Structural and Electrotransport Properties of Perfluorinated Sulfocationic Membranes Modified by Silica and Zirconium Hydrophosphate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Modification
2.3. Research Methods
3. Results and Discussion
3.1. Structural Characteristics
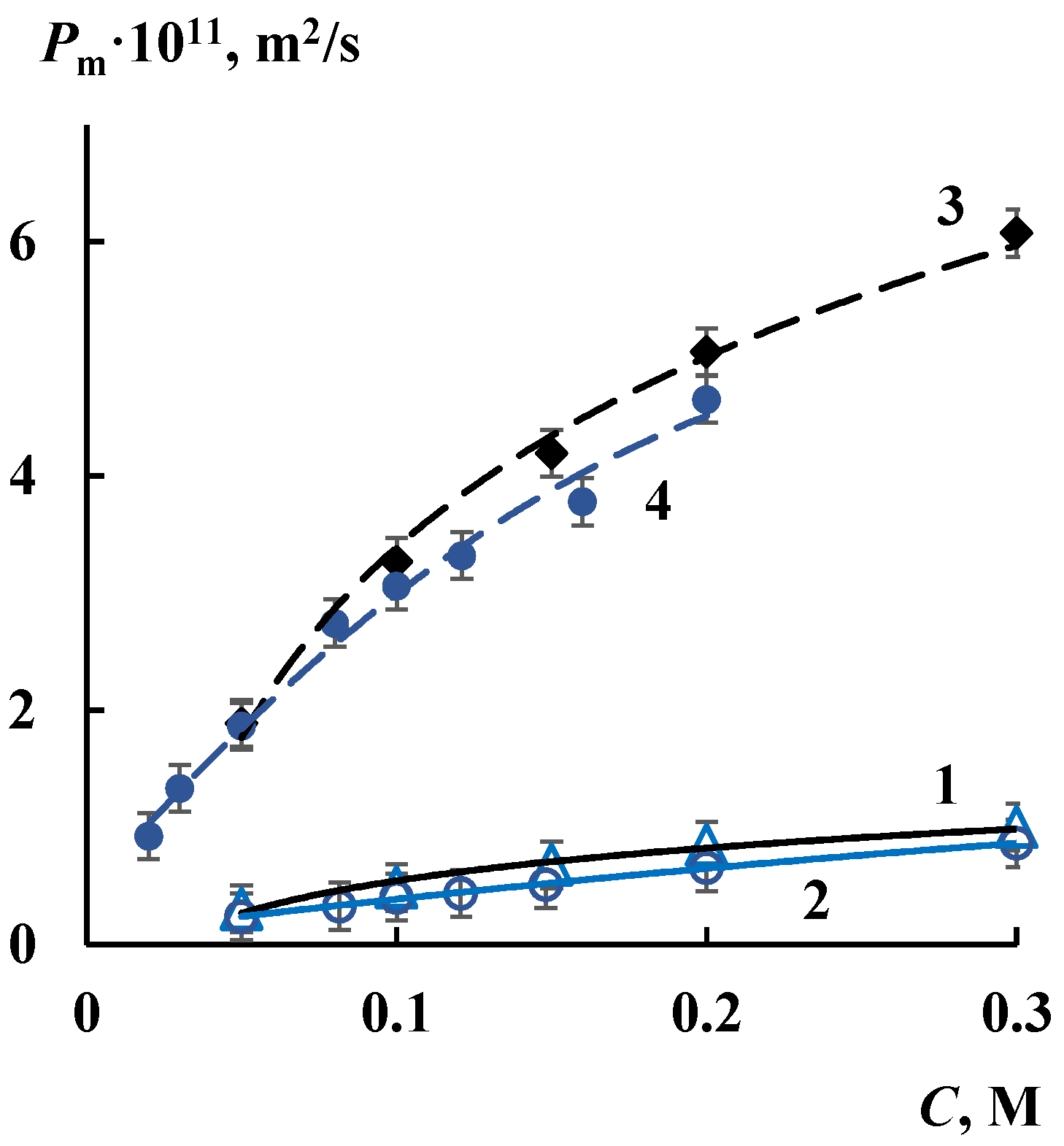
3.2. Transport Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HZP | zirconium hydrophosphate |
| ETWM | extended three-wire model |
| PEMFC | proton exchange membrane fuel cells |
References
- Filippov, S.P.; Yaroslavtsev, A.B. Hydrogen energy: Development prospects and materials. Russ. Chem. Rev. 2021, 90, 627–643. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B. Correlation between the properties of hybrid ion-exchange membranes and the nature and dimensions of dopant particles. Nanotechnol. Russ. 2012, 7, 437–451. [Google Scholar] [CrossRef]
- Vázquez-Rodríguez, G.; Torres-Rodríguez, L.M.; Montes-Rojas, A. Synthesis and characterization of commercial cation exchange membranes modified electrochemically by polypyrrole: Effect of synthesis conditions on the transport properties. Desalination 2017, 416, 94–105. [Google Scholar] [CrossRef]
- Kononenko, N.A.; Loza, N.V.; Shkirskaya, S.A.; Falina, I.V.; Khanukaeva, D.Y. Influence of conditions of polyaniline synthesis in perfluorinated membrane on electrotransport properties and surface morphology of composites. J. Solid State Electrochem. 2015, 19, 2623–2631. [Google Scholar] [CrossRef]
