The Relevance of GIRK Channels in Heart Function
Abstract
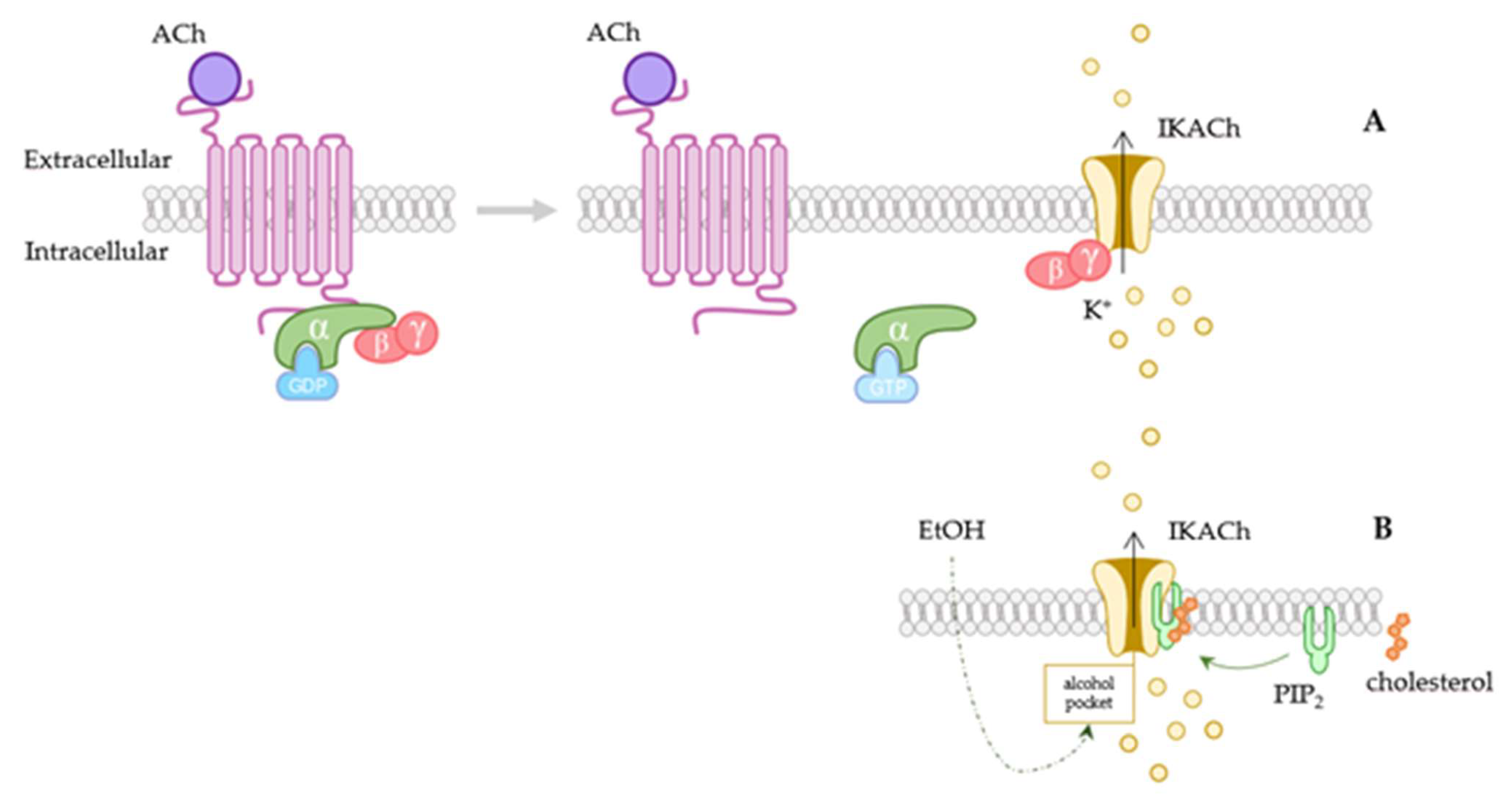
:1. Introduction
2. Structure and Signaling
2.1. GIRK1
2.2. GIRK2
2.3. GIRK3
2.4. GIRK4
3. GIRK Pharmacology
4. GIRK in the Heart
| Modification | Species | Effect in Cardiac Physiology | Reference |
|---|---|---|---|
| GIRK1 gene ablation GIRK4 gene ablation | Mouse | Loss of parasympathetic regulation Loss of heart rate dynamics | [55] |
| GIRK4 gene disruption | Mouse | IKACh effect in heart rate | [15] |
| GIRK4 knockout mice | Mouse | Atrial fibrillation | [88] |
| ↑ GIRK4 mRNA in sinoatrial myocytes | Dog | Heart failure | [89] |
| GIRK blockade | Dog | Suppression of atrial arrythmias | [90] |
| GIRK genetic variations | Human | Βγ-signaling pathway variations Heart-rate variations | [94] |
| GIRK4 mutation | Human | Familiar LQTS | [20] |
| IKACh constitutive activation | Human | Chronic atrial fibrillation | [95,96] |
| IKACh gradient current | Human | Paroxysmal atrial fibrillation | [97] |
| GIRK4 overexpression | Human | Protective against adenosine-induced atrial fibrillation | [98] |
| GIRK4 silencing | Human | Arrhythmia-control mechanism | [99] |
| GIRK4 gain-of-function | Human | Familial SND | [101] |
| GIRK overfunction | Human | Atrial fibrillation and SND | [102] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burg, S.; Attali, B. Targeting of Potassium Channels in Cardiac Arrhythmias. Trends Pharmacol. Sci. 2021, 42, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, J.; Caballero, R.; Gómez, R.; Valenzuela, C.; Delpón, E. Pharmacology of Cardiac Potassium Channels. Cardiovasc. Res. 2004, 62, 9–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyders, D.J. Structure and Function of Cardiac Potassium Channels. Cardiovasc. Res. 1999, 42, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Nichols, C.G.; Makhina, E.N.; Pearson, W.L.; Sha, Q.; Lopatin, A.N. Inward Rectification and Implications for Cardiac Excitability. Circ. Res. 1996, 78, 1–7. [Google Scholar] [CrossRef]
- Lüscher, C.; Slesinger, P.A. Emerging Concepts for G Protein-Gated Inwardly Rectifying Potassium (GIRK) Channels in Health and Disease. Nat. Rev. Neurosci. 2010, 11, 301. [Google Scholar] [CrossRef] [Green Version]
- Glaaser, I.W.; Slesinger, P.A. Structural Insights into GIRK Channel Function. Int. Rev. Neurobiol. 2015, 123, 117–160. [Google Scholar] [CrossRef]
- Pierce, K.L.; Premont, R.T.; Lefkowitz, R.J. Seven-Transmembrane Receptors. Nat. Rev. Mol. Cell Biol. 2002, 3, 639–650. [Google Scholar] [CrossRef]
