Effect of Cobalt Ferrite Nanoparticles in a Hydrophilic Shell on the Conductance of Bilayer Lipid Membrane
Abstract
:1. Introduction
2. Materials and Methods
3. Results
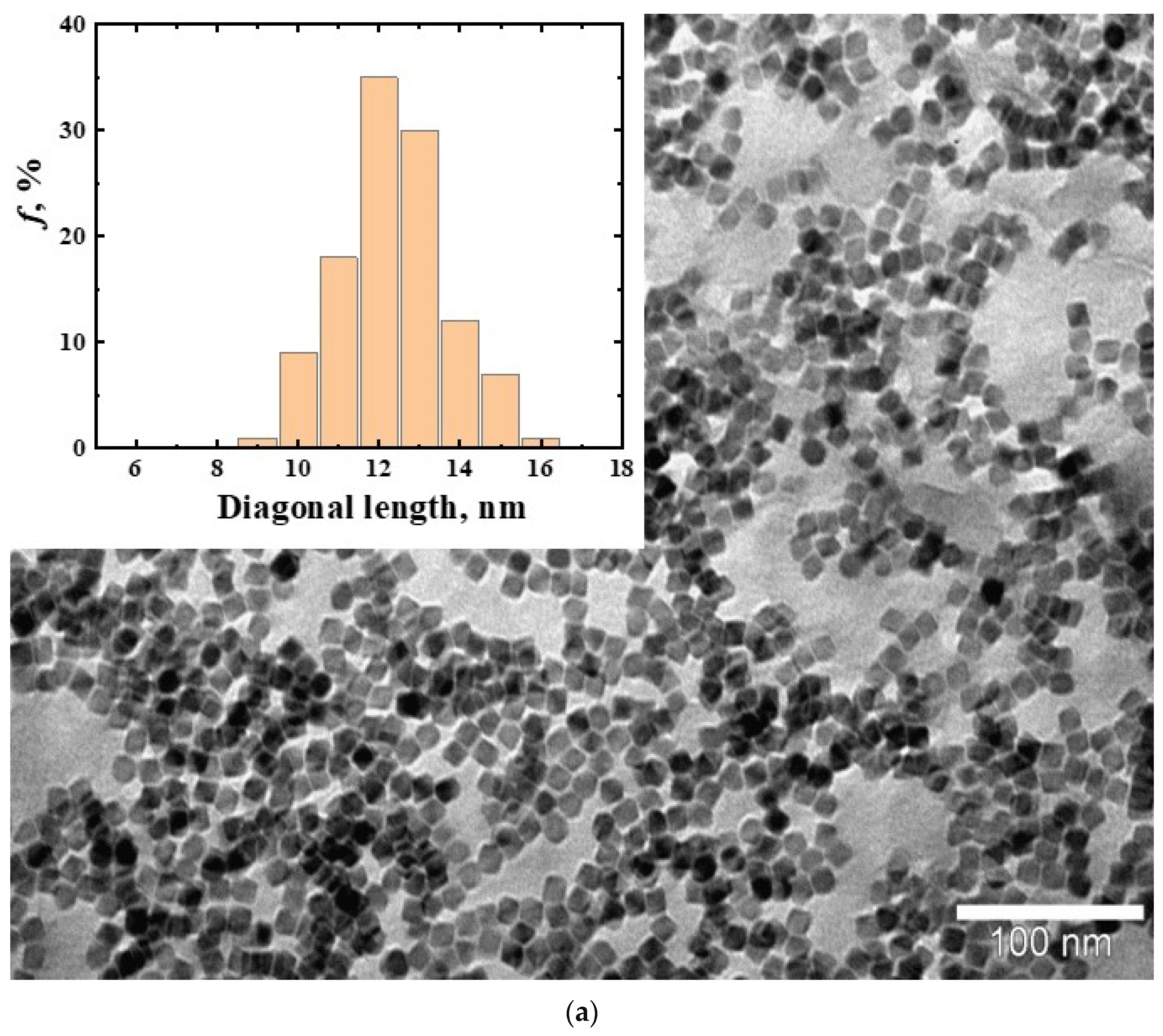