- Shkirskaya, S.; Kolechko, M.; Kononenko, N. Sensor properties of materials based on fluoride polymer F-4SF films modified by polyaniline. Curr. Appl. Phys. 2015, 15, 1587–1592. [Google Scholar] [CrossRef]
- Apel, P.Y.; Bobreshova, O.V.; Volkov, A.V.; Volkov, V.V.; Nikonenko, V.V.; Stenina, I.A.; Filippov, A.N.; Yampolskii, Y.P.; Yaroslavtsev, A.B. Prospects of Membrane Science Development. Membr. Membr. Technol. 2019, 1, 45–63. [Google Scholar] [CrossRef] [Green Version]
- Simonov, A.S.; Kondratenko, M.S.; Elmanovich, I.V.; Sizov, V.E.; Kharitonova, E.P.; Abramchuk, S.S.; Nikolaev, A.Y.; Fedosov, D.A.; Gallyamov, M.O.; Khokhlov, A.R. Modification of Nafion with silica nanoparticles in supercritical carbon dioxide for electrochemical applications. J. Membr. Sci. 2018, 564, 106–114. [Google Scholar] [CrossRef]
- Xu, G.; Wei, Z.; Li, S.; Li, J.; Yang, Z.; Grigoriev, S.A. In-situ sulfonation of targeted silica-filled Nafion for high-temperature PEM fuel cell application. Int. J. Hydrog. Energy 2019, l44, 29711–29716. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Mountz, D.A.; Reuschle, D.A.; Blackwell, R.I. Self-assembled organic/inorganic hybrids as membrane materials. Electrochim. Acta 2004, 50, 565–569. [Google Scholar] [CrossRef]
- Bauer, F.; Willert-Porada, M. Microstructural characterization of Zr-phosphate-Nafion membranes for direct methanol fuer cell (DMFC) applications. J. Membr. Sci. 2004, 233, 141–149. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Kumar, G.G.; Yoo, D.J. Sulfonated graphene oxide/Nafion composite membranes for high temperature and low humidity proton exchange membrane fuel cells. RSC Adv. 2018, 8, 7494–7508. [Google Scholar] [CrossRef] [Green Version]
