Antimicrobial Properties of a Copper/Silicone Composite Membrane Prepared Using a Two-Step Immersion Process in Iodine and Copper Sulfate Solutions
Abstract
:1. Introduction
2. Materials and Methods
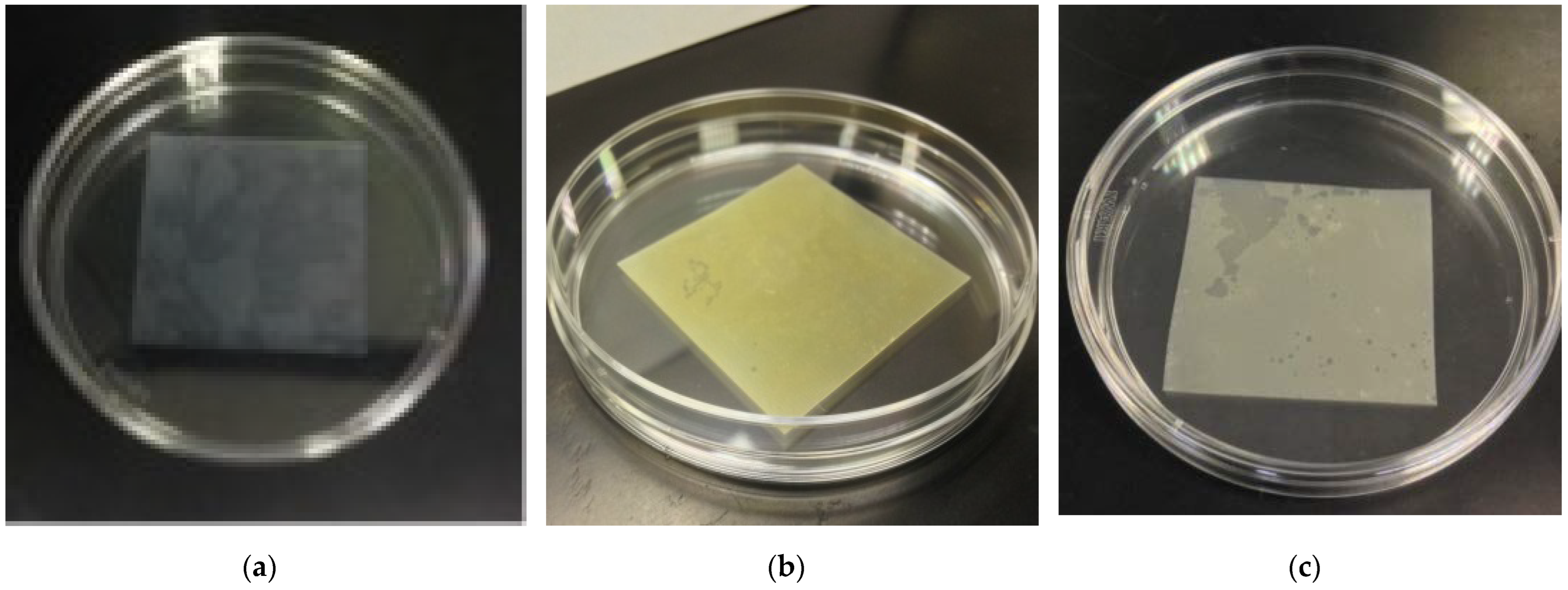
2.1. Preparation of Cu/Silicone Composite Membranes
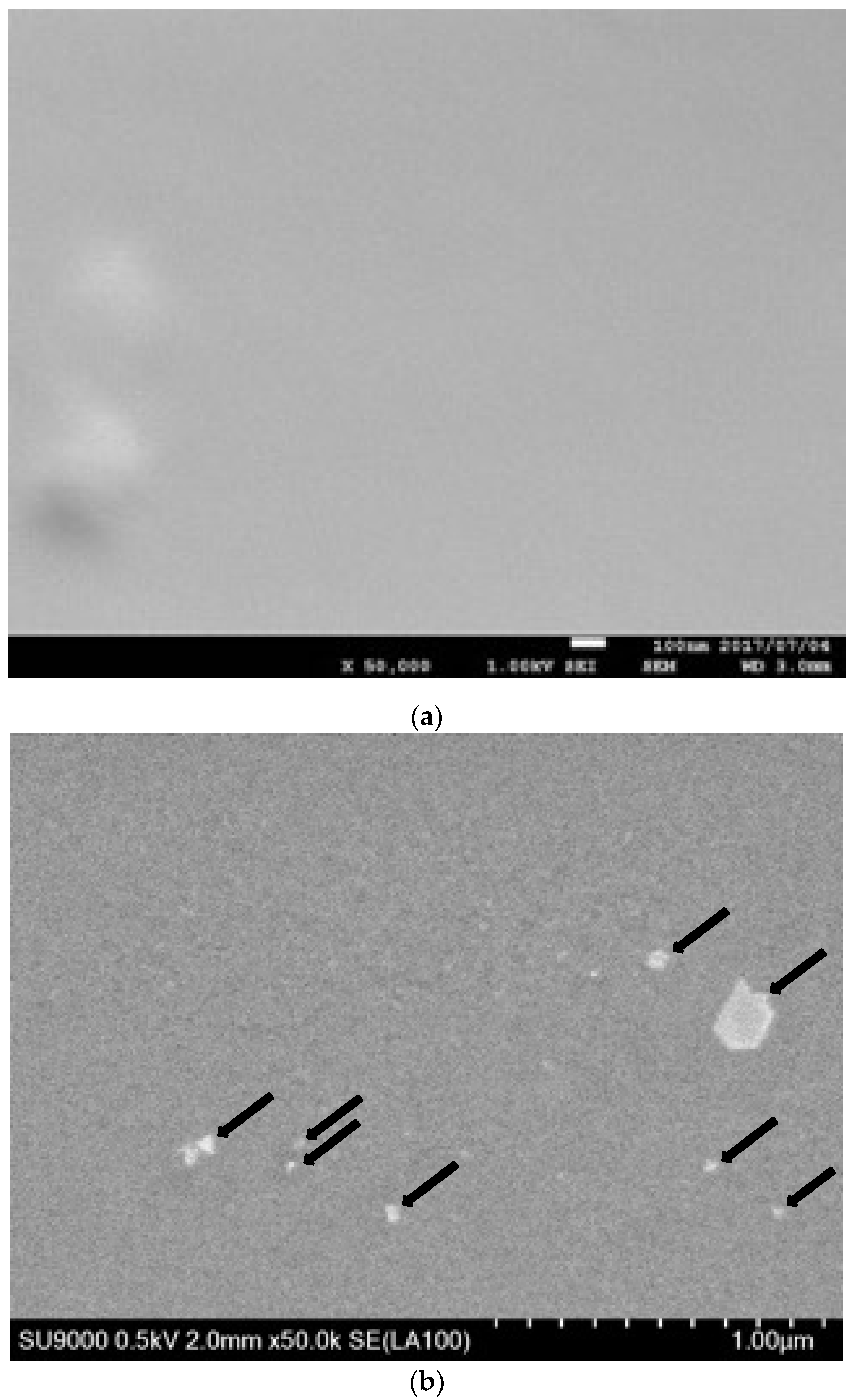
2.2. Scanning Electron Microscopy
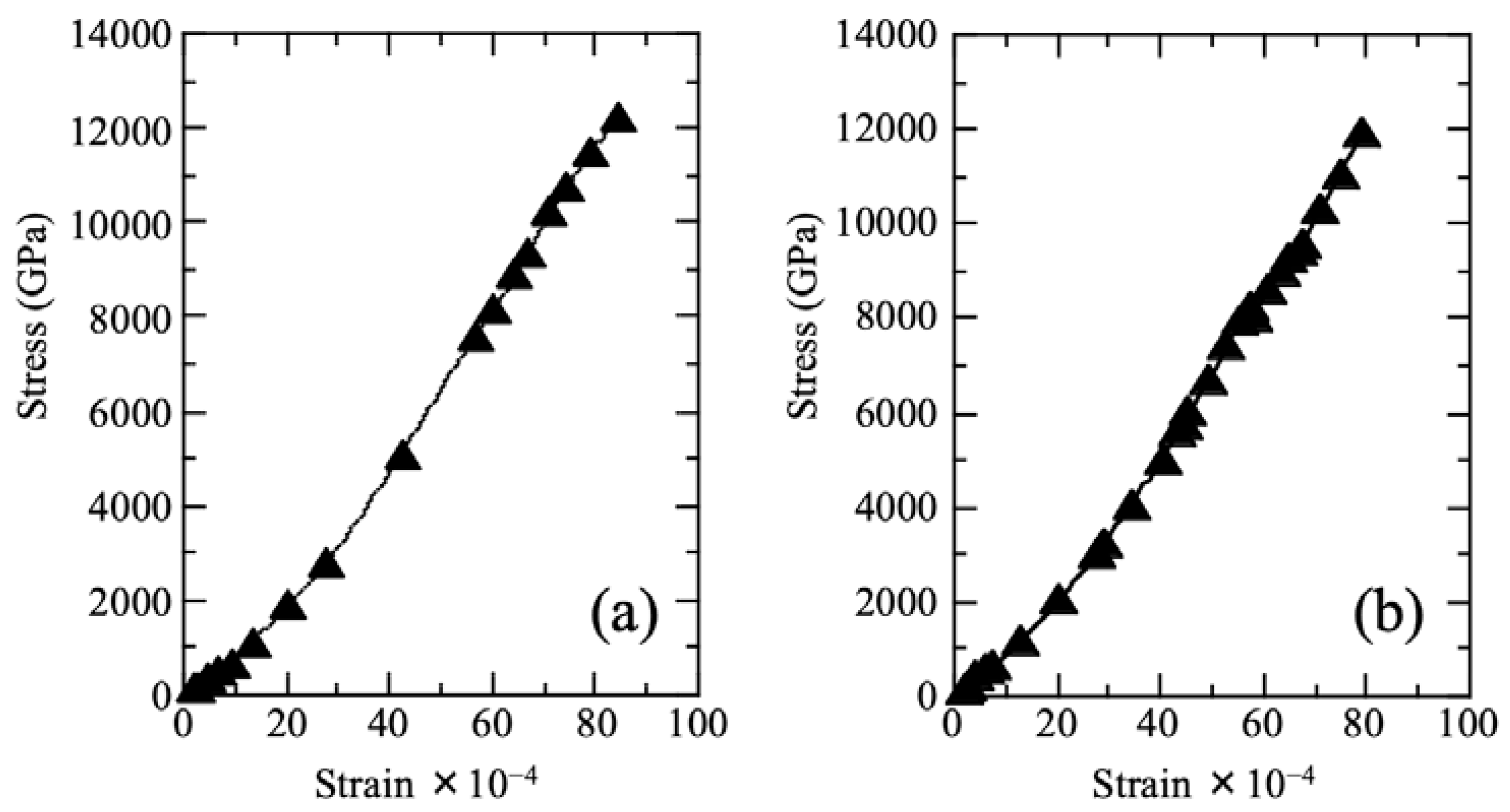
2.3. Tensile Strength Test
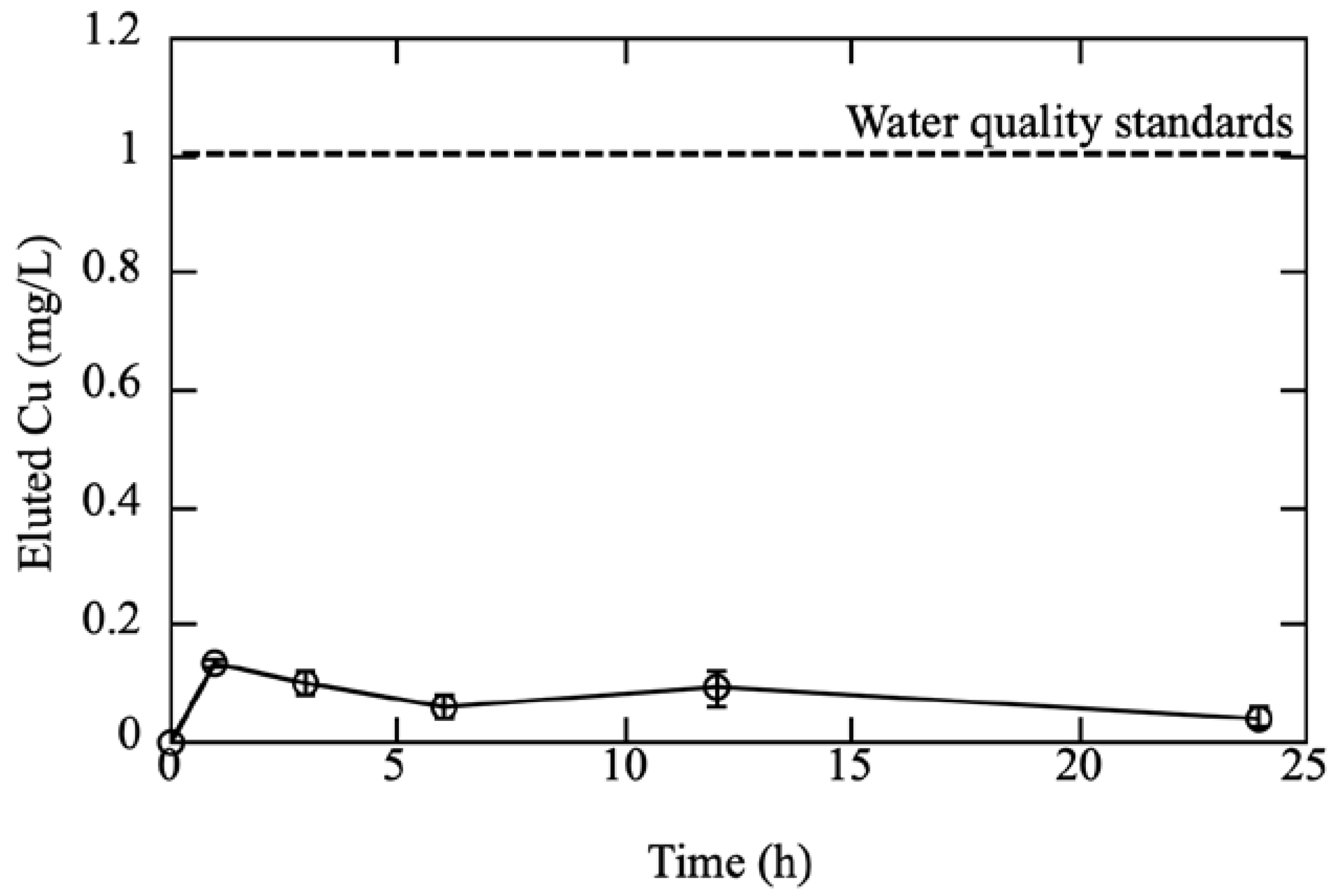
2.4. Elution Test
2.5. Antibacterial Efficacy Test
- U0: mean logarithm of the number of viable bacteria immediately after inoculation of the unprocessed silicone membrane.
- Ut: mean logarithm of the number of viable bacteria after 24 h of the untreated silicone membrane.
- At: mean logarithm of the number of viable bacteria after 24 h of the Cu/silicone membrane.
2.6. Durability Test
2.7. Statistical Analysis
3. Results and Discussion
3.1. Cu/Silicone Composite Membrane
3.2. Cu Elution from the Cu/Silicone Membrane
3.3. Antibacterial Efficacy and Its Durability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFU | Colony-forming unit |
| EDS | Energy-dispersive X-ray spectroscopy |
| E | Young’s modulus |
| E. coli | Escherichia coli |
| JIS | Japanese Industrial Standard |
| NB | Nutrient broth |
| NP | Nanoparticle |
| SCDLP | Soybean–casein digest broth with lecithin and polysorbate 80 |
| SEM | Scanning electron microscope |
| S. aureus | Staphylococcus aureus |
References
- Bae, W.J.; Choi, J.B.; Kim, K.S.; Kim, S.J.; Cho, H.J.; Ha, U.S.; Hong, S.H.; Lee, J.Y.; Kim, S.W. Evaluation of the biocompatibility of packing materials for a catheter. Transl. Androl. Urol. 2015, 4, AB168. [Google Scholar]
- Goveas, R.; Puttipisitchet, O.; Shrestha, B.; Thaworanunta, S.; Srithavaj, M.L. Silicone nasal prosthesis retained by an intranasal stent: A clinical report. J. Prosthet. Dent. 2012, 108, 129–132. [Google Scholar] [CrossRef]
- Hirahara, H.; Mori, K.; Kudo, T.; Matsuno, Y.; Mori, K. Adhesion between cured silicone rubbers. Int. Polym. Sci. Technol. 2014, 86, 327–332. [Google Scholar] [CrossRef]
- Shit, S.C.; Shah, P. A review on silicone rubber. Natl Acad. Sci. Lett. 2013, 36, 355–365. [Google Scholar] [CrossRef]
- Gu, G.; Erişen, D.E.; Yang, K.; Zhang, B.; Shen, M.; Zou, J.; Qi, X.; Chen, S.; Xu, X. Antibacterial and anti-inflammatory activities of chitosan/copper complex coating on medical catheters: In Vitro and In Vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Chang, H.Y.; Lu, J.K.; Huang, Y.C.; Harroun, S.G.; Tseng, Y.T.; Li, Y.J.; Huang, C.C.; Chang, H.T. Self-assembly of antimicrobial peptides on gold nanodots: Against multidrug-resistant bacteria and wound-healing application. Adv. Funct. Mater. 2015, 25, 7189–7199. [Google Scholar] [CrossRef]
- Sethulekshmi, A.S.; Jayan, J.S.; Saritha, A.; Joseph, K.; Aprem, A.S.; Sisupal, S.B. Antimicrobial studies in rubber nanocomposites—A mini review. Ind. Crops Prod. 2022, 187, 115374. [Google Scholar] [CrossRef]
- Lipovsky, A.; Thallinger, B.; Perelshtein, I.; Ludwig, R.; Sygmund, C.; Nyanhongo, G.S.; Guebitz, G.M.; Gedanken, A. Ultrasound coating of polydimethylsiloxanes with antimicrobial enzymes. J. Mater. Chem. B 2015, 3, 7014–7019. [Google Scholar] [CrossRef]
- Li, X.; Li, P.; Saravanan, R.; Basu, A.; Mishra, B.; Lim, S.H.; Su, X.; Tambyah, P.A.; Leong, S.S.J. Antimicrobial functionalization of silicone surfaces with engineered short peptides having broad spectrum antimicrobial and salt-resistant properties. Acta Biomater. 2014, 10, 258–266. [Google Scholar] [CrossRef]
- Meléndez-Ortiz, H.I.; Alvarez-Lorenzo, C.; Burillo, G.; Magariños, B.; Concheiro, A.; Bucio, E. Radiation-grafting of N-vinylimidazole onto silicone rubber for antimicrobial properties. Radiat. Phys. Chem. 2015, 110, 59–66. [Google Scholar] [CrossRef]
- Popa, C.L.; Groza, A.; Chapon, P.; Ciobanu, C.S.; Ghita, R.V.; Trusca, R.; Ganciu, M.; Predoi, D. Physicochemical analysis of the polydimethylsiloxane interlayer influence on a hydroxyapatite doped with silver coating. J. Nanomater. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Asghari, M.; Mahmoodi, N.M. Chitosan-wrapped multiwalled carbon nanotube as filler within PEBA thin film nanocomposite (TFN) membrane to improve dye removal. Carbohydr. Polym. 2020, 237, 116128. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M.; Oveisi, M.; Bakhtiari, M.; Hayati, B.; Shekarchi, A.A.; Bagheri, A.; Rahimi, S. Environmentally friendly ultrasound-assisted synthesis of magnetic zeolitic imidazolate framework-Graphene oxide nanocomposites and pollutant removal from water. J. Mol. Liq. 2019, 282, 115–130. [Google Scholar] [CrossRef]
- Aoki, S.; Yamakawa, K.; Kubo, K.; Takeshita, J.; Takeuchi, M.; Nobuoka, Y.; Wada, R.; Kikuchi, M.; Sawai, J. Antibacterial properties of silicone membranes after a simple two-step immersion process in iodine and silver nitrate solutions. Biocontrol Sci. 2018, 23, 97–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawakami, H.; Yoshida, K.; Nishida, Y.; Kikuchi, Y.; Sato, Y. Antibacterial properties of metallic elements for alloying evaluated with application of JIS Z 2801: 2000. ISIJ Int. 2008, 48, 1299–1304. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Yeo, S.Y.; Jeong, S.H. Antibacterial effect of nanosized silver colloidal solution on textile fabrics. J. Mater. Sci. 2003, 38, 2199–2204. [Google Scholar] [CrossRef]
- Cioffi, N.; Torsi, L.; Ditaranto, N.; Tantillo, G.; Ghibelli, L.; Sabbatini, L.; Bleve-Zacheo, T.; D’Alessio, M.; Zambonin, P.G.; Traversa, E. Copper nanoparticles/polymer composites with antifungal and bacteriostatic properties. Chem. Mater. 2005, 17, 5255–5262. [Google Scholar] [CrossRef]
- Song, X.; Xie, L.; Zhang, M.; Wang, W.; Li, L.; Lu, X.; Lei, P.; Liu, D.; Chen, Y.; Chen, H.; et al. Cu-decorated graphene oxide coatings with enhanced antibacterial activity for surface modification of implant. Mater. Res. Bull. 2021, 141, 111345. [Google Scholar] [CrossRef]
- Cioffi, N.; Torsi, L.; Ditaranto, N.; Sabbatini, L.; Zambonin, P.G.; Tantillo, G.; Ghibelli, L.; D’Alessio, M.; Bleve-Zacheo, T.; Traversa, E. Antifungal activity of polymer-based copper nanocomposite coatings. Appl. Phys. Lett. 2004, 85, 2417–2419. [Google Scholar] [CrossRef]
- Avery, S.V.; Howlett, N.G.; Radice, S. Copper toxicity towards Saccharomyces cerevisiae: Dependence on plasma membrane fatty acid composition. Appl. Environ. Microbiol. 1996, 62, 3960–3966. [Google Scholar] [CrossRef] [Green Version]
- Zoroddu, M.A.; Zanetti, S.; Pogni, R.; Basosi, R. An electron spin resonance study and antimicrobial activity of copper (II). J. Inorg. Biochem. 1996, 63, 291–300. [Google Scholar] [CrossRef]
- Kawakami, H.; Hayashi, T.; Nishikubo, H.; Morikawa, A.; Suzuki, S.; Sato, Y.; Kikuchi, Y. Effects of surface contamination and cleaning with hypochlorite wipes on the antibacterial activity of copper-alloyed antibacterial stainless steel. Biocontrol Sci. 2014, 19, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, B.; Lin, C.; Deng, Y.; Yuan, Z.; Shen, X.; Chen, M.; He, Y.; Peng, Z.; Hu, Y.; Cai, K. Copper-nanoparticle-embedded hydrogel for killing bacteria and promoting wound healing with photothermal therapy. J. Mater. Chem. B 2019, 7, 2534–2548. [Google Scholar] [CrossRef]
- JIS. Rubber, Vulcanized or Thermoplastics-Determination of Tensile Stress—Strain Properties; Japanese Standards Association: Tokyo, Japan, 2010. [Google Scholar]
- Wada, R.; Shimizu, H.; Okabe, M. Junction size and mechanical properties of atactic and syndiotactic high-molecular weight poly (vinyl alcohol) hydrogels. Jpn. J. Polym. Sci. Technol. 2012, 69, 623–630. (In Japanese) [Google Scholar]
- JIS. Antimicrobial Products-Test for Antimicrobial Activity and Efficacy; Japanese Standards Association: Tokyo, Japan, 2001. [Google Scholar]
- Kauffman, G.B.; Fang, L.Y.; Viswanathan, N.; Townsend, G. Purification of copper(I) iodide. In Inorganic Syntheses; Smith, L.H., Jr., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1983; Volume 22, pp. 101–103. [Google Scholar]
- Liu, C.; Yan, B.; Sun, J.; Dong, X.; Zheng, J.; Duan, J.; Hou, B. Cu@C core-shell nanoparticles modified polydimethylsiloxane-based coatings with improved static antifouling performance. Prog. Org. Coat. 2022, 171, 107026. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, D.; Gu, Q.; Miao, J.; Cen, X.; Golodok, R.P.; Savich, V.V.; Ilyushchenko, A.P.; Zhou, Z.; Wang, R. One-step coordination of metal—phenolic networks as antibacterial coatings with sustainable and controllable copper release for urinary catheter applications. RSC Adv. 2022, 12, 15685–15693. [Google Scholar] [CrossRef]
- Yamatani, M. Silicone, 1st ed.; The Chemical Daily: Tokyo, Japan, 2011; p. 88. (In Japanese) [Google Scholar]
- Water Quality Test Items and Their Standards (Date of Enforcement: April 1, 2010). Available online: http://www.mhlw.go.jp/english/wp/wp-hw4/dl/living_environment/2011071908.pdf (accessed on 10 September 2022).
- Ishida, T. Bactericidal continuous processes of Cu2+ solutions based on the antibacterial susceptibilities against Gram-negative and Gram-positive bacteria. Biseibutsu Seitai 2016, 31, 45–56. (In Japanese) [Google Scholar]
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Yawar, W.; ul Hasan, M.M. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef]
- Sharma, P.; Goyal, D.; Chudasama, B. Antibacterial activity of colloidal copper nanoparticles against Gram-negative (Escherichia coli and Proteus vulgaris) bacteria. Lett. Appl. Microbiol. 2022, 74, 695–706. [Google Scholar] [CrossRef]

| Membrane | The Number of Stomacher Treatment | R Value | |
|---|---|---|---|
| E. coli | S. aureus | ||
| Cu/silicone | 0 | 4.2 ± 0.5 | 4.5 ± 0.7 |
| 5 | 2.9 ± 0.5 | 3.1 ± 0.1 | |
| 10 | 3.1 ± 0.1 | 3.5 ± 0.1 | |
| Ag/silicone * | 0 | >6.0 | >6.0 |
| 5 | - | - | |
| 10 | >6.0 | >6.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeshita, J.; Aoki, S.; Wada, R.; Osawa, A.; Sawai, J. Antimicrobial Properties of a Copper/Silicone Composite Membrane Prepared Using a Two-Step Immersion Process in Iodine and Copper Sulfate Solutions. Membranes 2022, 12, 1049. https://doi.org/10.3390/membranes12111049
Takeshita J, Aoki S, Wada R, Osawa A, Sawai J. Antimicrobial Properties of a Copper/Silicone Composite Membrane Prepared Using a Two-Step Immersion Process in Iodine and Copper Sulfate Solutions. Membranes. 2022; 12(11):1049. https://doi.org/10.3390/membranes12111049
Chicago/Turabian StyleTakeshita, Junpei, Shiho Aoki, Risei Wada, Ayako Osawa, and Jun Sawai. 2022. "Antimicrobial Properties of a Copper/Silicone Composite Membrane Prepared Using a Two-Step Immersion Process in Iodine and Copper Sulfate Solutions" Membranes 12, no. 11: 1049. https://doi.org/10.3390/membranes12111049
APA StyleTakeshita, J., Aoki, S., Wada, R., Osawa, A., & Sawai, J. (2022). Antimicrobial Properties of a Copper/Silicone Composite Membrane Prepared Using a Two-Step Immersion Process in Iodine and Copper Sulfate Solutions. Membranes, 12(11), 1049. https://doi.org/10.3390/membranes12111049