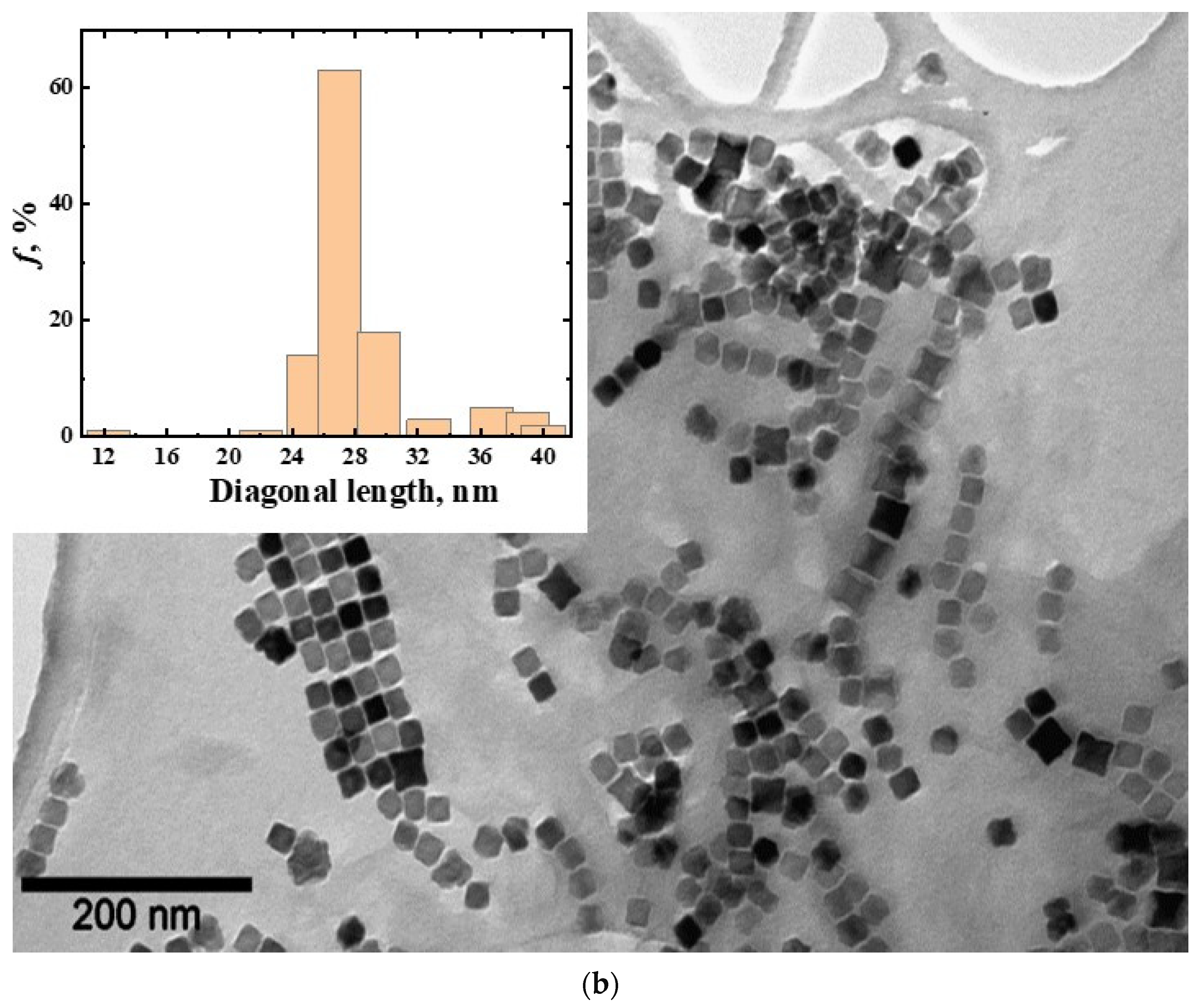
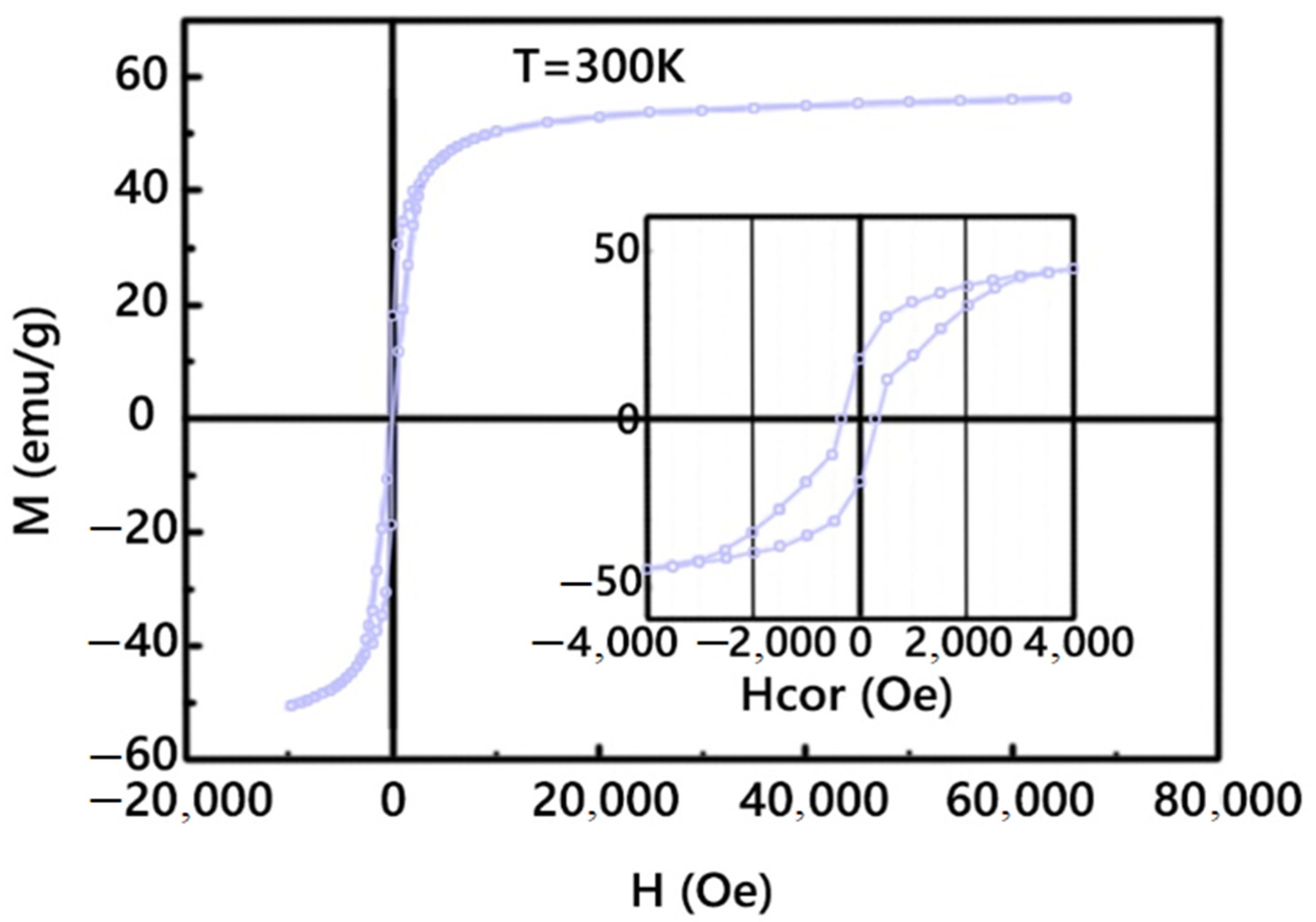
3.1. Magnetic Nanoparticles CoFe2O4
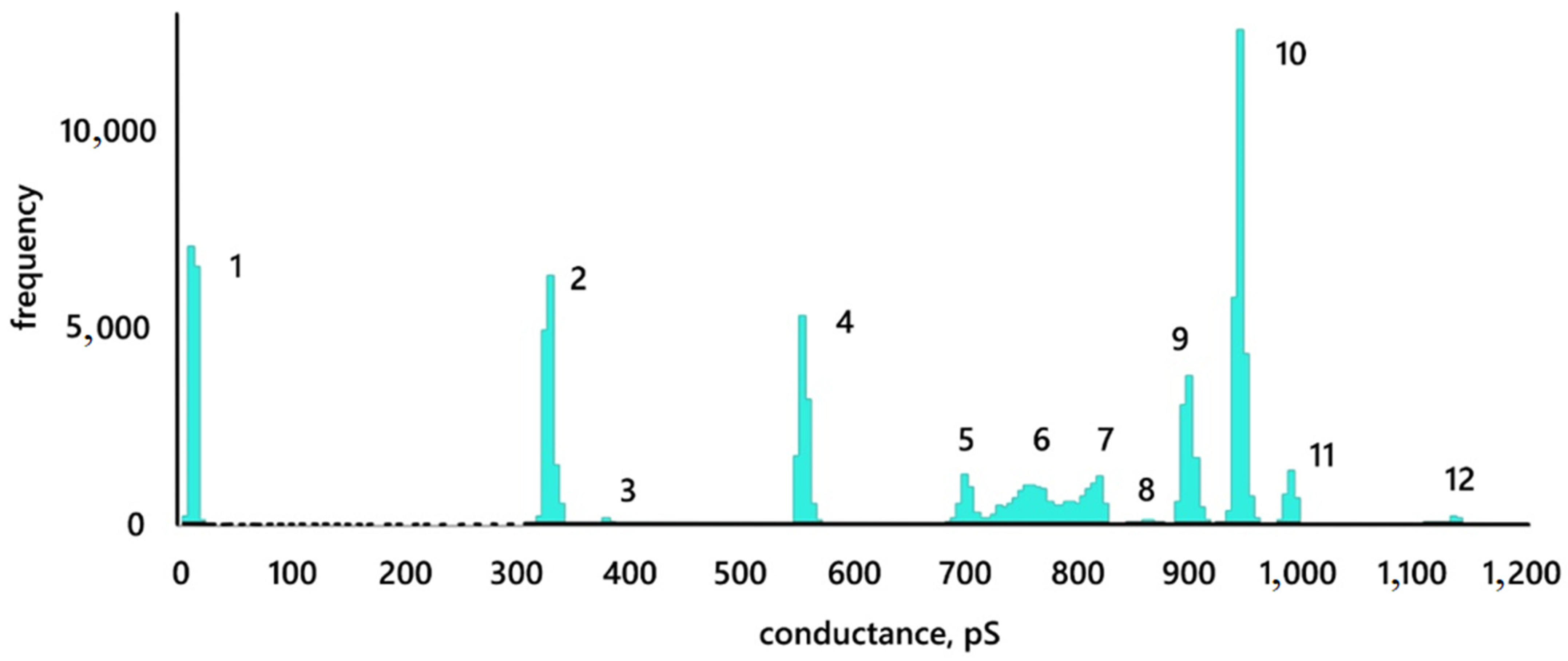
3.2. Conductance of Lipid Bilayer Membranes