- Safronova, E.Y.; Yaroslavtsev, A.B. Prospects of practical application of hybrid membranes. Pet. Chem. 2016, 56, 281–293. [Google Scholar] [CrossRef]
- Park, J.-S.; Shin, M.-S.; Kim, C.-S. Proton exchange membranes for fuel cell operation at low relative humidity and intermediatetemperature: An updated review. Curr. Opin. Electrochem. 2017, 5, 43–55. [Google Scholar] [CrossRef]
- Sahu, A.K.; Ketpang, K.; Shanmugam, S.; Kwon, O.; Lee, S.; Kim, H. Sulfonated graphene–Nafion composite membranes for polymer electrolyte fuel cells operating under reduced relative humidity. J. Phys. Chem. 2016, 120, 15855–15866. [Google Scholar] [CrossRef]
- Bakangura, E.; Wu, L.; Ge, L.; Yang, Z.; Xu, T. Mixed matrix proton exchange membranes for fuel cells: State of the art and perspectives. Prog. Polym. Sci. 2016, 57, 103–152. [Google Scholar] [CrossRef]
- Liu, Y.; Lehnert, W.; Janben, H.; Samsun, R.; Stolten, D. A review of high-temperature polymer electrolyte membrane fuel-cell (HT-PEMFC)-based auxiliary power units for diesel-powered road vehicles. J. Power Sources 2016, 311, 91–102. [Google Scholar] [CrossRef]
- Strathmann, H. Ion-Exchange Membrane Separation Processes, 1st ed.; Membrane Science and Technology Series 9; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Nagarale, R.K.; Gohil, G.S.; Shahi, V.K. Recent developments on ion-exchange membranes and electro-membrane processes. Adv. Colloid Interface Sci. 2006, 119, 97–130. [Google Scholar] [CrossRef]
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B.; Stenina, I.A. Current progress in membranes for fuel cells and reverse electrodialysis. Mendeleev Commun. 2021, 31, 423–432. [Google Scholar] [CrossRef]
- Zabolotskii, V.I.; Protasov, K.V.; Sharafan, M.V. Sodium chloride concentration by electrodialysis with hybrid organic-inorganic ion-exchange membranes: An investigation of the process. Russ. J. Electrochem. 2010, 46, 979–986. [Google Scholar] [CrossRef]
- Yen, C.; Lee, C.; Lin, Y.; Lin, H.; Hsiao, Y.; Liao, S.; Chuanga, C.; Ma, C.M. Sol–gel derived sulfonated-silica/Nafion composite membrane for direct methanol fuel cell. J. Power Sources 2007, 173, 36–44. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Yaroslavtsev, A.B. Relationship between properties of hybrid ion-exchange membranes and dopant nature. Solid State Ion. 2013, 251, 23–27. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Yaroslavtsev, A.B. Nafion-type membranes doped with silica nanoparticles with modified surface. Solid State Ion. 2012, 221, 6–10. [Google Scholar] [CrossRef]
- Tang, H.; Wan, Z.; Pan, M.; Jiang, S.P. Self-assembled Nafion–silica nanoparticles for elevated-high temperature polymer electrolyte membrane fuel cells. Electrochem. Commun. 2007, 9, 2003–2008. [Google Scholar] [CrossRef]
- Voropaeva, E.Y.; Stenina, I.A.; Yaroslavtsev, A.B. Transport Properties of Hydrous-Silica-Modified MF-4SK Membranes. Russ. J. Inorg. Chem. 2008, 53, 1531–1535. [Google Scholar] [CrossRef]