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly Rectifying Potassium Channels: Their Structure, Function, and Physiological Roles. Physiol. Rev. 2010, 90, 291–366. [Google Scholar] [CrossRef] [Green Version]
- Kano, H.; Toyama, Y.; Imai, S.; Iwahashi, Y.; Mase, Y.; Yokogawa, M.; Osawa, M.; Shimada, I. Structural Mechanism Underlying G Protein Family-Specific Regulation of G Protein-Gated Inwardly Rectifying Potassium Channel. Nat. Commun. 2019, 10, 2008. [Google Scholar] [CrossRef] [Green Version]
- Dascal, N.; Kahanovitch, U. The Roles of Gβγ and Gα in Gating and Regulation of GIRK Channels. Int. Rev. Neurobiol. 2015, 123, 27–85. [Google Scholar] [CrossRef]
- Logothetis, D.E.; Kurachi, Y.; Galper, J.; Neer, E.J.; Clapham, D.E. The Βγ Subunits of GTP-Binding Proteins Activate the Muscarinic K+ Channel in Heart. Nature 1987, 325, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Wickman, K.D.; Iñiguez-Lluhi, J.A.; Davenport, P.A.; Taussig, R.; Krapivinsky, G.B.; Linder, M.E.; Gilman, A.G.; Clapham, D.E. Recombinant G-Protein Beta Gamma-Subunits Activate the Muscarinic-Gated Atrial Potassium Channel. Nature 1994, 368, 255–257. [Google Scholar] [CrossRef] [PubMed]
- DiFrancesco, D. Pacemaker Mechanisms in Cardiac Tissue. Annu. Rev. Physiol. 1993, 55, 455–472. [Google Scholar] [CrossRef] [PubMed]
- Gordan, R.; Gwathmey, J.K.; Xie, L.-H. Autonomic and Endocrine Control of Cardiovascular Function. World J. Cardiol. 2015, 7, 204. [Google Scholar] [CrossRef] [PubMed]
- Wickman, K.; Nemec, J.; Gendler, S.J.; Clapham, D.E. Abnormal Heart Rate Regulation in GIRK4 Knockout Mice. Neuron 1998, 20, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Luján, R.; Marron Fernandez de Velasco, E.; Aguado, C.; Wickman, K. New Insights into the Therapeutic Potential of Girk Channels. Trends Neurosci. 2014, 37, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Rai, D.; Akagi, T.; Shimohata, A.; Ishii, T.; Gangi, M.; Maruyama, T.; Wada-Kiyama, Y.; Ogiwara, I.; Kaneda, M. Involvement of the C-Terminal Domain in Cell Surface Localization and G-Protein Coupling of MGluR6. J. Neurochem. 2021, 158, 837–848. [Google Scholar] [CrossRef]
- Jeremic, D.; Sanchez-Rodriguez, I.; Jimenez-Diaz, L.; Navarro-Lopez, J.D. Therapeutic Potential of Targeting G Protein-Gated Inwardly Rectifying Potassium (GIRK) Channels in the Central Nervous System. Pharmacol. Ther. 2021, 223, 107808. [Google Scholar] [CrossRef]
- Jabbari, J.; Olesen, M.S.; Holst, A.G.; Nielsen, J.B.; Haunso, S.; Svendsen, J.H. Common Polymorphisms in KNCJ5 Are Associated with Early-Onset Lone Atrial Fibrillation in Caucasians. Cardiology 2011, 118, 116–120. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Liang, B.; Liu, J.; Li, J.; Grunnet, M.; Olesen, S.-P.; Rasmussen, H.B.; Ellinor, P.T.; Gao, L.; et al. Identification of a Kir3.4 Mutation in Congenital Long QT Syndrome. Am. J. Hum. Genet. 2010, 86, 872–880. [Google Scholar] [CrossRef]