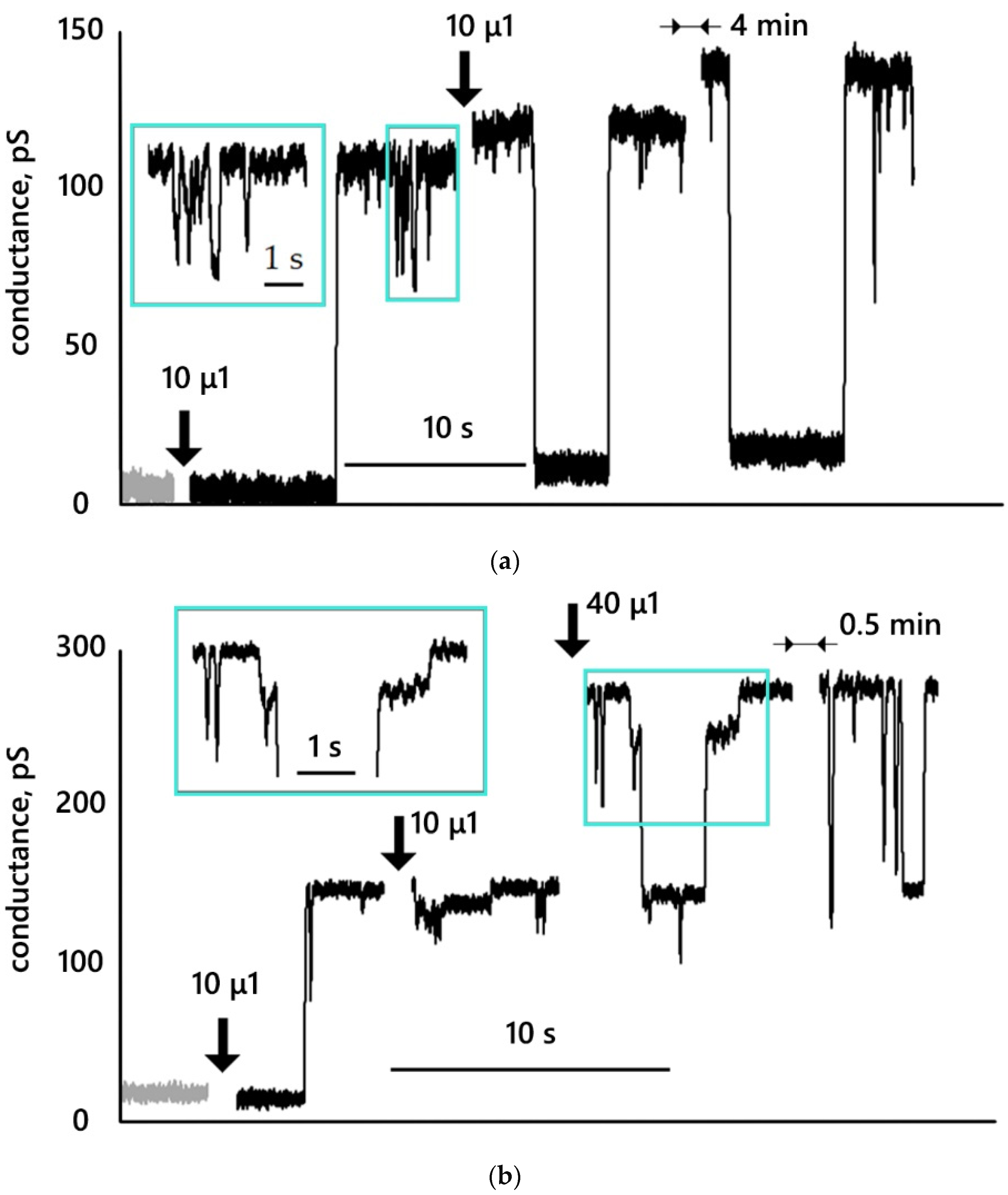
3.3. Conductance Traces
4. Discussion
5. Conclusions
- MNP12 and MNP27 with a hydrophilic coating interact with DPhPC bilayer membranes, which can lead to the appearance of metastable conductive pores, which, in turn, increase the integral conductance of the membranes. The conductance of membranes in the presence of MNPs with PEG is significantly lower than in the presence of MNPs with HSA.
- Conductive pores resulting from the interaction of nanoparticles with a membrane can change their size while remaining metastable structures. Those conductive pores can have several metastable states. If we assume the shape of the pore to be cylindrical, then the radii of the registered pores lie in the range from 0.4 to 1.3 nm.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Farnoud, A.M.; Nazemidashtarjandi, S. Emerging investigator series: Interactions of engineered nanomaterials with the cell plasma membrane; what have we learned from membrane models? Environ. Sci. Nano 2019, 6, 13–40. [Google Scholar] [CrossRef]
- Ruenraroengsak, P.; Novak, P.; Berhanu, D.; Thorley, A.J.; Valsami-Jones, E.; Gorelik, J.; Korchev, Y.; Tetley, T.D. Respiratory epithelial cytotoxicity and membrane damage (holes) caused by amine-modified nanoparticles. Nanotoxicology 2012, 6, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, X.; Ji, Y.; Bai, R.; Zhao, Y.; Wu, X.; Chen, C. Surface chemistry of gold nanorods: Origin of cell membrane damage and cytotoxicity. Nanoscale 2013, 5, 8384–8391. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Li, S.-J.; Feng, J.; Mei, P.; Ren, Z.-H.; Chang, Y.-L.; Liu, Y. Effects of surface charges on the bactericide activity of CdTe/ZnS quantum dots: A cell membrane disruption perspective. Langmuir 2017, 33, 2378–2386. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Malugin, A.; Ghandehari, H. Impact of silica nanoparticle design on cellular toxicity and hemolytic activity. ACS Nano 2011, 5, 5717–5728. [Google Scholar] [CrossRef] [PubMed]
- Rascol, E.; Devoisselle, J.-M.; Chopineau, J. The relevance of membrane models to understand nanoparticles–cell membrane interactions. Nanoscale 2016, 8, 4780–4798. [Google Scholar] [CrossRef]
- Broda, J.; Setzler, J.; Leifert, A.; Steitz, J.; Benz, R.; Simon, U.; Wenzel, W. Ligand-lipid and ligand-core affinity control the interaction of gold nanoparticles with artificial lipid bilayers and cell membranes. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1409–1419. [Google Scholar] [CrossRef]
- Lu, B.; Smith, T.; Schmidt, J.J. Nanoparticle–lipid Bilayer Interactions Studied with Lipid Bilayer Arrays. Nanoscale 2015, 7, 7858–7866. [Google Scholar] [CrossRef]
- Acharya, S.; Lu, B.; Edwards, S.; Toh, C.; Petersen, A.; Yong, C.; Lyu, P.; Huang, A.; Schmidt, J.J. Disruption of artificial lipid bilayers in the presence of transition metal oxide and rare earth metal oxide nanoparticle. J. Phys. D Appl. Phys. 2019, 52, 044002. [Google Scholar] [CrossRef]
- Ramachandran, S.; Merrill, N.E.; Blick, R.H.; van der Weide, D.W. Colloidal quantum dots initiating current bursts in lipid bilayers. Biosens. Bioelectron. 2005, 20, 2173–2176. [Google Scholar] [CrossRef]
- Chen, J.; Hessler, J.A.; Putchakayala, K.; Panama, B.K.; Khan, D.P.; Hong, S.; Mullen, D.G.; DiMaggio, S.C.; Som, A.; Tew, G.N.; et al. Cationic nanoparticles induce nanoscale disruption in living cell plasma membranes. J. Phys. Chem. B 2009, 113, 11179–11185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michel, R.; Gradzielski, M. Experimental aspects of colloidal interactions in mixed systems of liposome and inorganic nanoparticle and their applications. Int. J. Mol. Sci. 2012, 13, 11610–11642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulyaev, Y.V.; Cherepenin, V.A.; Vdovin, V.A.; Taranov, I.V.; Yaroslavov, A.A.; Kim, V.P.; Khomutov, G.B. Pulsed electric field-induced remote decapsulation of nanocomposite liposomes with implanted conducting nanoparticles. J. Commun. Technol. Electron. 2015, 60, 1097–1108. [Google Scholar] [CrossRef]
- Franco, V.; Dodrill, B. Magnetic Measurement Techniques for Materials Characterization, 1st ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2021; 820p. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Narayanaswamy, V.; Alaabed, S.; Sambasivam, S.; Muralee Gopi, C.V. Principles of magnetic hyperthermia: A focus on using multifunctional hybrid magnetic nanoparticles. Magnetochemistry 2019, 5, 67. [Google Scholar] [CrossRef] [Green Version]
- Asín, L.; Ibarra, M.R.; Tres, A.; Goya, G.F. Controlled cell death by magnetic hyperthermia: Effects of exposure time, field amplitude, and nanoparticle concentration. Pharm. Res. 2012, 29, 1319–1327. [Google Scholar] [CrossRef]
- Garanina, A.S.; Naumenko, V.A.; Nikitin, A.A.; Myrovali, E.; Petukhova, A.Y.; Klimyuk, S.V.; Nalench, Y.A.; Ilyasov, A.R.; Vodopyanov, S.S.; Erofeev, A.S.; et al. Temperature-controlled magnetic nanoparticles hyperthermia inhibits primary tumor growth and metastases dissemination. Nanomed. Nanotechnol. Biol. Med. 2020, 25, 102171. [Google Scholar] [CrossRef]
- Drašler, B.; Drobne, D.; Novak, S.; Valant, J.; Boljte, S.; Otrin, L.; Rappolt, M.; Sartori, B.; Iglič, A.; Kralj-Iglič, V.; et al. Effects of magnetic cobalt ferrite nanoparticles on biological and artificial lipid membranes. Int. J. Nanomed. 2014, 9, 1559–1581. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Zhang, L.F.; Bae, S.C.; Granick, S. Nanoparticle-induced surface reconstruction of phospholipid membranes. Proc. Natl. Acad. Sci. USA 2008, 105, 18171–18175. [Google Scholar] [CrossRef] [Green Version]
- de Planque, M.R.R.; Aghdaei, S.; Roose, T.; Morgan, H. Electrophysiological characterization of membrane disruption by nanoparticles. ACS Nano 2011, 5, 3599–3606. [Google Scholar] [CrossRef]
- Dawson, K.A.; Salvati, A.; Lynch, I. Nanoparticles reconstruct lipids. Nat. Nanotechnol. 2009, 4, 84–85. [Google Scholar] [CrossRef]
- Chen, R.; Choudhary, P.; Schurr, R.N.; Bhattacharya, P.; Brown, J.M.; Ke, P.C. Interaction of lipid vesicle with silver nanoparticle-serum albumin protein corona. Appl. Phys. Lett. 2012, 100, 13703–137034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anosov, A.A.; Korepanova, E.A.; Koplak, O.V.; Kazamanov, V.A.; Derunets, A.S.; Morgunov, R.B. The increase in electrical conductivity and the appearance of lipid pores induced by magnetic nanoparticles CoFe2O4 in bilayer lipid membranes. Russ. J. Electrochem. 2022, 58, 321–328. [Google Scholar] [CrossRef]
- Chubarov, A.S. Serum albumin for magnetic nanoparticles coating. Magnetochemistry 2022, 8, 13. [Google Scholar] [CrossRef]
- Sun, C.; Sze, R.; Zhang, M. Folic acid-PEG conjugated superparamagnetic nanoparticles for targeted cellular uptake and detection by MRI. J. Biomed. Mater. Res. A. 2006, 78, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, V.; Nikitin, A.; Kapitanova, K.; Melnikov, P.; Vodopyanov, S.; Garanina, A.; Valikhov, M.; Ilyasov, A.; Vishnevskiy, D.; Majouga, A.; et al. Intravital microscopy reveals a novel mechanism of nanoparticles excretion in kidney. J. Control. Release 2019, 307, 368–378. [Google Scholar] [CrossRef]
- Nikitin, A.A.; Yurenya, A.Y.; Zatsepin, T.S.; Aparin, I.O.; Chekhonin, V.P.; Majouga, A.G.; Farle, M.; Wiedwald, U.; Abakumov, M.A. Magnetic nanoparticles as a tool for remote DNA manipulations at a single-molecule level. ACS Appl. Mater. Interfaces 2021, 13, 14458–14469. [Google Scholar] [CrossRef]
- Mueller, P.; Rudin, D.; Tien, H.; Wescott, W. Reconstruction of cell membrane structure in vitro and its transformation into an excitable system. Nature 1962, 194, 979–980. [Google Scholar] [CrossRef]
- Johnson, M.E.; Simon, S.; Kauffman, J.W.; Macdonald, R.C. A synthetic lecithin containing branched-chain fatty acids: Physical properties and membrane studies. Biochim. Biophys. Acta (BBA) Biomembr. 1973, 291, 587–591. [Google Scholar] [CrossRef]
- Wang, S.; Larson, R.G. Water channel formation and ion transport in linear and branched lipid bilayers. Phys. Chem. Chem. Phys. 2014, 16, 7251–7262. [Google Scholar] [CrossRef]
- Tristram-Nagle, S.; Kim, D.J.; Akhunzada, N.; Kučerka, N.; Mathai, J.C.; Katsaras, J.; Zeidel, M.; Nagle, J.F. Structure and water permeability of fully hydrated diphytanoylPC. Chem. Phys. Lipids 2010, 163, 630–637. [Google Scholar] [CrossRef] [Green Version]
- Zecchi, K.A.; Heimburg, T. Nonlinear conductance, rectification and mechanosensitive channel formation of lipid membranes. Front. Cell. Dev. Biol. 2021, 26, 592520. [Google Scholar] [CrossRef] [PubMed]
- DeBlois, R.W.; Bean, C.P.; Wesley, R.K. Electrokinetic measurements with submicron particles and pores by the resistive pulse technique. J. Colloid Interface Sci. 1977, 61, 323–335. [Google Scholar] [CrossRef]
- Glaser, R.W.; Leikin, S.L.; Chernomordik, L.V.; Pastushenko, V.F.; Sokirko, A.I. Reversible electrical breakdown of lipid bilayers: Formation and evolution of pores. Biochim. Biophys. Acta 1988, 940, 275–287. [Google Scholar] [CrossRef]
- Lehtonen, J.Y.A.; Kinnunen, P.K.J. Changes in the lipid dynamic of liposomal membranes induced by poly(ethylene glycol): Free volume alterations revealed by inter- and intramolecular excimer forming phospholipid analogs. Biophys. J. 1994, 66, 1981–1990. [Google Scholar] [CrossRef] [Green Version]
- Antonov, V.F.; Smirnova, E.Y.; Anosov, A.A.; Norik, V.P.; Nemchenko, O.Y. PEG blocking of single pores arising on phase transitions in unmodified lipid bilayers. Biophysics 2008, 53, 390–395. [Google Scholar] [CrossRef]
- Anosov, A.A.; Sharakshane, A.A.; Smirnova, E.Y.; Nemchenko, O.Y. Bilayer permeability during phase transition as an Erlang flow of hydrophilic pores resulting from diffusion in the radius space. Biochem. Moscow Suppl. Ser. A. 2017, 11, 8–16. [Google Scholar] [CrossRef]
- Negoda, A.; Kim, K.J.; Crandall, E.D.; Worden, R.M. Polystyrene nanoparticle exposure induces ion-selective pores in lipid bilayers. Biochim. Biophys. Acta (BBA) Biomembr. 2013, 1828, 2215–2222. [Google Scholar] [CrossRef] [Green Version]
- Continia, C.; Schneemilcha, M.; Gaisfordb, S.; Quirkea, N. Nanoparticle–membrane interactions. J. Exp. Nanosci. 2018, 13, 62–81. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, X.; Gu, N. Computational investigation of interaction between nanoparticles and membranes: Hydrophobic/hydrophilic effect. J. Phys. Chem. B 2008, 112, 16647–16653. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle–cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef]
- Wohlert, J.; den Otter, W.K.; Edholm, O.; Briels, W.J. Free energy of a trans-membrane pore calculated from atomistic molecular dynamics simulations. J. Chem. Phys. 2006, 124, 154905. [Google Scholar] [CrossRef] [PubMed]
- Melikov, K.C.; Frolov, V.A.; Shcherbakov, A.; Samsonov, A.V.; Chizmadzhev, Y.A.; Chernomordik, L.V. Voltage-induced nonconductive pre-pores and metastable single pores in unmodified planar lipid bilayer. Biophys. J. 2001, 80, 1829–1836. [Google Scholar] [CrossRef] [Green Version]
- Awasthi, N.; Hub, J.S. Simulations of pore formation in lipid membranes: Reaction coordinates, convergence, hysteresis, and finite-size effects. J. Chem. Theory Comput. 2016, 12, 3261–3269. [Google Scholar] [CrossRef] [PubMed]
- Schüller, F.; Benz, R.; Sahl, H.G. The peptide antibiotic subtilin acts by formation of voltage-dependent multi-state pores in bacterial and artificial membranes. Eur. J. Biochem. 1989, 182, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Moll, G.N.; Roberts, G.C.; Konings, W.N.; Driessen, A.J.M. Mechanism of lantibiotic-induced pore-formation. Antonie Van Leeuwenhoek 1996, 69, 185–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaulin, Y.A.; Schagina, L.V.; Bezrukov, S.M.; Malev, V.V.; Feigin, A.M.; Takemoto, J.Y.; Teeter, J.H.; Brand, J.G. Cluster organization of ion channels formed by the antibiotic syringomycin E in bilayer lipid membranes. Biophys. J. 1998, 74, 2918–2925. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, T.; Sakoh, M.; Nagaoka, Y.; Asami, K. Ion channels of alamethicin dimer N-terminally linked by disulfide bond. Biophys. J. 2003, 85, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Laub, K.R.; Witschas, K.; Blicher, A.; Madsen, S.B.; Lückhoff, A.; Heimburg, T. Comparing ion conductance recordings of synthetic lipid bilayers with cell membranes containing TRP channels. Biochim. Biophys. Acta (BBA) Biomembr. 2012, 1818, 1123–1134. [Google Scholar] [CrossRef] [Green Version]
- Freeman, S.A.; Wang, M.A.; Weaver, J.C. Theory of electroporation of planar bilayer membranes: Predictions of the aqueous area, change in capacitance, and pore-pore separation. Biophys. J. 1994, 67, 42–56. [Google Scholar] [CrossRef]
- Powell, K.T.; Derrick, E.G.; Weaver, J.C. A quantitative theory of reversible electrical breakdown in bilayer membranes. Bioelectrochem. Bioenerg. 1986, 15, 243–255. [Google Scholar] [CrossRef]
- Anosov, A.A.; Smirnova, E.Y.; Ryleeva, E.D.; Gligonov, I.A.; Korepanova, E.A.; Sharakshane, A.A. Estimation of the parameters of the Smoluchowski equation describing the occurrence of pores in a bilayer lipid membrane under soft poration. Eur. Phys. J. E 2020, 43, 66. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.C.; Mintzer, R.A. Decreased bilayer stability due to transmembrane potentials. Physics Letters A 1981, 86, 57–59. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, G.; Wei, W. Simulation of nanoparticles interacting with a cell membrane: Probing the structural basis and potential biomedical application. NPG Asia Mater. 2021, 13, 52. [Google Scholar] [CrossRef]
- Gupta, R.; Rai, B. Effect of size and surface charge of gold nanoparticles on their skin permeability: A molecular dynamics study. Sci. Rep. 2017, 7, 45292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polak, A.; Tarek, M.; Tomšič, M.; Valant, J.; Ulrih, N.P.; Jamnik, A.; Kramar, P.; Miklavčič, D. Electroporation of archaeal lipid membranes using MD simulations. Bioelectrochemistry 2014, 100, 18–26. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anosov, A.; Koplak, O.; Smirnova, E.; Borisova, E.; Korepanova, E.; Derunets, A. Effect of Cobalt Ferrite Nanoparticles in a Hydrophilic Shell on the Conductance of Bilayer Lipid Membrane. Membranes 2022, 12, 1106. https://doi.org/10.3390/membranes12111106
Anosov A, Koplak O, Smirnova E, Borisova E, Korepanova E, Derunets A. Effect of Cobalt Ferrite Nanoparticles in a Hydrophilic Shell on the Conductance of Bilayer Lipid Membrane. Membranes. 2022; 12(11):1106. https://doi.org/10.3390/membranes12111106
Chicago/Turabian StyleAnosov, Andrey, Oksana Koplak, Elena Smirnova, Elizaveta Borisova, Eugenia Korepanova, and Alice Derunets. 2022. "Effect of Cobalt Ferrite Nanoparticles in a Hydrophilic Shell on the Conductance of Bilayer Lipid Membrane" Membranes 12, no. 11: 1106. https://doi.org/10.3390/membranes12111106
APA StyleAnosov, A., Koplak, O., Smirnova, E., Borisova, E., Korepanova, E., & Derunets, A. (2022). Effect of Cobalt Ferrite Nanoparticles in a Hydrophilic Shell on the Conductance of Bilayer Lipid Membrane. Membranes, 12(11), 1106. https://doi.org/10.3390/membranes12111106