- Tominaga, Y.; Hong, I.-C.; Asai, S.; Sumita, M. Proton conduction in Nafion composite membranes filled with mesoporous silica. J. Power Sources 2007, 171, 530–534. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Yaroslavtsev, A.B. Transport properties of materials based on MF-4SC membranes and silica manufactured by a casting method. Russ. J. Inorg. Chem. 2010, 55, 1499–1502. [Google Scholar] [CrossRef]
- Shalimov, A.S.; Novikova, S.A.; Stenina, I.A.; Yaroslavtsev, A.B. Ion transport in MF-4SK cation-exchange membranes modified with acid zirconium phosphate. Russ. J. Inorg. Chem. 2006, 51, 700–705. [Google Scholar] [CrossRef]
- Aziz, M.A.; Shanmugam, S. Zirconium oxide nanotube-Nafion composite as high performance membrane for all vanadium redox flow battery. J. Power Sources 2017, 337, 36–44. [Google Scholar] [CrossRef]
- Hara, S.; Sakamoto, H.; Miyayama, M.; Kudo, T. Proton-conducting properties of hydrated tin dioxide as an electrolyte for fuel cells at intermediate temperature. Solid State Ion. 2002, 154, 679–685. [Google Scholar] [CrossRef]
- Wang, C.; Chalkova, E.; Lute, C.D.; Fedkin, M.V.; Komarneni, S.; Chung, T.C.M.; Lvov, S.N. Proton Conductive Inorganic Materials for Temperatures Up to 120 °C and Relative Humidity Down to 5%. J. Electrochem. Soc. 2010, 157, B1634–B1642. [Google Scholar] [CrossRef]
- Gerasimova, E.; Safronova, E.; Ukshe, A.; Dobrovolsky, Y.; Yaroslavtsev, A. Electrocatalytic and transport properties of hybrid Nafion membranes doped with silica and cesium acid salt of phosphotungstic acid in hydrogen fuel cells. Chem. Eng. J. 2016, 305, 121–128. [Google Scholar] [CrossRef]