- Loewi, O. Über Humorale Übertragbarkeit Der Herznervenwirkung. Pflüger’s Arch. Gesamte Physiol. Menschen Tiere 1921, 189, 239–242. [Google Scholar] [CrossRef]
- Borges, R.; García, A.G. One Hundred Years from Otto Loewi Experiment, a Dream That Revolutionized Our View of Neurotransmission. Pflügers Arch. Eur. J. Physiol. 2021, 473, 977–981. [Google Scholar] [CrossRef]
- Touhara, K.K.; Mackinnon, R. Molecular Basis of Signaling Specificity between GIRK Channels and GPCRs. eLife 2018, e42908. [Google Scholar] [CrossRef]
- Digby, G.J.; Sethi, P.R.; Lambert, N.A. Differential Dissociation of G Protein Heterotrimers. J. Physiol. 2008, 586, 3325–3335. [Google Scholar] [CrossRef]
- Wickman, K.; Pu, W.T.; Clapham, D.E. Structural Characterization of the Mouse Girk Genes. Gene 2002, 284, 241–250. [Google Scholar] [CrossRef]
- Wellner-Kienitz, M.C.; Bender, K.; Pott, L. Overexpression of Beta 1 and Beta 2 Adrenergic Receptors in Rat Atrial Myocytes. Differential Coupling to G Protein-Gated Inward Rectifier K(+) Channels via G(s) and G(i)/O. J. Biol. Chem. 2001, 276, 37347–37354. [Google Scholar] [CrossRef] [Green Version]
- Kuang, Q.; Purhonen, P.; Hebert, H. Structure of Potassium Channels. Cell Mol Life Sci. 2015, 72, 3677–3693. [Google Scholar] [CrossRef] [Green Version]
- Kofuji, P.; Davidson, N.; Lester, H.A. Evidence That Neuronal G-Protein-Gated Inwardly Rectifying K+ Channels Are Activated by G Beta Gamma Subunits and Function as Heteromultimers. Proc. Natl. Acad. Sci. USA 1995, 92, 6542–6546. [Google Scholar] [CrossRef] [Green Version]
- Stoffel, M.; Espinosa, R.; Powell, K.L.; Philipson, L.H.; Le Beau, M.M.; Bell, G.I. Human G-Protein-Coupled Inwardly Rectifying Potassium Channel (GIRK1) Gene (KCNJ3): Localization to Chromosome 2 and Identification of a Simple Tandem Repeat Polymorphism. Genomics 1994, 21, 254–256. [Google Scholar] [CrossRef]
- Kubo, Y.; Reuveny, E.; Slesinger, P.A.; Jan, Y.N.; Jan, L.Y. Primary Structure and Functional Expression of a Rat G-Protein-Coupled Muscarinic Potassium Channel. Nature 1993, 364, 802–806. [Google Scholar] [CrossRef]
