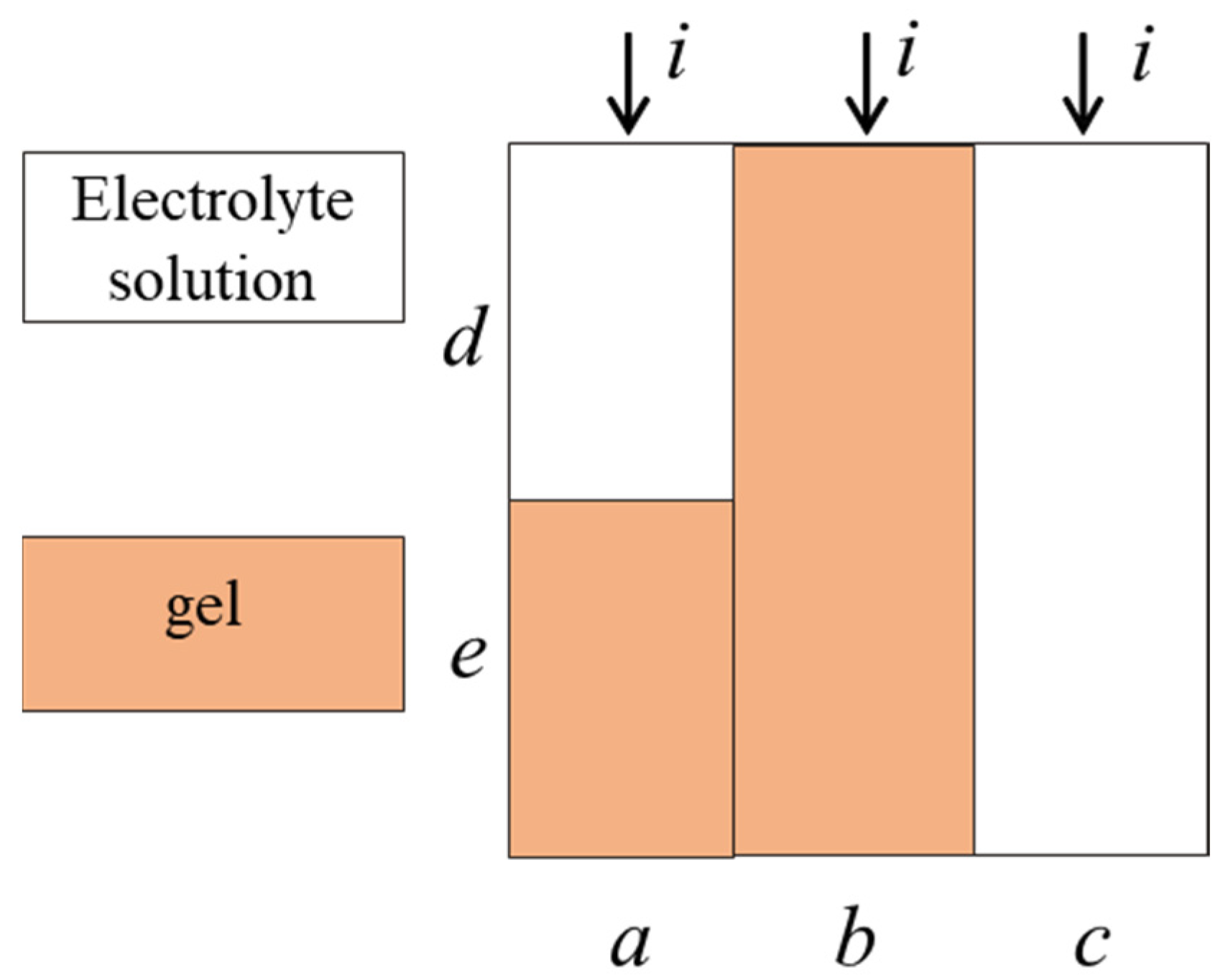
3.3. Parameters of Extended Three-Wire Model
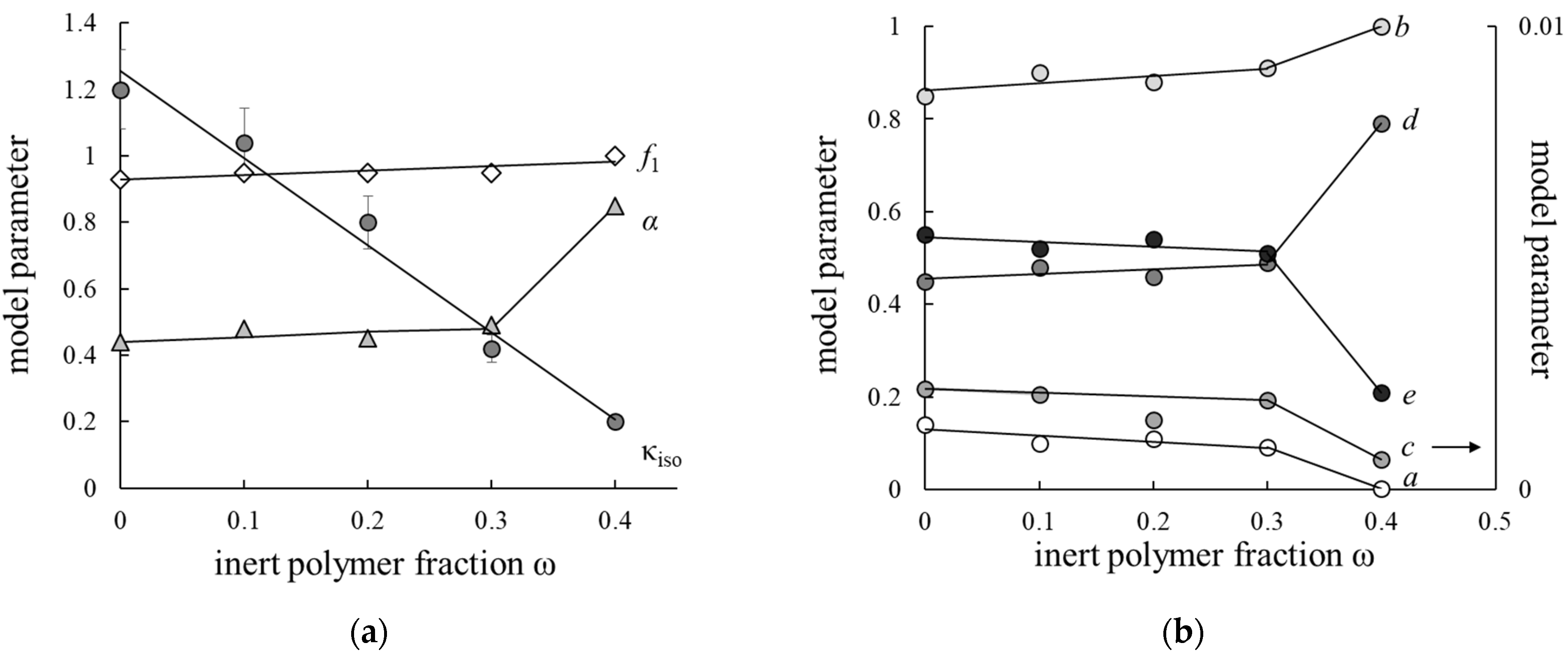
3.4. Microheterogeneous Model
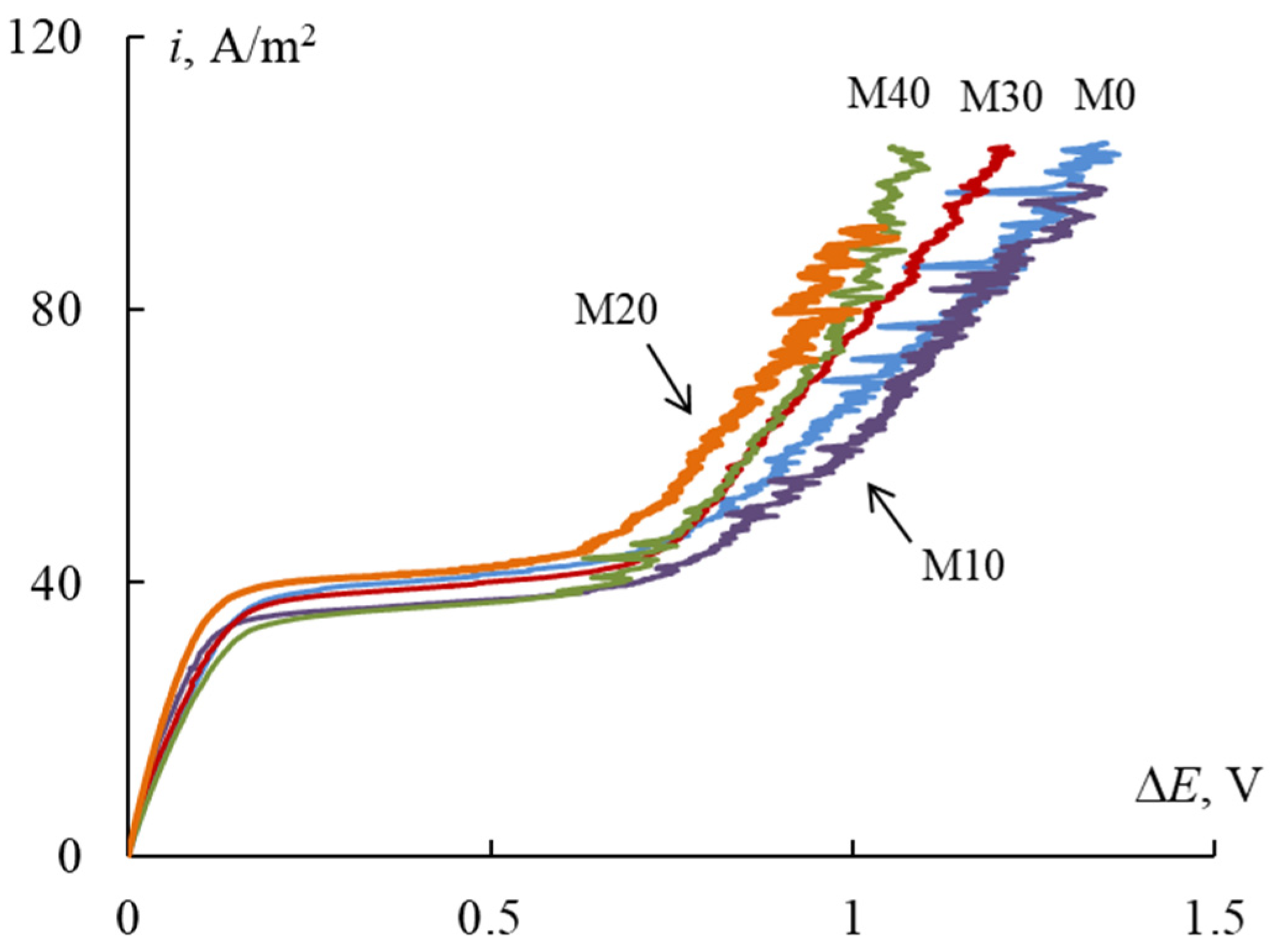
3.5. CV-Curves
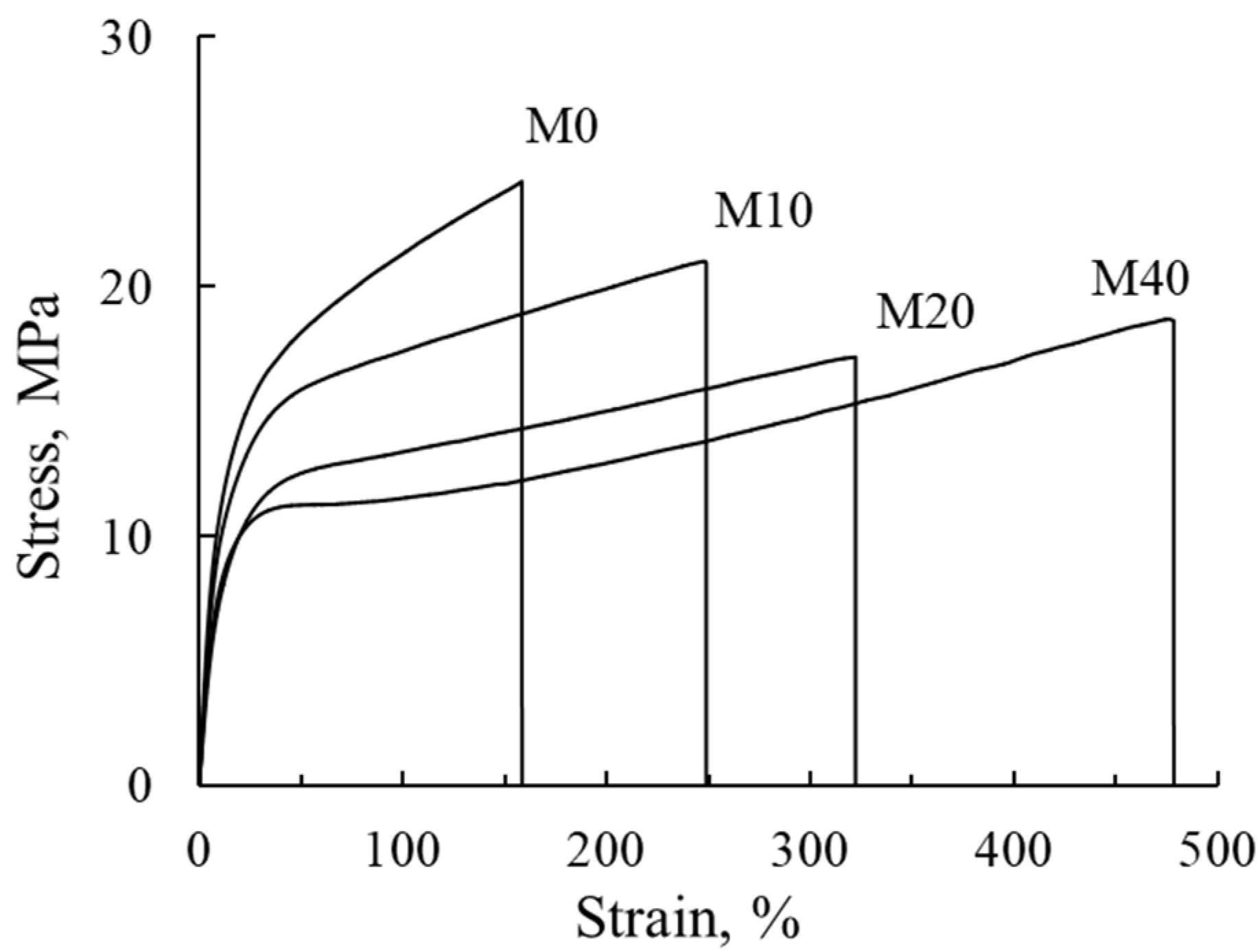
3.6. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, J.; Pan, Z.-Z.; Jin, Y.; Qiu, Q.; Zhang, C.; Zhao, Y.; Li, Y. Membranes in non-aqueous redox flow battery: A review. J. Power Source 2021, 500, 229983. [Google Scholar] [CrossRef]
- Petrov, M.M.; Modestov, A.D.; Konev, D.V.; Antipov, A.E.; Loktionov, P.A.; Pichugov, R.D.; Kartashova, N.V.; Glazkov, A.T.; Abunaeva, L.Z.; Andreev, V.N.; et al. Redox flow batteries: Role in modern electric power industry and com-parative characteristics of the main types. Russ. Chem. Rev. 2021, 90, 677–702. [Google Scholar] [CrossRef]
- Modestov, A.D.; Konev, D.V.; Tripachev, O.V.; Antipov, A.E.; Tolmachev, Y.V.; Vorotyntsev, M.A. A Hydrogen–bromate flow battery for air-deficient environments. Energy Technol. 2018, 6, 242–245. [Google Scholar] [CrossRef]
- Shi, Y.; Eze, C.; Xiong, B.; He, W.; Zhang, H.; Lim, T.M.; Ukil, A.; Zhao, J. Recent development of membrane for vanadium redox flow battery applications: A review. Appl. Energy 2019, 238, 202–224. [Google Scholar] [CrossRef]
- Tang, L.; Lu, W.; Zhang, H.; Li, X. Progress and perspective of the cathode materials towards bromine-based flow batteries. Energy Mater. Adv. 2022, 2022, 9850712. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3)x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Ng, W.W.; Thiam, H.S.; Pang, Y.L.; Chong, K.C.; Lai, S.O. A State-of-Art on the development of Nafion-based membrane for performance improvement in direct methanol fuel cells. Membranes 2022, 12, 506. [Google Scholar] [CrossRef]
- Li, Y.; Hui, J.; Kawchuk, J.; O’Brien, A.; Jiang, Z.; Hoorfar, M. Composite membranes of PVDF nanofibers impregnated with Nafion for increased fuel concentrations in direct methanol fuel cells. Fuel Cells 2019, 19, 43–50. [Google Scholar] [CrossRef]
- Choi, H.J.; Youn, C.; Kim, S.C.; Jeong, D.; Lim, S.N.; Chang, D.R.; Bae, J.W.; Park, J. Nafion/functionalized metal–organic framework composite membrane for vanadium redox flow battery. Microporous Mesoporous Mater. 2022, 341, 112054. [Google Scholar] [CrossRef]
- Rambabu, G.; Nagaraju, N.; Bhat, S.D. Functionalized fullerene embedded in Nafion matrix: A modified composite membrane electrolyte for direct methanol fuel cells. Chem. Eng. J. 2016, 306, 43–52. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, D.; Zhao, L.; Wang, S.; Liu, J.; Yan, C. Excellent ion selectivity of Nafion membrane modified by PBI via acid-base pair effect for vanadium flow battery. Electrochim. Acta 2021, 394, 139144. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Shaydullin, R.R.; Yaroslavtsev, A.B. Improving the conductivity and permselectivity of ion-exchange membranes by introduction of inorganic oxide nanoparticles: Impact of acid–base properties. Colloid Polym. Sci. 2019, 297, 741–748. [Google Scholar] [CrossRef]
- Falina, I.; Loza, N.; Loza, S.; Titskaya, E.; Romanyuk, N. Permselectivity of cation exchange membranes modified by polyaniline. Membranes 2021, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-X.; Zhang, L.-J.; Wang, Y.-X. Enhanced performance of a Nafion membrane through ionomer self-organization in the casting solution. J. Power Sources 2013, 233, 309–312. [Google Scholar] [CrossRef]
- Park, J.W.; Wycisk, R.; Pintauro, P.N. Nafion/PVDF nanofiber composite membranes for regenerative hydrogen/bromine fuel cells. J. Membr. Sci. 2015, 490, 103–112. [Google Scholar] [CrossRef]
- Mollá, S.; Compañ, V. Performance of composite Nafion/PVA membranes for direct methanol fuel cells. J. Power Sources 2011, 196, 2699–2708. [Google Scholar] [CrossRef]
- Ru, C.; Gu, Y.; Duan, Y.; Zhao, C.; Na, H. Enhancement in proton conductivity and methanol resistance of Nafion membrane induced by blending sulfonated poly(arylene ether ketones) for direct methanol fuel cells. J. Membr. Sci. 2019, 573, 439–447. [Google Scholar] [CrossRef]
- Porada, S.; van Egmond, W.J.; Post, J.W.; Saakes, M.; Hamelers, H.V.M. Tailoring ion exchange membranes to enable low osmotic water transport and energy efficient electrodialysis. J. Membr. Sci. 2018, 552, 22–30. [Google Scholar] [CrossRef]
- Kim, R.; Kim, G.H.; Doo, G.; Choi, C.; Kim, S.; Lee, J.-H.; Heo, J.; Jung, H.-Y.; Kim, H.-T. Ultrathin Nafion-filled porous membrane for zinc/bromine redox flow batteries. Sci. Rep. 2017, 7, 10503. [Google Scholar] [CrossRef]
- Klose, C.; Breitwieser, M.; Vierrath, S.; Klingele, M.; Cho, H.; Büchler, A.; Kerres, J.; Thiele, S. Electrospun sulfonated poly(ether ketone) nanofibers as proton conductive reinforcement for durable Nafion composite membranes. J. Power Sources 2017, 361, 237–242. [Google Scholar] [CrossRef]
- Small, L.J.; Pratt III, H.D.; Anderson, T.M. Crossover in Membranes for Aqueous Soluble Organic Redox Flow Batteries. J. Electrochem. Soc. 2019, 166, A2536–A2542. [Google Scholar] [CrossRef]
- Briot, L.; Petit, M.; Cacciuttolo, Q.; Pera, M.-C. Aging phenomena and their modelling in aqueous organic redox flow batteries: A review. J. Power Sources 2022, 536, 231427. [Google Scholar] [CrossRef]
- Kononenko, N.A.; Fomenko, M.A.; Volfkovich, Y.M. Structure of perfluorinated membranes investigated by method of standard contact porosimetry. Adv. Colloid Interface Sci. 2015, 222, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Recent approaches to improve Nafion performance for fuel cell applications: A review. Int. J. Hydrogen Energy 2019, 44, 28919–28938. [Google Scholar] [CrossRef]
- Berezina, N.P.; Kononenko, N.A.; Dyomina, O.A.; Gnusin, N.P. Characterization of ion-exchange membrane materials: Properties vs structure. Adv. Colloid Interface Sci. 2008, 139, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Jalani, N.H.; Datta, R. The effect of equivalent weight, temperature, cationic forms, sorbates, and nanoinorganic additives on the sorption behavior of Nafion. J. Membr. Sci. 2005, 264, 167–175. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids (Technical Report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Zabolotsky, V.I.; Nikonenko, V.V. Effect of structural membrane inhomogeneity on transport properties. J. Membr. Sci. 1993, 79, 181–198. [Google Scholar] [CrossRef]
- Falina, I.V.; Demina, O.A.; Kononenko, N.A.; Annikova, L.A. Influence of inert components on the formation of conducting channels in ion-exchange membranes. J. Solid State Electrochem. 2017, 21, 767–775. [Google Scholar] [CrossRef]
- Gnusin, N.P.; Demina, O.A.; Berezina, N.P.; Kononenko, N.A. Modeling of mass electrotransfer in terms of the transport and structural properties of ion-exchange membranes. Theor. Found. Chem. Eng. 2004, 38, 394–398. [Google Scholar] [CrossRef]
- Filippov, A.N.; Kononenko, N.A.; Loza, N.V.; Kopitsyna, D.S.; Petrova, D.A. Modelling of transport properties of perfluorinated one- and bilayer membranes modified by polyaniline decorated clay nanotubes. Electrochim. Acta 2021, 389, 138768. [Google Scholar] [CrossRef]
- Ibanez, R.; Stamatialis, D.F.; Wessling, M. Role of membrane surface in concentration polarization at cation exchange membranes. J. Memb. Sci. 2004, 239, 119–128. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Mareev, S.A.; Pis’menskaya, N.D.; Uzdenova, A.M.; Kovalenko, A.V.; Urtenov, M.K.; Pourcelly, G. Effect of electroconvection and its use in intensifying the mass transfer in electrodialysis (Review). Russ. J. Electrochem. 2017, 53, 1122–1144. [Google Scholar] [CrossRef]
- Safronova, E.; Golubenko, D.; Pourcelly, G.; Yaroslavtsev, A. Mechanical properties and influence of straining on ion coductivityof perfluorosulfonic acid Nafions-type membranes depending on water uptake. J. Membr. Sci. 2015, 473, 218–225. [Google Scholar] [CrossRef]
- Kundu, S.; Simon, L.C.; Fowler, M.; Grot, S. Mechanical properties of Nafion electrolyte membranes under hydrated conditions. Polymer 2005, 46, 11707–11715. [Google Scholar] [CrossRef]
- Zatoń, M.; Rozière, J.; Jones, D.J. Current understanding of chemical degradation mechanisms of perfluorosulfonic acid membranes and their mitigation strategies: A review. Sustain. Energy Fuels 2017, 1, 409. [Google Scholar] [CrossRef]
| Membrane | ω, % | l, μm | Q, mmol/gdry | W, % | nm, molH2O/molSO3− |
|---|---|---|---|---|---|
| M0 | 0 | 359 ± 20 | 1.02 | 21.3 | 14 |
| M10 | 10 | 398 ± 20 | 0.91 ± 0.01 | 17.8 | 13 |
| M20 | 20 | 437 ± 20 | 0.80 ± 0.01 | 16.1 | 13 |
| M30 | 30 | 368 ± 20 | 0.67 ± 0.01 | 12.9 | 12 |
| M40 | 40 | 367 ± 20 | 0.60 ± 0.01 | 9.9 | 10 |
| Membrane | β | f2 | κiso, S/m | α | G∙1015, m5 mol −1 s−1 |
|---|---|---|---|---|---|
| M0 | 1.54 | 0.06 | 1.19 | 0.38 | 2.89 |
| M10 | 1.50 | 0.04 | 1.03 | 0.45 | 2.94 |
| M20 | 1.54 | 0.04 | 0.80 | 0.40 | 2.06 |
| M30 | 1.35 | 0.04 | 0.42 | 0.40 | 0.20 |
| M40 | 1.00 | 0.0002 | 0.21 | 1 | - |
| Membrane | ilim, A/m2 | ΔElim, V | ΔEover, V | Δ, V | tgohm | tglim | tgover |
|---|---|---|---|---|---|---|---|
| M0 | 37.6 ± 1.6 | 0.13 ± 0.004 | 0.88 ± 0.13 | 0.84 ± 0.14 | 283.06 ± 5.29 | 8.04 ± 0.41 | 127.82 ± 27.84 |
| M10 | 34.8 ± 0.7 | 0.09 ± 0.001 | 0.81 ± 0.03 | 0.72 ± 0.03 | 369.94 ± 6.37 | 7.34 ± 0.14 | 115.88 ± 8.41 |
| M20 | 38.2 ±0.5 | 0.11 ± 0.003 | 0.63 ± 0.03 | 0.52 ± 0.03 | 326.68 ± 6.44 | 11.39 ± 1.7 | 126.12 ± 14.11 |
| M30 | 36.9 ± 0.1 | 0.12 ± 0.001 | 0.71 ± 0.01 | 0.59 ± 0.01 | 317.46 ± 6.45 | 7.74 ± 0.22 | 120.20 ± 1.98 |
| M40 | 36.4 ± 0.9 | 0.11 ± 0.001 | 0.80 ± 0.07 | 0.72 ± 0.07 | 299.39 ± 0.85 | 7.71 ± 4.35 | 121.30 ± 27.26 |
| Membrane | E, MPa | Yield Strength, MPa | ε, % | Tensile Strength, MPa |
|---|---|---|---|---|
| M0 | 176 | 16.7 | 160 | 24.2 |
| M10 | 142 | 15.4 | 250 | 20.9 |
| M20 | 108 | 12.1 | 320 | 17.2 |
| M40 | 135 | 11.0 | 480 | 18.7 |
| F-26 | – | – | 520 | 40.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falina, I.; Kononenko, N.; Timofeev, S.; Rybalko, M.; Demidenko, K. Nanocomposite Membranes Based on Fluoropolymers for Electrochemical Energy Sources. Membranes 2022, 12, 935. https://doi.org/10.3390/membranes12100935
Falina I, Kononenko N, Timofeev S, Rybalko M, Demidenko K. Nanocomposite Membranes Based on Fluoropolymers for Electrochemical Energy Sources. Membranes. 2022; 12(10):935. https://doi.org/10.3390/membranes12100935
Chicago/Turabian StyleFalina, Irina, Natalia Kononenko, Sergey Timofeev, Michail Rybalko, and Ksenia Demidenko. 2022. "Nanocomposite Membranes Based on Fluoropolymers for Electrochemical Energy Sources" Membranes 12, no. 10: 935. https://doi.org/10.3390/membranes12100935
APA StyleFalina, I., Kononenko, N., Timofeev, S., Rybalko, M., & Demidenko, K. (2022). Nanocomposite Membranes Based on Fluoropolymers for Electrochemical Energy Sources. Membranes, 12(10), 935. https://doi.org/10.3390/membranes12100935