- Strathmann, H.; Grabowski, A.; Eigenberger, G. Ion-exchange membranes in the chemical process industry. Ind. Eng. Chem. Res. 2013, 52, 10364–10379. [Google Scholar] [CrossRef]
- Berezina, N.P.; Kononenko, N.A.; Dyomina, O.A.; Gnusin, N.P. Characterization of ion-exchange membrane materials: Properties vs structure. Adv. Colloid Interface Sci. 2008, 139, 3–28. [Google Scholar] [CrossRef]
- Volfkovich, Y.M.; Filippov, A.N.; Bagotsky, V.S. Structural Properties of Porous Materials and Powders Used in Different Fields of Science and Technology; Springer: London, UK, 2014. [Google Scholar]
- Volfkovich, Y.M.; Bagotzky, V.S.; Sosenkin, V.E.; Blinov, I.A. The standard contact porosimetry. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187–188, 349–365. [Google Scholar] [CrossRef]
- Rouquerol, J.; Baron, G.; Denoyel, R.; Giesche, H.; Groen, J.; Klobes, P.; Levitz, P.; Neimark, A.V.; Rigby, S.; Skudas, R.; et al. Liquid Intrusion and Alternative Methods for the Characterization of Macroporous Materials (IUPAC Technical Report). Pure Appl. Chem. 2011, 84, 107–136. [Google Scholar] [CrossRef]
- Kononenko, N.A.; Fomenko, M.A.; Volfkovich, Y.M. Structure of perfluorinated membranes investigated by method of standard contact porosimetry. Adv. Colloid Interface Sci. 2015, 222, 425–435. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids (Technical Report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Kononenko, N.; Nikonenko, V.; Grande, D.; Larchet, C.; Dammak, L.; Fomenko, M.; Volfkovich, Y. Porous structure of ion exchange membranes investigated by various techniques. Adv. Colloid Interface Sci. 2017, 246, 196–216. [Google Scholar] [CrossRef]
- Volfkovich, Y.M.; Sosenkin, V.E. Porous structure and wetting of fuel cell components as the factors determining their electrochemical characteristics. Russ. Chem. Rev. 2012, 81, 936–959. [Google Scholar] [CrossRef]
- Marcus, Y. The hydration of ions and their effects on the structure of water. J. Chem. Soc. Faraday Trans. 1986, 82, 233–242. [Google Scholar] [CrossRef]
- Berezina, N.P.; Shkirskaya, S.A.; Kolechko, M.V.; Popova, O.V.; Senchikhin, I.N.; Roldugin, V.I. Barrier effects of polyaniline layer in surface modified MF-4SK/polyaniline membranes. Russ. J. Electrochem. 2011, 47, 995–1005. [Google Scholar] [CrossRef]
- Falina, I.V.; Demina, O.A.; Kononenko, N.A.; Annikova, L.A. Influence of inert components on the formation of conducting channels in ion-exchange membranes. J. Solid State Electrochem. 2017, 21, 767–775. [Google Scholar] [CrossRef]
- Demina, O.A.; Kononenko, N.A.; Falina, I.V. New Approach to the Characterization of Ion Exchange Membranes Using a Set of Model Parameters. Petrol. Chem. 2014, 54, 515–525. [Google Scholar] [CrossRef]
- Voropaeva, E.Y.; Stenina, I.A.; Yaroslavtsev, A.B. Ion transport in MF-4SK membranes modified with hydrous zirconia. Russ. J. Inorg. Chem. 2008, 53, 1677–1680. [Google Scholar] [CrossRef]
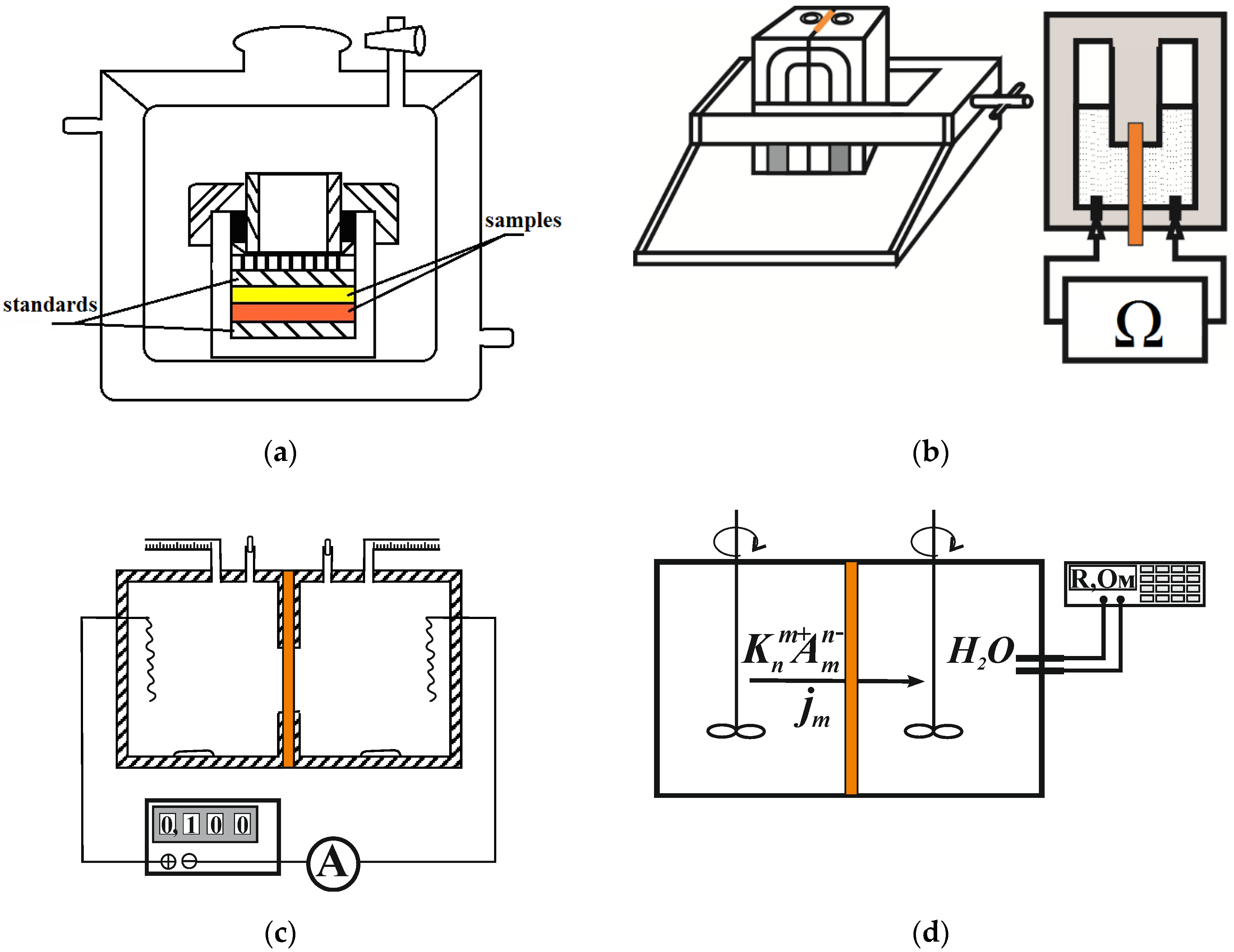

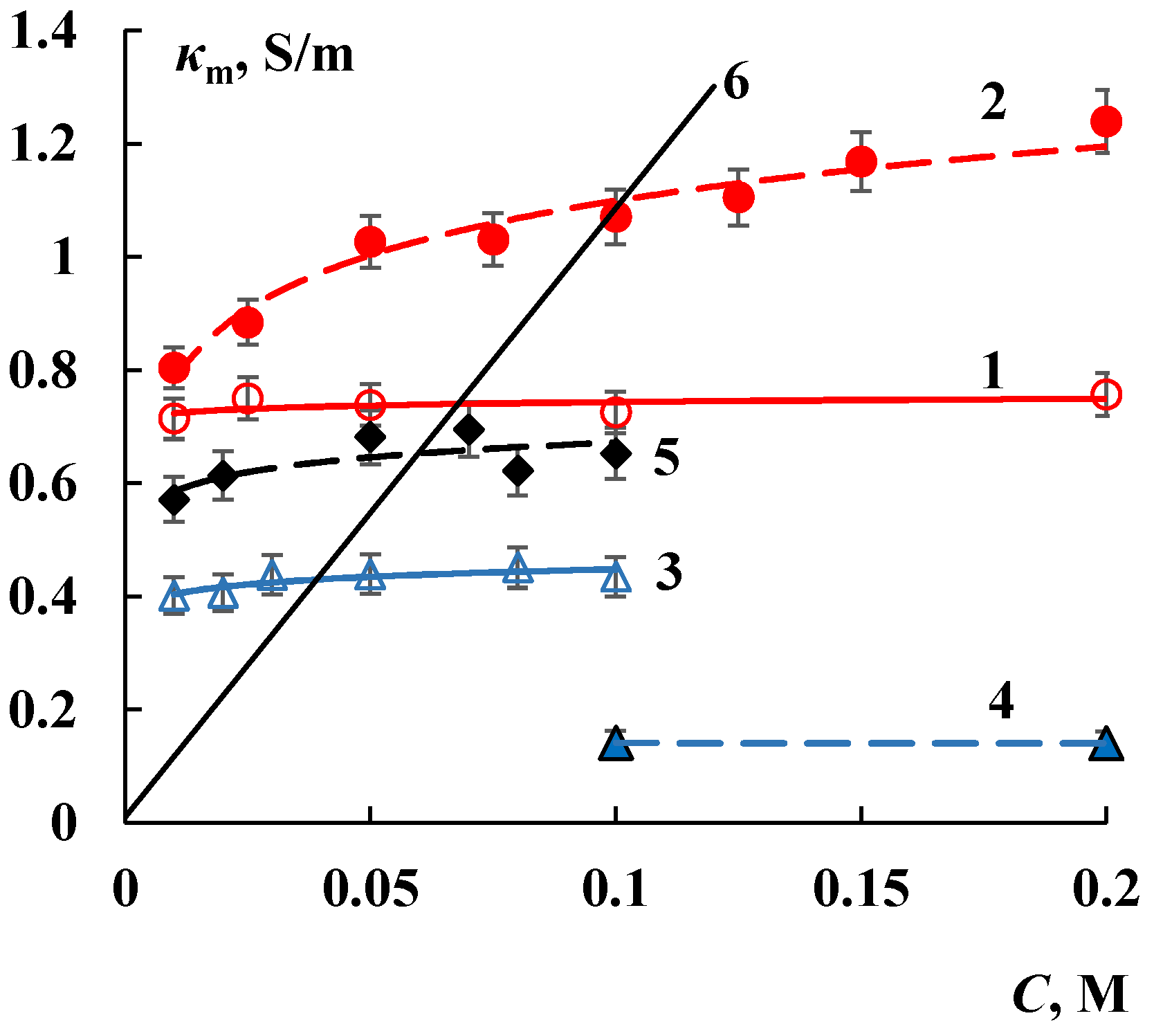
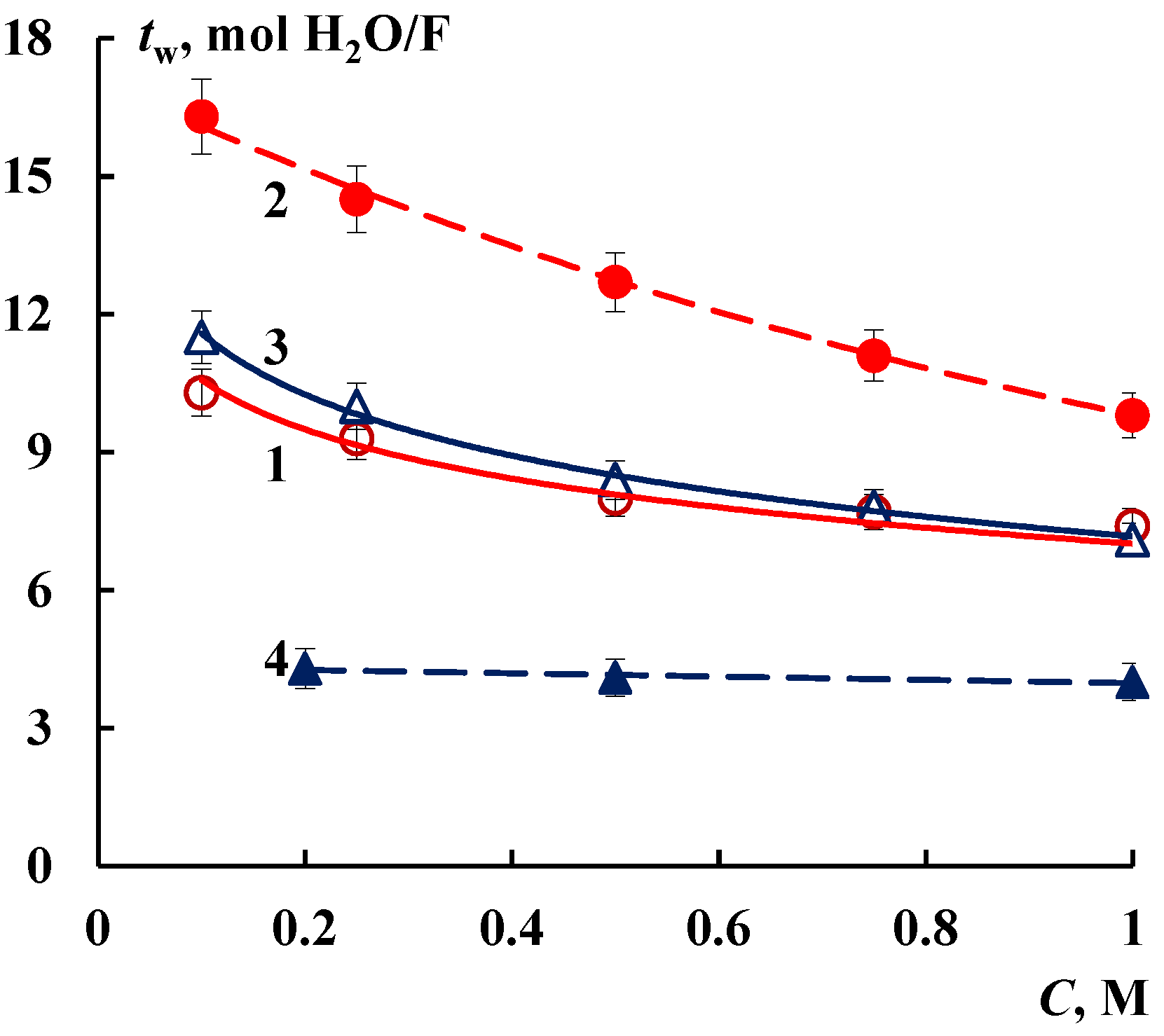
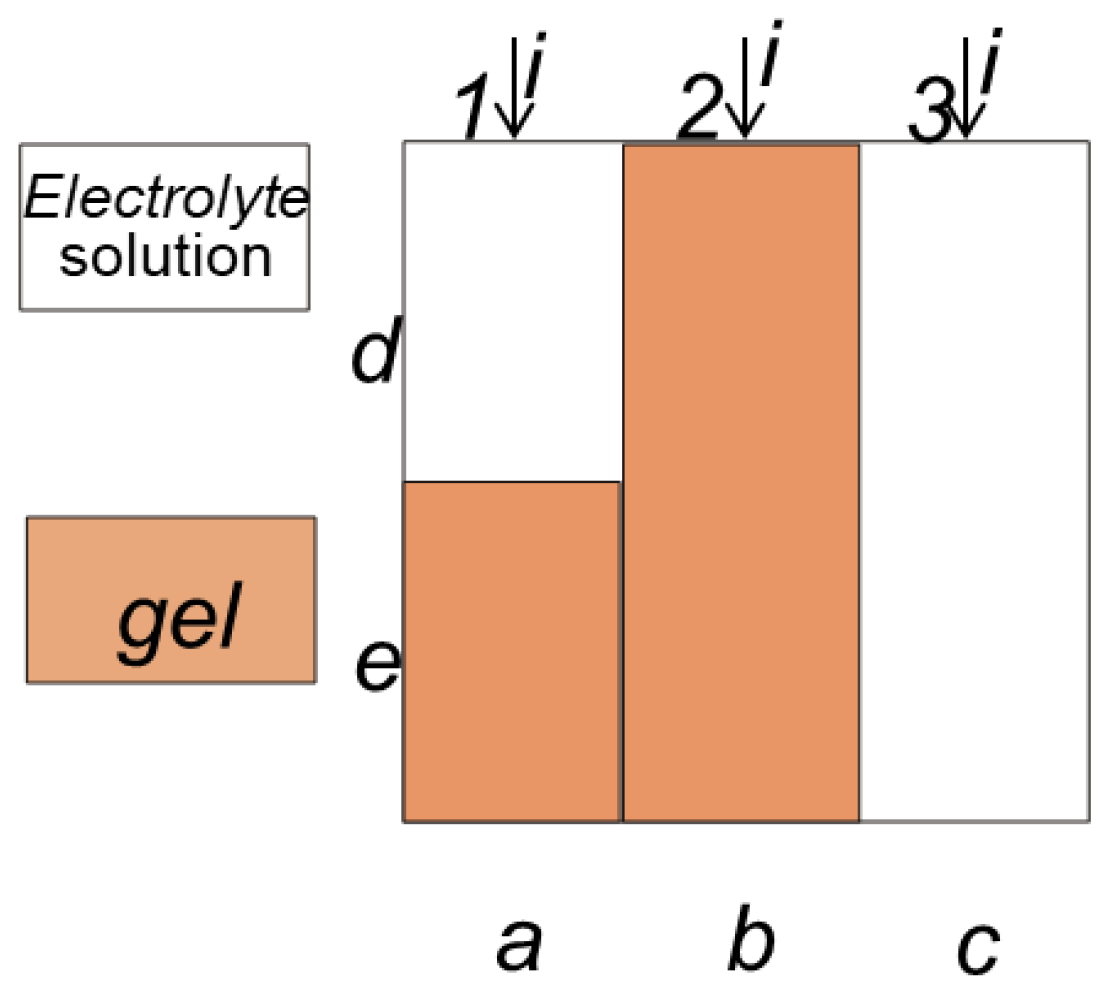

| Membrane | V0, cm3/gdry | Q, mmol/gdry | nm, mol H2O/mol SO3− | S, m2/g | L, nm | ||
|---|---|---|---|---|---|---|---|
| Nafion | 0.26 | 0.87 | 16.1 | 0.82 | 0.06 | 199 | 0.62 |
| Nafion/SiO2 | 0.37 | 0.86 | 23.5 | 0.74 | 0.10 | 218 | 0.65 |
| MF-4SK | 0.23 | 0.80 | 16.2 | 0.85 | 0.05 | 194 | 0.63 |
| MF-4SK/SiO2 | 0.18 | 0.72 | 13.7 | 0.88 | 0.03 | 194 | 0.67 |
| MF-4SK/HZF | 0.30 | 1.93 | 8.6 | 0.82 | 0.09 | 250 | 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shkirskaya, S.A.; Kononenko, N.A.; Timofeev, S.V. Structural and Electrotransport Properties of Perfluorinated Sulfocationic Membranes Modified by Silica and Zirconium Hydrophosphate. Membranes 2022, 12, 979. https://doi.org/10.3390/membranes12100979
Shkirskaya SA, Kononenko NA, Timofeev SV. Structural and Electrotransport Properties of Perfluorinated Sulfocationic Membranes Modified by Silica and Zirconium Hydrophosphate. Membranes. 2022; 12(10):979. https://doi.org/10.3390/membranes12100979
Chicago/Turabian StyleShkirskaya, Svetlana A., Natalia A. Kononenko, and Sergej V. Timofeev. 2022. "Structural and Electrotransport Properties of Perfluorinated Sulfocationic Membranes Modified by Silica and Zirconium Hydrophosphate" Membranes 12, no. 10: 979. https://doi.org/10.3390/membranes12100979
APA StyleShkirskaya, S. A., Kononenko, N. A., & Timofeev, S. V. (2022). Structural and Electrotransport Properties of Perfluorinated Sulfocationic Membranes Modified by Silica and Zirconium Hydrophosphate. Membranes, 12(10), 979. https://doi.org/10.3390/membranes12100979