- Mett, A.; Karbat, I.; Tsoory, M.; Fine, S.; Iwanir, S.; Reuveny, E. Reduced Activity of GIRK1-Containing Heterotetramers Is Sufficient to Affect Neuronal Functions, Including Synaptic Plasticity and Spatial Learning and Memory. J. Physiol. 2021, 599, 521–545. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.W.; Sui, J.L.; Vivaudou, M.; Logothetis, D.E. Control of Channel Activity through a Unique Amino Acid Residue of a g Protein-Gated Inwardly Rectifying K+ Channel Subunit. Proc. Natl. Acad. Sci. USA 1996, 93, 14193–14198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krapivinsky, G.; Gordon, E.A.; Wickman, K.; Velimirović, B.; Krapivinsky, L.; Clapham, D.E. The G-Protein-Gated Atrial K+ Channel IKACh Is a Heteromultimer of Two Inwardly Rectifying K(+)-Channel Proteins. Nature 1995, 374, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Bukiya, A.N.; Osborn, C.V.; Kuntamallappanavar, G.; Toth, P.T.; Baki, L.; Kowalsky, G.; Oh, M.J.; Dopico, A.M.; Levitan, I.; Rosenhouse-Dantsker, A. Cholesterol Increases the Open Probability of Cardiac KACh Currents. Biochim. Biophys. Acta 2015, 1848, 2406–2413. [Google Scholar] [CrossRef] [Green Version]
- Lippiello, P.; Hoxha, E.; Tempia, F.; Miniaci, M.C. GIRK1-Mediated Inwardly Rectifying Potassium Current Is a Candidate Mechanism Behind Purkinje Cell Excitability, Plasticity, and Neuromodulation. Cerebellum 2020, 19, 751–761. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Kong, S.; Zang, K.; Jiang, S.; Wan, L.; Chen, L.; Wang, G.; Jiang, M.; Wang, X.; et al. GIRK1-Mediated Inwardly Rectifying Potassium Current Suppresses the Epileptiform Burst Activities and the Potential Antiepileptic Effect of ML297. Biomed. Pharmacother. 2018, 101, 362–370. [Google Scholar] [CrossRef]
- Ponce, A.; Bueno, E.; Kentros, C.; Vega-Saenz de Miera, E.; Chow, A.; Hillman, D.; Chen, S.; Zhu, L.; Wu, M.; Wu, X.; et al. G-Protein-Gated Inward Rectifier K+ Channel Proteins (GIRK1) Are Present in the Soma and Dendrites as Well as in Nerve Terminals of Specific Neurons in the Brain. J. Neurosci. 1996, 16, 1990–2001. [Google Scholar] [CrossRef] [Green Version]
- Wagner, V.; Stadelmeyer, E.; Riederer, M.; Regitnig, P.; Gorischek, A.; DeVaney, T.; Schmidt, K.; Tritthart, H.A.; Hirschberg, K.; Bauernhofer, T.; et al. Cloning and Characterisation of GIRK1 Variants Resulting from Alternative RNA Editing of the KCNJ3 Gene Transcript in a Human Breast Cancer Cell Line. J. Cell. Biochem. 2010, 110, 598–608. [Google Scholar] [CrossRef]
- Kammerer, S.; Sokolowski, A.; Hackl, H.; Platzer, D.; Jahn, S.W.; El-Heliebi, A.; Schwarzenbacher, D.; Stiegelbauer, V.; Pichler, M.; Rezania, S.; et al. KCNJ3 Is a New Independent Prognostic Marker for Estrogen Receptor Positive Breast Cancer Patients. Oncotarget 2016, 7, 84705–84717. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K.; Iwayama, Y.; Toyota, T.; Ohnishi, T.; Ohba, H.; Maekawa, M.; Yoshikawa, T. Association Study of the KCNJ3 Gene as a Susceptibility Candidate for Schizophrenia in the Chinese Population. Hum. Genet. 2012, 131, 443–451. [Google Scholar] [CrossRef]
- Jelacic, T.M.; Sims, S.M.; Clapham, D.E. Functional Expression and Characterization of G-Protein-Gated Inwardly Rectifying K+ Channels Containing GIRK3. J. Membr. Biol. 1999, 169, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.J.; Jan, Y.N.; Jan, L.Y. Heteromultimerization of G-Protein-Gated Inwardly Rectifying K+ Channel Proteins GIRK1 and GIRK2 and Their Altered Expression in Weaver Brain. J. Neurosci. 1996, 16, 7137–7150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lüscher, C.; Jan, L.Y.; Stoffel, M.; Malenka, R.C.; Nicoll, R.A. G Protein-Coupled Inwardly Rectifying K+ Channels (GIRKs) Mediate Postsynaptic but Not Presynaptic Transmitter Actions in Hippocampal Neurons. Neuron 1997, 19, 687–695. [Google Scholar] [CrossRef] [Green Version]
- Hee, J.C.; Qian, X.; Ehlers, M.; Yuh, N.J.; Jan, L.Y. Neuronal Activity Regulates Phosphorylation-Dependent Surface Delivery of G Protein-Activated Inwardly Rectifying Potassium Channels. Proc. Natl. Acad. Sci. USA 2009, 106, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Inanobe, A.; Yoshimoto, Y.; Horio, Y.; Morishige, K.I.; Hibino, H.; Matsumoto, S.; Tokunaga, Y.; Maeda, T.; Hata, Y.; Takai, Y.; et al. Characterization of G-Protein-Gated K+ Channels Composed of Kir3.2 Subunits in Dopaminergic Neurons of the Substantia Nigra. J. Neurosci. 1999, 19, 1006–1017. [Google Scholar] [CrossRef] [Green Version]
- Arora, D.; Haluk, D.M.; Kourrich, S.; Pravetoni, M.; Fernández-Alacid, L.; Nicolau, J.C.; Luján, R.; Wickman, K. Altered Neurotransmission in the Mesolimbic Reward System of Girk−/− Mice. J. Neurochem. 2010, 114, 1487. [Google Scholar] [CrossRef] [Green Version]
- Whorton, M.R.; MacKinnon, R. X-Ray Structure of the Mammalian GIRK2-Βγ G-Protein Complex. Nature 2013, 498, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Ikeda, K.; Ichikawa, T.; Abe, S.; Togashi, S.; Kumanishi, T. Molecular Cloning of a Mouse G-Protein-Activated K+ Channel (MGIRK1) and Distinct Distributions of 3 GIRK (GIRK1, 2 and 3) MRNAs in Mouse Brain. Biochem. Biophys. Res. Commun. 1995, 208, 1166–1173. [Google Scholar] [CrossRef]
- Karschin, C.; Schreibmayer, W.; Dascal, N.; Lester, H.; Davidson, N.; Karschin, A. Distribution and Localization of a G Protein-Coupled Inwardly Rectifying K+ Channel in the Rat. FEBS Lett. 1994, 348, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.C.; Ehrhard, P.; Goldowitz, D.; Smeyne, R.J. Developmental Expression of the GIRK Family of Inward Rectifying Potassium Channels: Implications for Abnormalities in the Weaver Mutant Mouse. Brain Res. 1997, 778, 251–264. [Google Scholar] [CrossRef]
- Herman, M.A.; Sidhu, H.; Stouffer, D.G.; Kreifeldt, M.; Le, D.; Cates-Gatto, C.; Munoz, M.B.; Roberts, A.J.; Parsons, L.H.; Roberto, M.; et al. GIRK3 Gates Activation of the Mesolimbic Dopaminergic Pathway by Ethanol. Proc. Natl. Acad. Sci. USA. 2015, 112, 7091–7096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, A.D.; Carroll, M.E.; Loth, A.K.; Stoffel, M.; Wickman, K. Decreased Cocaine Self-Administration in Kir3 Potassium Channel Subunit Knockout Mice. Neuropsychopharmacology 2003, 285, 932–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, M.; Inanobe, A.; Kurachi, Y. G Protein Regulation of Potassium Ion Channels. Pharmacol. Rev. 1998, 50, 723. [Google Scholar] [PubMed]
- Dobrzynski, H.; Marples, D.D.R.; Musa, H.; Yamanushi, T.T.; Henderson, Z.; Takagishi, Y.; Honjo, H.; Kodama, I.; Boyett, M.R. Distribution of the Muscarinic K+ Channel Proteins Kir3.1 and Kir3.4 in the Ventricle, Atrium, and Sinoatrial Node of Heart. J. Histochem. Cytochem. 2001, 49, 1221–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.W.; Anderson, A.; Guzman, P.A.; Nakano, A.; Tolkacheva, E.G.; Wickman, K. Atrial GIRK Channels Mediate the Effects of Vagus Nerve Stimulation on Heart Rate Dynamics and Arrhythmogenesis. Front. Physiol. 2018, 9, 943. [Google Scholar] [CrossRef]
- Wickman, K.; Karschin, C.; Karschin, A.; Picciotto, M.R.; Clapham, D.E. Brain Localization and Behavioral Impact of the G-Protein-Gated K+ Channel Subunit GIRK4. J. Neurosci. 2000, 20, 5608. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.; Scholl, U.I.; Yue, P.; Björklund, P.; Zhao, B.; Nelson-Williams, C.; Ji, W.; Cho, Y.; Patel, A.; Men, C.J.; et al. K+ Channel Mutations in Adrenal Aldosterone-Producing Adenomas and Hereditary Hypertension. Science 2011, 331, 768–772. [Google Scholar] [CrossRef] [Green Version]
- Scholl, U.I.; Nelson-Williams, C.; Yue, P.; Grekin, R.; Wyatt, R.J.; Dillon, M.J.; Couch, R.; Hammer, L.K.; Harley, F.L.; Farhi, A.; et al. Hypertension with or without Adrenal Hyperplasia Due to Different Inherited Mutations in the Potassium Channel KCNJ5. Proc. Natl. Acad. Sci. USA 2012, 109, 2533–2538. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Ikeda, K.; Kojima, H.; Niki, H.; Yano, R.; Yoshioka, T.; Kumanishi, T. Ethanol Opens G-Protein-Activated Inwardly Rectifying K+ Channels. Nat. Neurosci. 1999, 212, 1091–1097. [Google Scholar] [CrossRef]
- Lewohl, J.M.; Wilson, W.R.; Mayfield, R.D.; Brozowski, S.J.; Morrisett, R.A.; Harris, R.A. G-Protein-Coupled Inwardly Rectifying Potassium Channels Are Targets of Alcohol Action. Nat. Neurosci. 1999, 2, 1084–1090. [Google Scholar] [CrossRef]
- Aryal, P.; Dvir, H.; Choe, S.; Slesinger, P.A. A Discrete Alcohol Pocket Involved in GIRK Channel Activation. Nat. Neurosci. 2009, 12, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Bodhinathan, K.; Slesinger, P.A. Molecular Mechanism Underlying Ethanol Activation of G-Protein-Gated Inwardly Rectifying Potassium Channels. Proc. Natl. Acad. Sci. USA 2013, 110, 18309–18314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Ung, P.M.U.; Zahoránszky-Kőhalmi, G.; Zakharov, A.V.; Martinez, N.J.; Simeonov, A.; Glaaser, I.W.; Rai, G.; Schlessinger, A.; Marugan, J.J.; et al. Identification of a G-Protein-Independent Activator of GIRK Channels. Cell Rep. 2020, 31, 107770. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, K.; Romaine, I.; Days, E.; Pascual, C.; Malik, A.; Yang, L.; Zou, B.; Du, Y.; Sliwoski, G.; Morrison, R.D.; et al. ML297 (VU0456810), the First Potent and Selective Activator of the GIRK Potassium Channel, Displays Antiepileptic Properties in Mice. ACS Chem. Neurosci. 2013, 4, 1278–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabata, T.; Haruki, S.; Nakayama, H.; Kano, M. GABAergic Activation of an Inwardly Rectifying K+ Current in Mouse Cerebellar Purkinje Cells. J. Physiol. 2005, 563, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Bodhinathan, K.; Slesinger, P.A. Alcohol Modulation of G-Protein-Gated Inwardly Rectifying Potassium Channels: From Binding to Therapeutics. Front. Physiol. 2014, 5, 76. [Google Scholar] [CrossRef] [Green Version]
- Mathiharan, Y.K.; Glaaser, I.W.; Zhao, Y.; Robertson, M.J.; Skiniotis, G.; Slesinger, P.A. Structural Insights into GIRK2 Channel Modulation by Cholesterol and PIP2. Cell Rep. 2021, 36, 109619. [Google Scholar] [CrossRef]
- Glaaser, I.W.; Slesinger, P.A. Dual Activation of Neuronal G Protein-Gated Inwardly Rectifying Potassium (GIRK) Channels by Cholesterol and Alcohol. Sci. Rep. 2017, 7, 4592. [Google Scholar] [CrossRef] [Green Version]
- Chen, I.S.; Tateyama, M.; Fukata, Y.; Uesugi, M.; Kubo, Y. Ivermectin Activates GIRK Channels in a PIP2-Dependent, Gβγ-Independent Manner and an Amino Acid Residue at the Slide Helix Governs the Activation. J. Physiol. 2017, 595, 5895–5912. [Google Scholar] [CrossRef] [Green Version]
- Logothetis, D.E.; Mahajan, R.; Adney, S.K.; Ha, J.; Kawano, T.; Meng, X.Y.; Cui, M. Unifying Mechanism of Controlling Kir3 Channel Activity by G Proteins and Phosphoinositides. