Characterization of Commercial Polymer–Carbon Composite Bipolar Plates Used in PEM Fuel Cells
Abstract
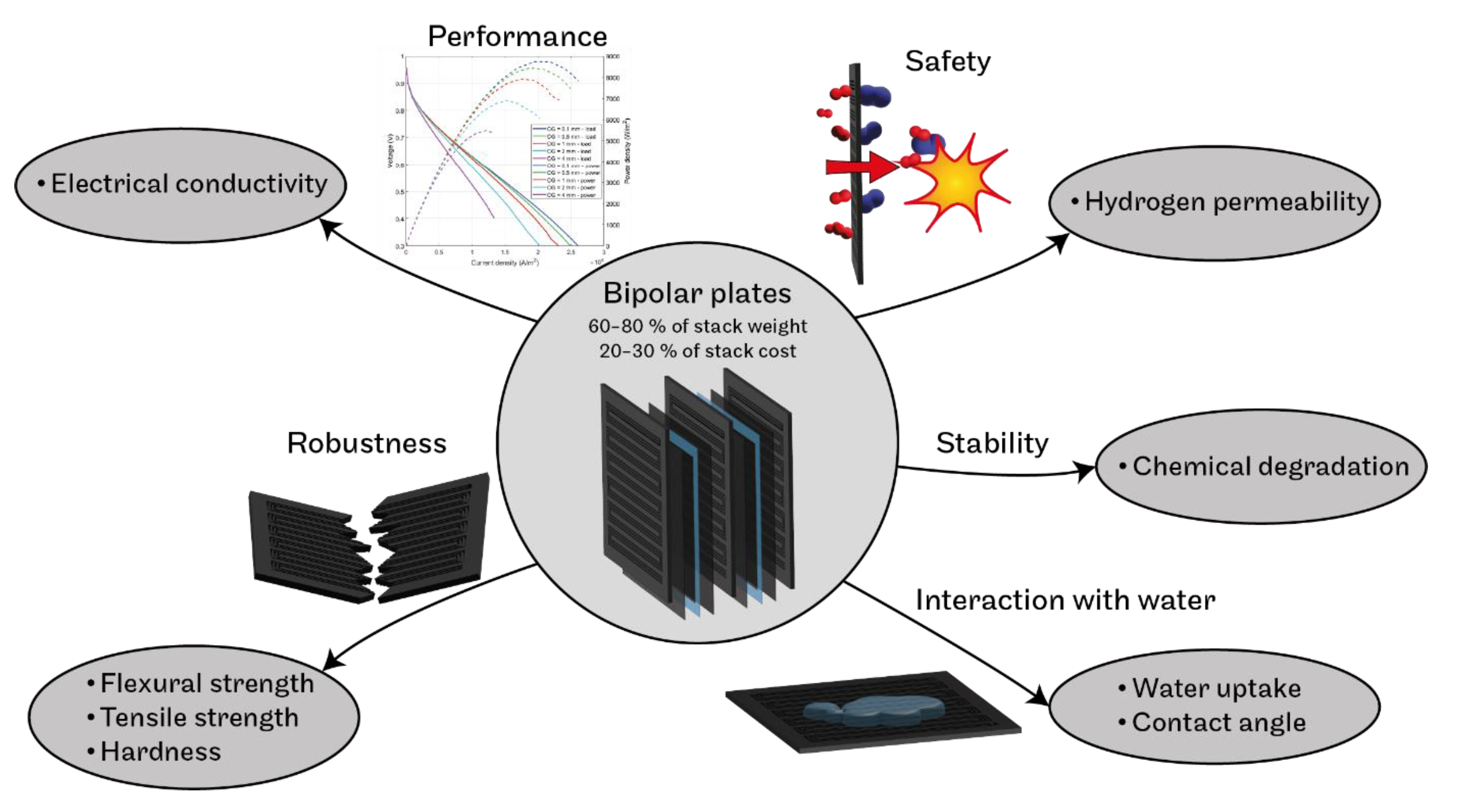
:1. Introduction
2. Materials and Methods
2.1. Sample Materials
2.2. Experimental Methods
2.2.1. Electrical Conductivity
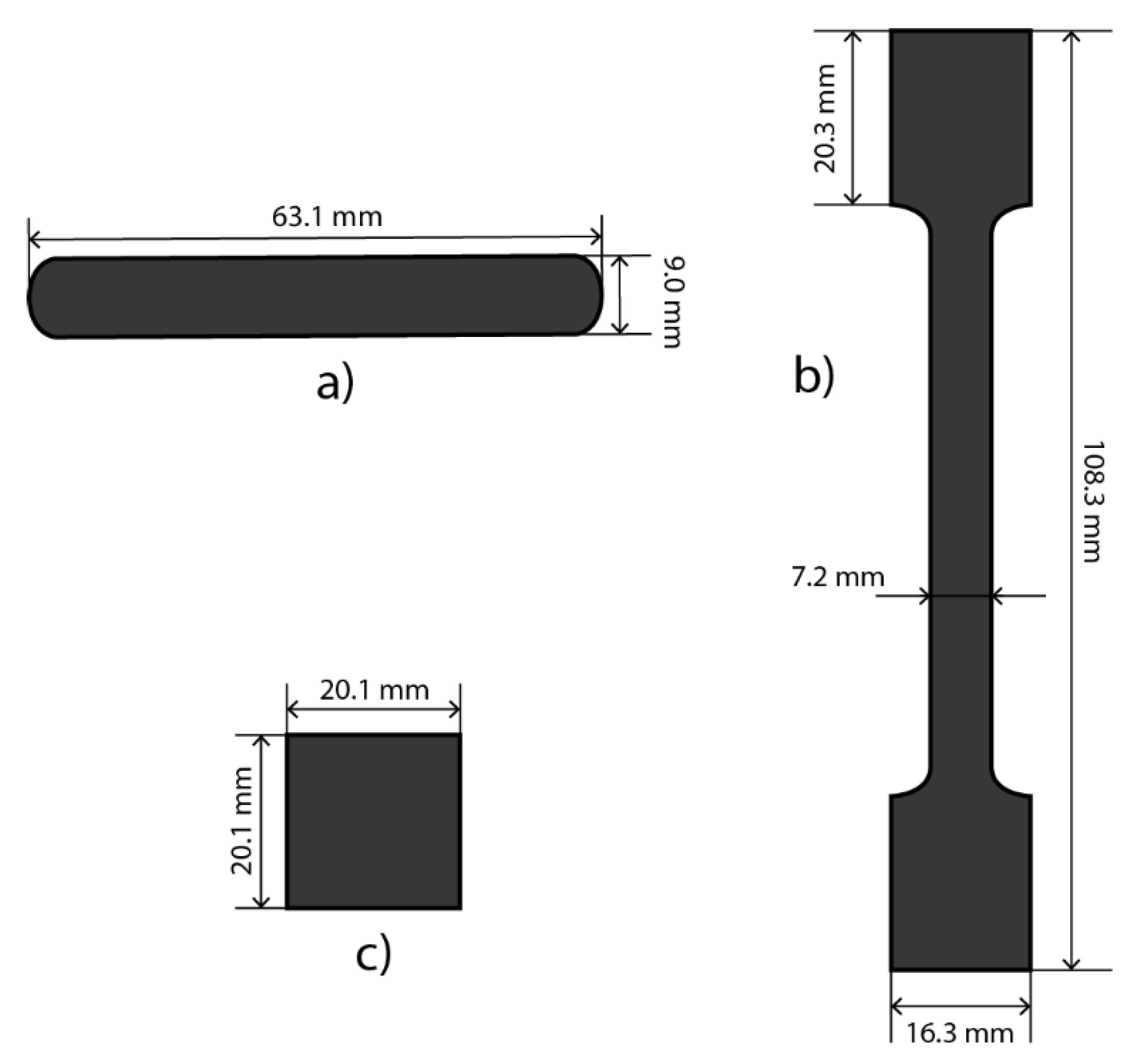
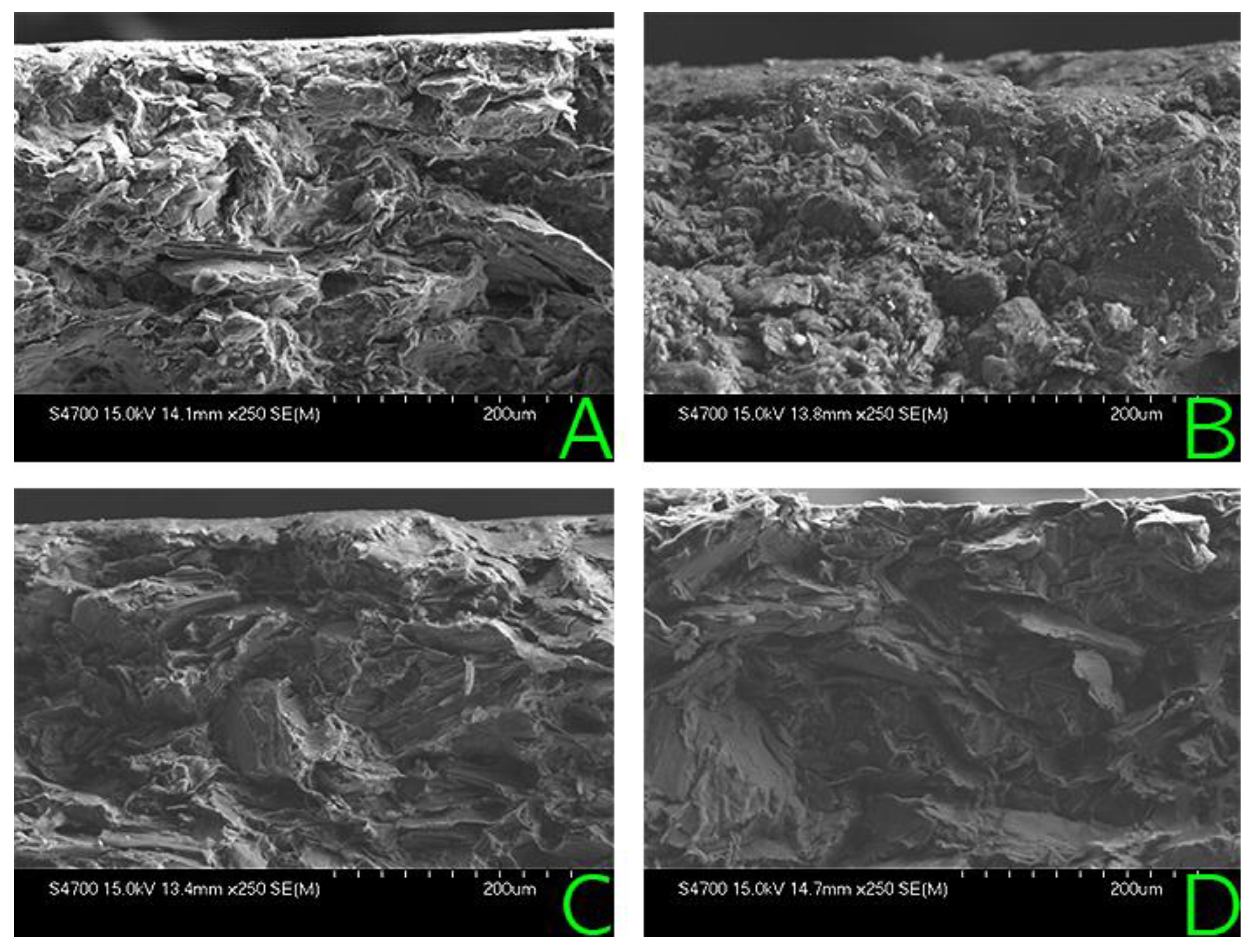
2.2.2. Mechanical Strength
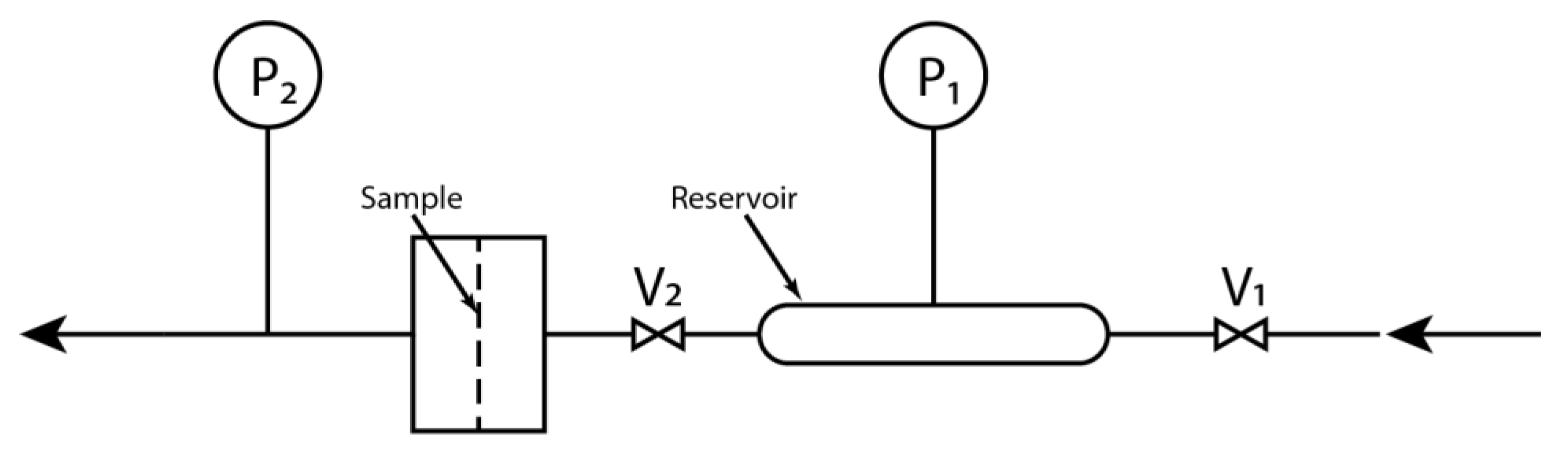
2.2.3. Permeability for Hydrogen
2.2.4. Water Uptake
2.2.5. Chemical Stability
2.2.6. Contact Angle
3. Results and Discussion
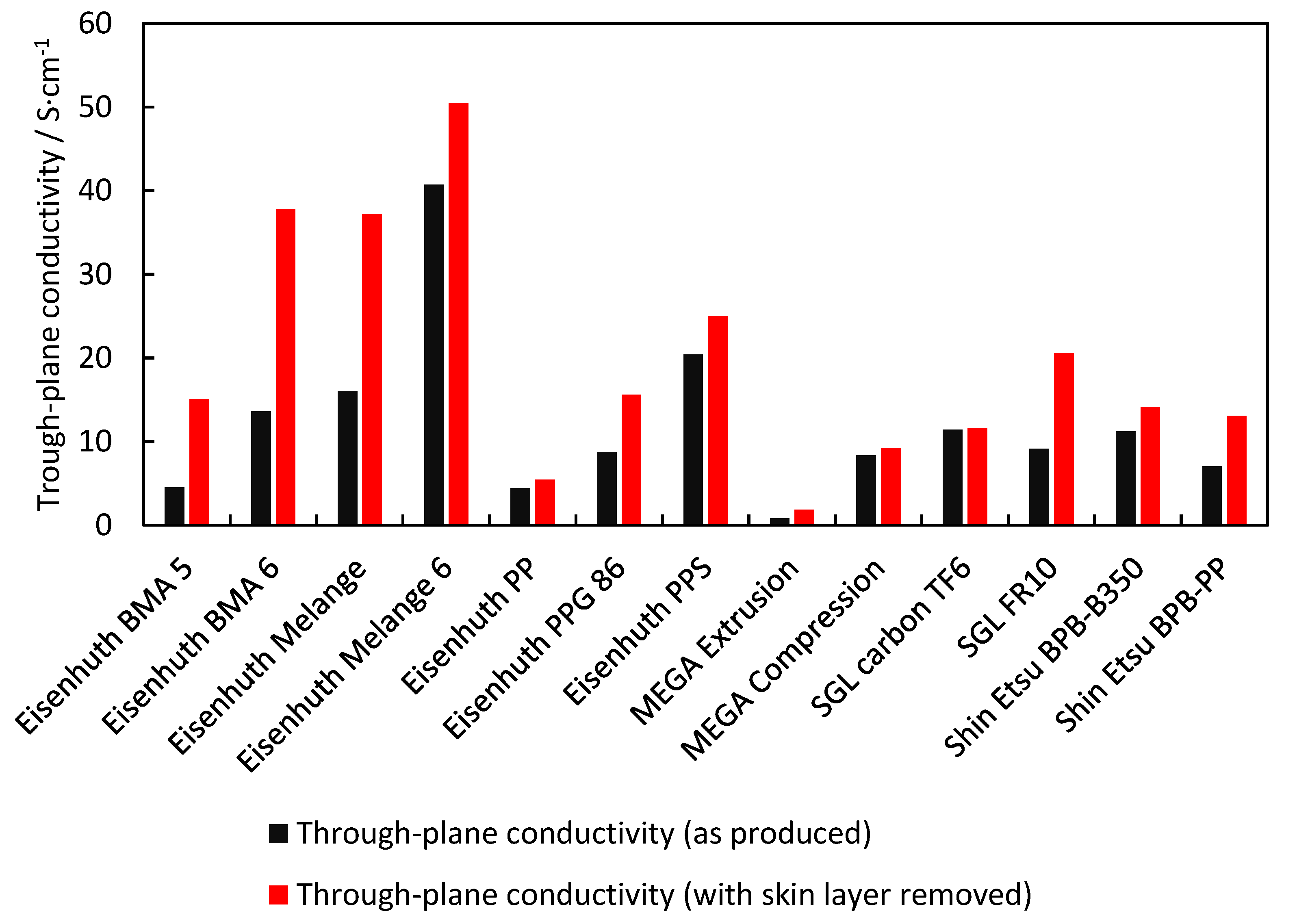
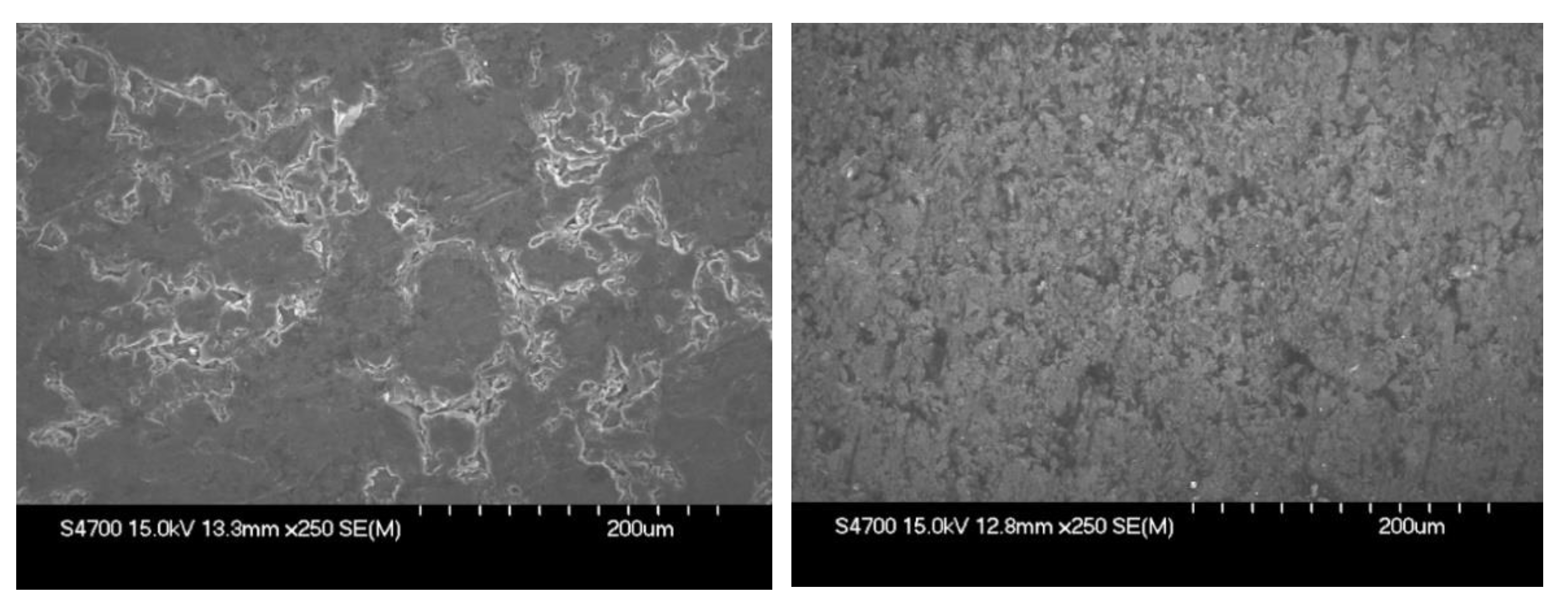
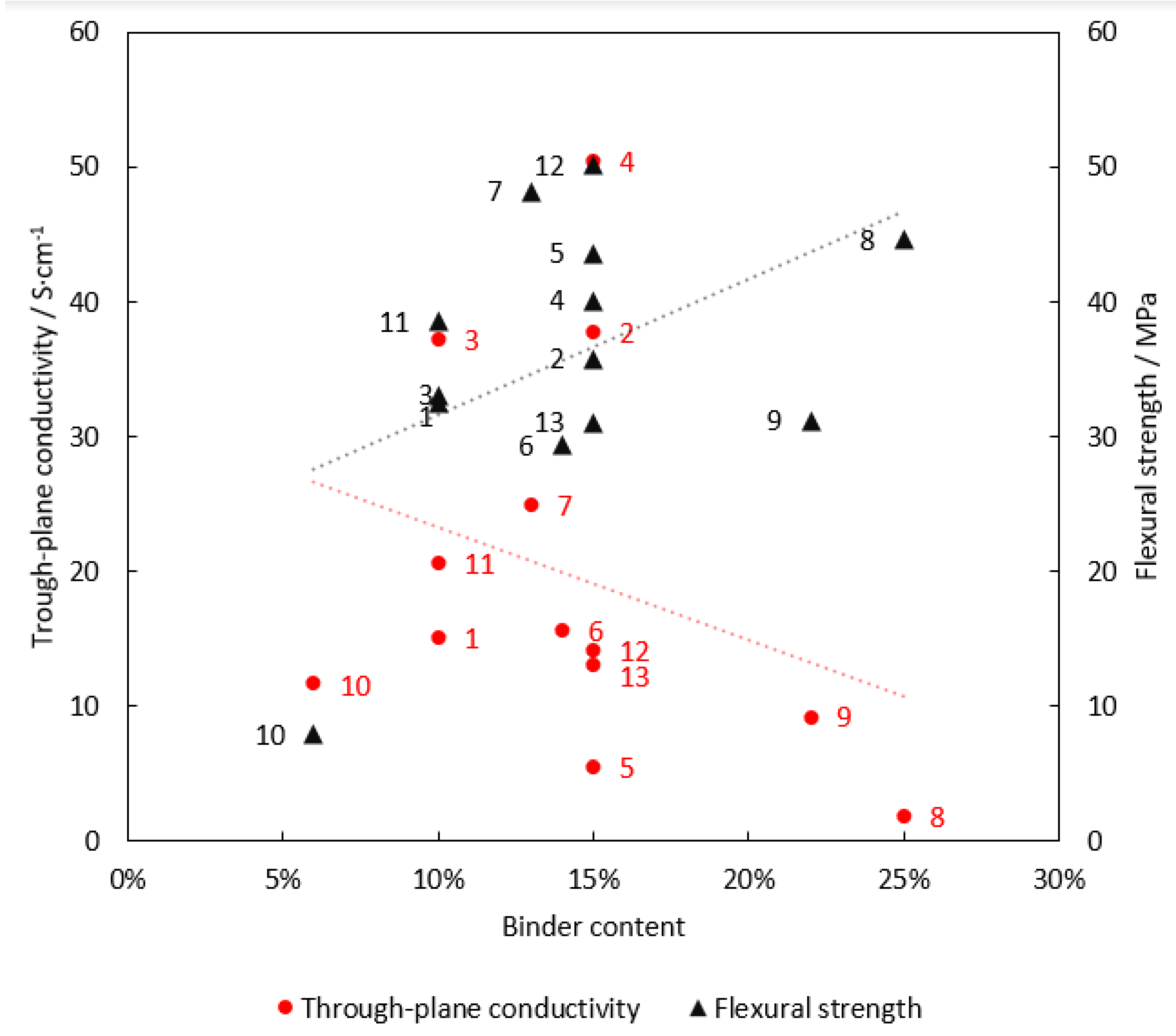
3.1. Electrical Conductivity
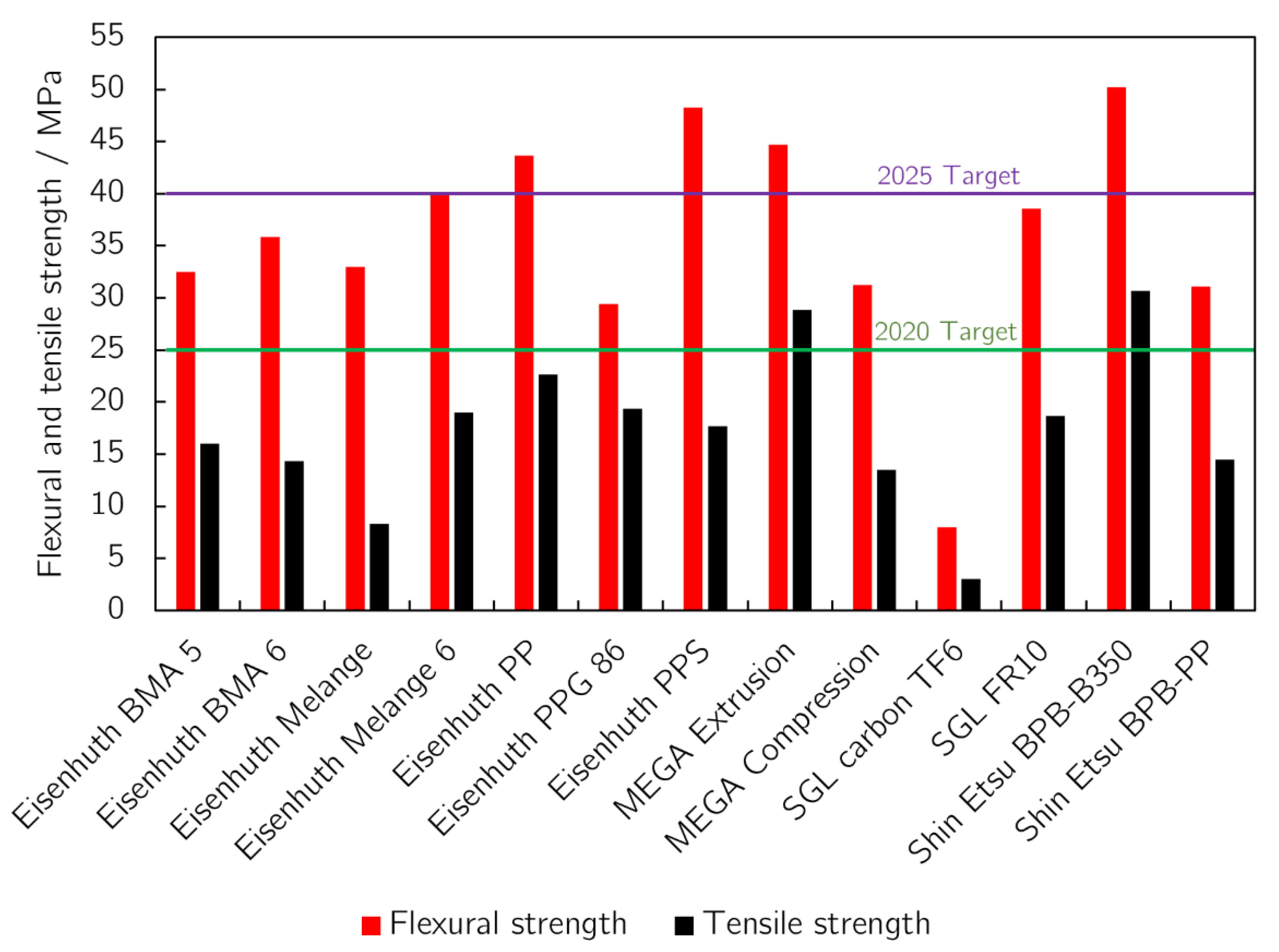
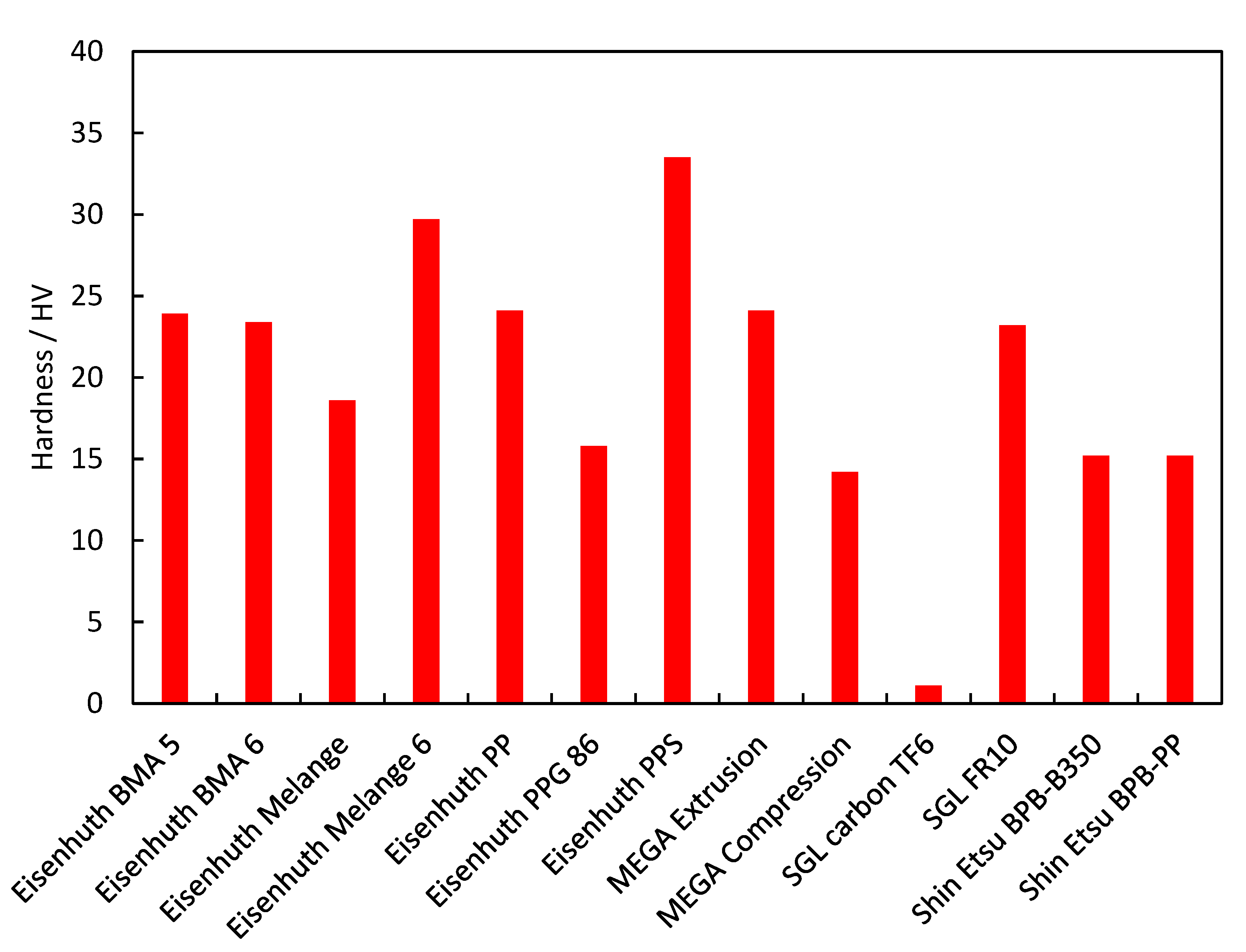
3.2. Mechanical Strength
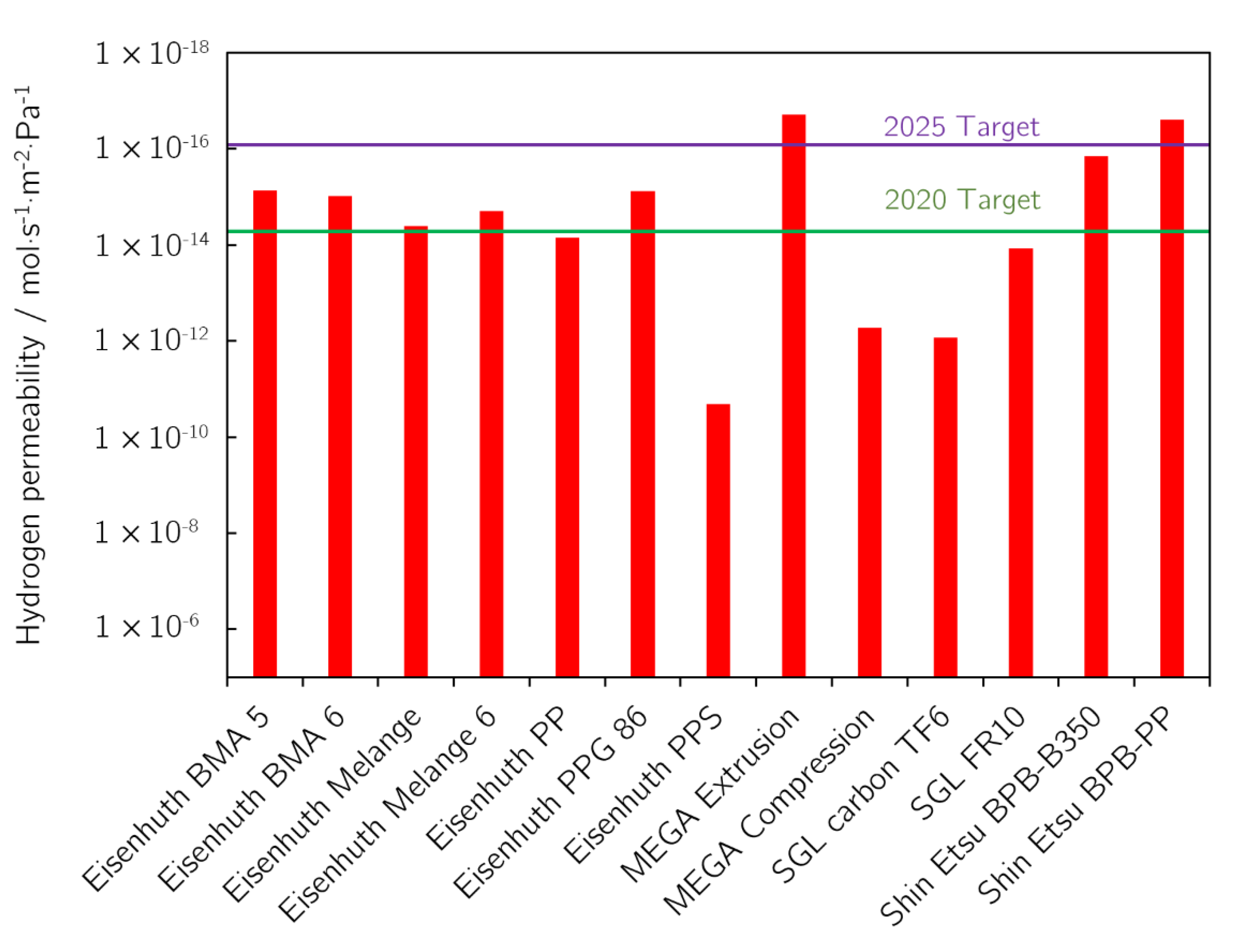
3.3. Permeability of Bipolar Plates for Hydrogen
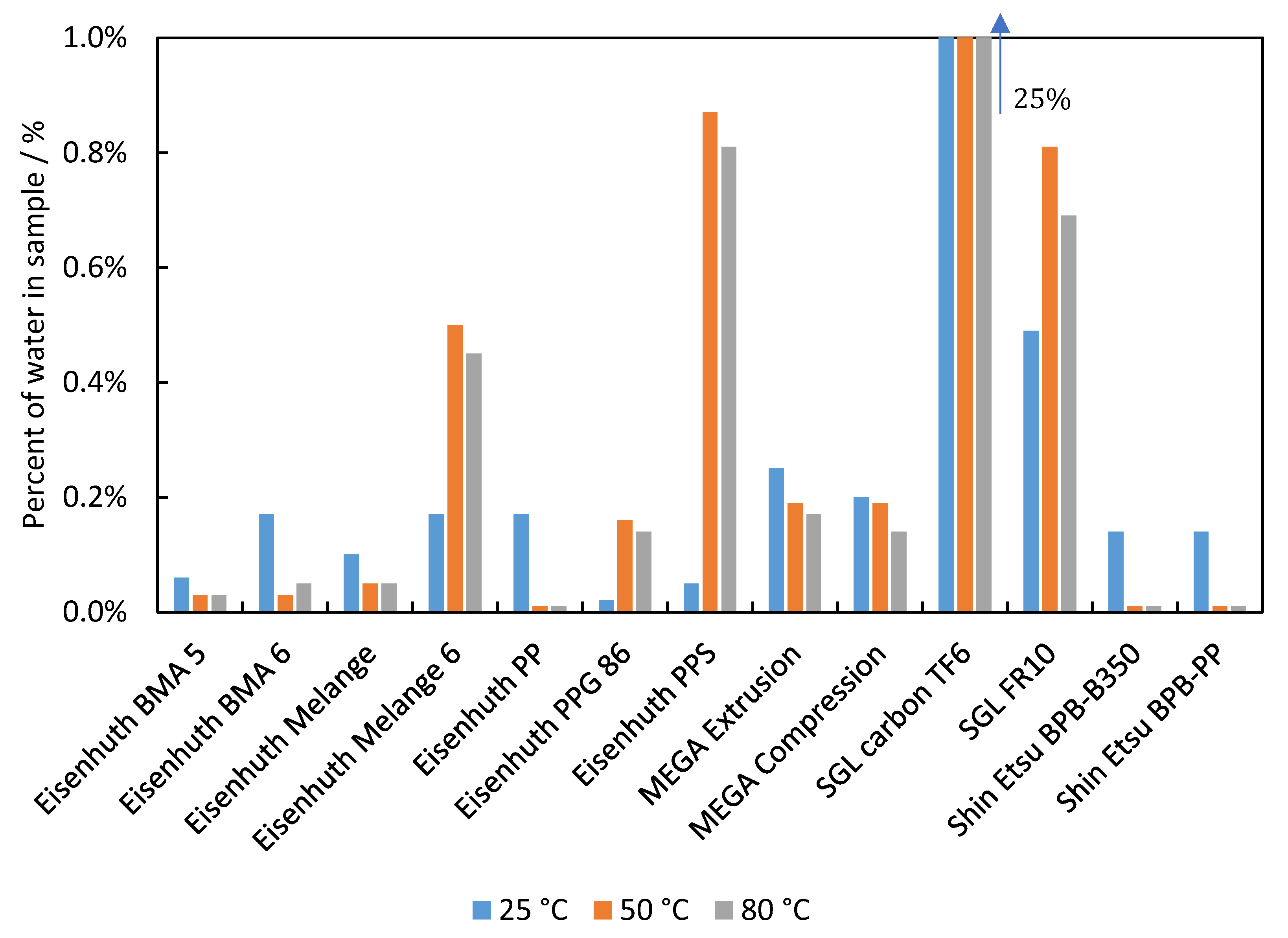
3.4. Water Uptake
3.5. Chemical Stability
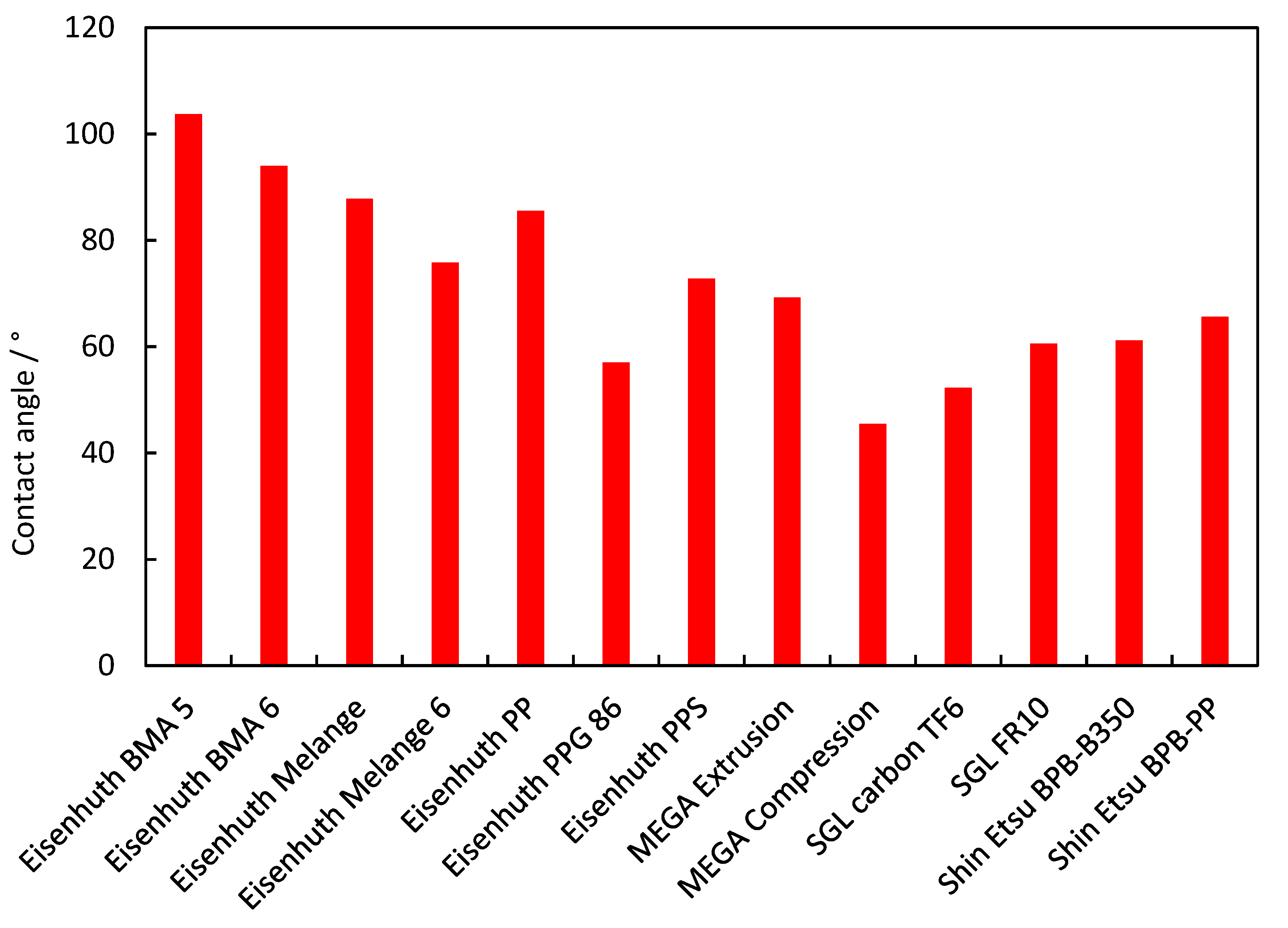
3.6. Contact Angle
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ajanovic, A.; Haas, R. Prospects and impediments for hydrogen and fuel cell vehicles in the transport sector. Int. J. Hydrogen Energy 2021, 46, 10049–10058. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Herve Bang, Y.; Di Noto, V. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Nazir, H.; Muthuswamy, N.; Louis, C.; Jose, S.; Prakash, J.; Buan, M.E.M.; Flox, C.; Chavan, S.; Shi, X.; Kauranen, P.; et al. Is the H2 economy realizable in the foreseeable future? Part III: H2 usage technologies, applications, and challenges and opportunities. Int. J. Hydrogen Energy 2020, 45, 28217–28239. [Google Scholar] [CrossRef] [PubMed]
- Guerrero Moreno, N.; Cisneros Molina, M.; Gervasio, D.; Pérez Robles, J.F. Approaches to polymer electrolyte membrane fuel cells (PEMFCs) and their cost. Renew. Sustain. Energy Rev. 2015, 52, 897–906. [Google Scholar] [CrossRef]
- Thompson, S.T.; James, B.D.; Huya-Kouadio, J.M.; Houchins, C.; DeSantis, D.A.; Ahluwalia, R.; Wilson, A.R.; Kleen, G.; Papageorgopoulos, D. Direct hydrogen fuel cell electric vehicle cost analysis: System and high-volume manufacturing description, validation, and outlook. J. Power Sources 2018, 399, 304–313. [Google Scholar] [CrossRef]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; et al. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Huya-Kouadio, J.M.; James, B.D.; Houchins, C. Meeting Cost and Manufacturing Expectations for Automotive Fuel Cell Bipolar Plates. ECS Trans. 2018, 83, 93–109. [Google Scholar] [CrossRef]
- Hermann, A.; Chaudhuri, T.; Spagnol, P. Bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2005, 30, 1297–1302. [Google Scholar] [CrossRef]
- Jung, S.-P.; Lee, C.-I.; Chen, C.-C.; Chang, W.-S.; Yang, C.-C. Development of novel proton exchange membrane fuel cells using stamped metallic bipolar plates. J. Power Sources 2015, 283, 429–442. [Google Scholar] [CrossRef]
- Bohackova, T.; Ludvik, J.; Kouril, M. Metallic Material Selection and Prospective Surface Treatments for Proton Exchange Membrane Fuel Cell Bipolar Plates—A Review. Materials 2021, 14, 2682. [Google Scholar] [CrossRef]
- Porstmann, S.; Wannemacher, T.; Drossel, W.G. A comprehensive comparison of state-of-the-art manufacturing methods for fuel cell bipolar plates including anticipated future industry trends. J. Manuf. Processes 2020, 60, 366–383. [Google Scholar] [CrossRef]
- Leng, Y.; Ming, P.; Yang, D.; Zhang, C. Stainless steel bipolar plates for proton exchange membrane fuel cells: Materials, flow channel design and forming processes. J. Power Sources 2020, 451. [Google Scholar] [CrossRef]
- Dundar, F.; Dur, E.; Mahabunphachai, S.; Koç, M. Corrosion resistance characteristics of stamped and hydroformed proton exchange membrane fuel cell metallic bipolar plates. J. Power Sources 2010, 195, 3546–3552. [Google Scholar] [CrossRef]
- Antunes, R.A.; Oliveira, M.C.L.; Ett, G.; Ett, V. Corrosion of metal bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2010, 35, 3632–3647. [Google Scholar] [CrossRef]
- Wu, S.; Yang, W.; Yan, H.; Zuo, X.; Cao, Z.; Li, H.; Shi, M.; Chen, H. A review of modified metal bipolar plates for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2021, 46, 8672–8701. [Google Scholar] [CrossRef]
- Saadat, N.; Dhakal, H.N.; Tjong, J.; Jaffer, S.; Yang, W.; Sain, M. Recent advances and future perspectives of carbon materials for fuel cell. Renew. Sustain. Energy Rev. 2021, 138. [Google Scholar] [CrossRef]
- Kang, K.; Park, S.; Ju, H. Effects of type of graphite conductive filler on the performance of a composite bipolar plate for fuel cells. Solid State Ionics 2014, 262, 332–336. [Google Scholar] [CrossRef]
- Saadat, N.; Dhakal, H.N.; Jaffer, S.; Tjong, J.; Yang, W.; Tan, J.; Sain, M. Expanded and nano-structured carbonaceous graphite for high performance anisotropic fuel cell polymer composites. Compos. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Wlodarczyk, R. Carbon-based materials for bipolar plates for low-temperatures PEM fuel cells—A review. Funct. Mater. Lett. 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Antunes, R.A.; de Oliveira, M.C.L.; Ett, G.; Ett, V. Carbon materials in composite bipolar plates for polymer electrolyte membrane fuel cells: A review of the main challenges to improve electrical performance. J. Power Sources 2011, 196, 2945–2961. [Google Scholar] [CrossRef]
- Wilberforce, T.; Ijaodola, O.; Ogungbemi, E.; Khatib, F.N.; Leslie, T.; El-Hassan, Z.; Thomposon, J.; Olabi, A.G. Technical evaluation of proton exchange membrane (PEM) fuel cell performance—A review of the effects of bipolar plates coating. Renew. Sustain. Energy Rev. 2019, 113, 109286. [Google Scholar] [CrossRef]
- Satola, B. Review—Bipolar Plates for the Vanadium Redox Flow Battery. J. Electrochem. Soc. 2021, 168, 060503. [Google Scholar] [CrossRef]
- U.S. DRIVE Fuel Cell Technical Team Roadmap; 2017; p. 10. Available online: https://www.energy.gov/sites/default/files/2017/11/f46/FCTT_Roadmap_Nov_2017_FINAL.pdf (accessed on 29 June 2022).
- DOE Technical Targets for Polymer Electrolyte Membrane Fuel Cell Components. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-polymer-electrolyte-membrane-fuel-cell-components (accessed on 29 June 2022).
- Jeong, K.I.; Oh, J.; Song, S.A.; Lee, D.; Lee, D.G.; Kim, S.S. A review of composite bipolar plates in proton exchange membrane fuel cells: Electrical properties and gas permeability. Compos. Struct. 2021, 262, 113617. [Google Scholar] [CrossRef]
- Kakati, B.K.; Deka, D. Differences in physico-mechanical behaviors of resol(e) and novolac type phenolic resin based composite bipolar plate for proton exchange membrane (PEM) fuel cell. Electrochim. Acta 2007, 52, 7330–7336. [Google Scholar] [CrossRef]
- Xia, L.-G.; Li, A.-J.; Wang, W.-Q.; Yin, Q.; Lin, H.; Zhao, Y.-B. Effects of resin content and preparing conditions on the properties of polyphenylene sulfide resin/graphite composite for bipolar plate. J. Power Sources 2008, 178, 363–367. [Google Scholar] [CrossRef]
- Kakati, B.K.; Ghosh, A.; Verma, A. Efficient composite bipolar plate reinforced with carbon fiber and graphene for proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2013, 38, 9362–9369. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3)x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- de Oliveira, M.C.L.; Ett, G.; Antunes, R.A. Materials selection for bipolar plates for polymer electrolyte membrane fuel cells using the Ashby approach. J. Power Sources 2012, 206, 3–13. [Google Scholar] [CrossRef]
- Liao, J.; Yang, J.; Li, Q.; Cleemann, L.N.; Jensen, J.O.; Bjerrum, N.J.; He, R.; Xing, W. Oxidative degradation of acid doped polybenzimidazole membranes and fuel cell durability in the presence of ferrous ions. J. Power Sources 2013, 238, 516–522. [Google Scholar] [CrossRef]
- Ghosh, A.; Goswami, P.; Mahanta, P.; Verma, A. Effect of carbon fiber length and graphene on carbon-polymer composite bipolar plate for PEMFC. J. Solid State Electrochem. 2014, 18, 3427–3436. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Xu, Y.; Guo, X.; Cao, Y.; Ming, W. Review: Modeling and Simulation of Membrane Electrode Material Structure for Proton Exchange Membrane Fuel Cells. Coatings 2022, 12, 1145. [Google Scholar] [CrossRef]

| Characteristics | Units | 2015 Status | 2020 Targets | 2025 Targets |
|---|---|---|---|---|
| Cost | USD·kWnet−1 | 7 | 3 | 2 |
| Plate weight | kg·kWnet−1 | <0.4 | 0.4 | 0.18 |
| Plate H2 permeation coefficient | Std cm3·s−1·cm−2·Pa @ 80 °C, 3 atm, 100% RH | 0 | <1.3 × 10−14 | 2 × 10−16 |
| Corrosion anode | µA·cm−2 | no active peak | 1 and no active peak | <1 and no active peak |
| Corrosion cathode | µA·cm−2 | <0,1 | <1 | <1 |
| Electrical conductivity | S·cm−1 | >100 | 100 | >100 |
| Area-specific resistance | Ω·cm2 | 0.006 | 0.01 | <0.01 |
| Flexural strength | MPa | >34 (carbon plate) | >25 | >40 |
| Manufacturer | Name | Binder Proportion | Manufacturing Process | Thickness/mm |
|---|---|---|---|---|
| Eisenhuth | BMA 5 | 10% fluoropolymer | extrusion | 4.34 |
| Eisenhuth | BMA 6 | 15% fluoropolymer | extrusion | 3.87 |
| Eisenhuth | Melange | 10% polyethylene | extrusion | 3.80 |
| Eisenhuth | Melange 6 | 15% polyethylene | extrusion | 3.74 |
| Eisenhuth | PP | 15% polypropylene | extrusion | 5.73 |
| Eisenhuth | PPG 86 | 14% polypropylene | extrusion | 2.04 |
| Eisenhuth | PPS | 13% polyphenylene sulfide | compression | 3.91 |
| MEGA | Extrusion | 25% polypropylene derivative | extrusion | 1.44 |
| MEGA | Compression | 22% polypropylene derivative | compression | 5.04 |
| SGL | carbon TF6 | 6% fluoropolymer | compression | 3.10 |
| SGL | FR10 | 10% thermoset | compression | 3.57 |
| Shin Etsu | BPB-B350 | 15% polyphenylene sulfide | extrusion | 3.04 |
| Shin Etsu | BPB-PP | 15% polypropylene | extrusion | 3.03 |
| Material | Thickness/mm | Conductivity (as produced)/S·cm−1 | Conductivity (skin layer removed)/S·cm−1 | Hydrogen permeability/mol·s−1·m−2·Pa−1 | Flexural strength/MPa | Tensile strength/MPa | Hardness/HV | Water uptake 25 °C/wt.% H2O | Water uptake 50 °C/wt.% H2O | Water uptake 80 °C/wt.% H2O | Density/kg·m−3 | Degradation (weight loss)/% | Contact angle /° |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2025 DOE Targets [23] | - | 100 | 100 | <2 × 10−16 | 40 | - | - | - | - | - | - | - | |
| Eisenhuth BMA 5 | 4.338 | 4.6 | 15.1 | 7.4 × 10−16 | 32.5 | 16 | 23.9 | 0.06 | 0.03 | 0.03 | 1822 | 0.01 | 103.7 |
| Eisenhuth BMA 6 | 3.874 | 13.6 | 37.7 | 9.8 × 10−16 | 35.8 | 14.3 | 23.4 | 0.17 | 0.03 | 0.05 | 2046 | 0.05 | 94 |
| Eisenhuth Melange | 3.802 | 16 | 37.2 | 4.0 × 10−15 | 33 | 8.3 | 18.6 | 0.10 | 0.05 | 0.05 | 2069 | 0.01 | 87.8 |
| Eisenhuth Melange 6 | 3.741 | 40.7 | 50.4 | 2.0 × 10−15 | 40.1 | 19 | 29.7 | 0.17 | 0.50 | 0.45 | 2020 | 0.08 | 75.8 |
| Eisenhuth PP | 5.728 | 4.4 | 5.5 | 6.9 × 10−15 | 43.7 | 22.7 | 24.1 | 0.17 | 0.01 | 0.01 | 1985 | 0.28 | 85.5 |
| Eisenhuth PPG 86 | 2.044 | 8.7 | 15.6 | 7.7 × 10−16 | 29.4 | 19.4 | 15.8 | 0.02 | 0.16 | 0.14 | 1800 | 0.08 | 57 |
| Eisenhuth PPS | 3.911 | 20.4 | 25 | 2.1 × 10−11 | 48.3 | 17.7 | 33.5 | 0.05 | 0.87 | 0.81 | 1946 | 2.52 | 72.8 |
| MEGA Extrusion | 1.439 | 0.8 | 1.8 | 1.9 × 10−17 | 44.7 | 28.9 | 24.1 | 0.25 | 0.19 | 0.17 | 1487 | 0.13 | 69.2 |
| MEGA Compression | 5.035 | 8.4 | 9.2 | 5.4 × 10−13 | 31.2 | 13.5 | 14.2 | 0.20 | 0.19 | 0.14 | 1565 | 0.00 | 45.5 |
| SGL Carbon TF6 | 3.086 | 11.5 | 11.6 | 8.5 × 10−13 | 8 | 3 | 1.1 | 24.01 | 24.39 | 23.74 | 957 | 0.00 | 52.3 |
| SGL FR10 | 3.569 | 9.1 | 20.6 | 1.2 × 10−14 | 38.6 | 18.7 | 23.2 | 0.49 | 0.81 | 0.69 | 1976 | 0.00 | 60.6/ 21.9 |
| Shin Etsu BPB-B350 | 3.036 | 11.2 | 14.1 | 1.4 × 10−16 | 50.2 | 30.7 | 15.2 | 0.14 | 0.01 | 0.01 | 1900 | 0.45 | 61.2 |
| Shin Etsu BPB-PP | 3.034 | 7.1 | 13.1 | 2.5 × 10−17 | 31.1 | 14.5 | 15.2 | 0.14 | 0.01 | 0.01 | 1740 | 0.02 | 65.6 |
| 2020 electrical conductivity satisfaction /% | 2025 electrical conductivity satisfaction /% | 2020 hydrogen permeability satisfaction /% | 2025 hydrogen permeability satisfaction /% | 2020 flexural strength satisfaction /% | 2025 flexural strength satisfaction /% | |
|---|---|---|---|---|---|---|
| 2025 DOE Targets | 100 | <5.3 × 10−15 | >25 | |||
| 2025 DOE Targets | 100 | <8.2 × 10−17 | >40 | |||
| Eisenhuth BMA 5 | 15 | 15 | 106 | 94 | 130 | 81 |
| Eisenhuth BMA 6 | 38 | 38 | 105 | 93 | 143 | 90 |
| Eisenhuth Melange | 37 | 37 | 101 | 89 | 132 | 83 |
| Eisenhuth Melange 6 | 50 | 50 | 103 | 91 | 160 | 100 |
| Eisenhuth PP | 6 | 6 | 99 | 88 | 175 | 109 |
| Eisenhuth PPG 86 | 16 | 16 | 106 | 94 | 118 | 74 |
| Eisenhuth PPS | 25 | 25 | 75 | 66 | 193 | 121 |
| MEGA Extrusion | 2 | 2 | 86 | 76 | 179 | 112 |
| MEGA Compression | 9 | 9 | 117 | 104 | 125 | 78 |
| SGL Carbon TF6 | 12 | 12 | 85 | 75 | 32 | 20 |
| SGL FR10 | 21 | 21 | 98 | 87 | 154 | 97 |
| Shin Etsu BPB-B350 | 14 | 14 | 111 | 99 | 201 | 126 |
| Shin Etsu BPB-PP | 13 | 13 | 116 | 103 | 124 | 78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hala, M.; Mališ, J.; Paidar, M.; Bouzek, K. Characterization of Commercial Polymer–Carbon Composite Bipolar Plates Used in PEM Fuel Cells. Membranes 2022, 12, 1050. https://doi.org/10.3390/membranes12111050
Hala M, Mališ J, Paidar M, Bouzek K. Characterization of Commercial Polymer–Carbon Composite Bipolar Plates Used in PEM Fuel Cells. Membranes. 2022; 12(11):1050. https://doi.org/10.3390/membranes12111050
Chicago/Turabian StyleHala, Miroslav, Jakub Mališ, Martin Paidar, and Karel Bouzek. 2022. "Characterization of Commercial Polymer–Carbon Composite Bipolar Plates Used in PEM Fuel Cells" Membranes 12, no. 11: 1050. https://doi.org/10.3390/membranes12111050
APA StyleHala, M., Mališ, J., Paidar, M., & Bouzek, K. (2022). Characterization of Commercial Polymer–Carbon Composite Bipolar Plates Used in PEM Fuel Cells. Membranes, 12(11), 1050. https://doi.org/10.3390/membranes12111050






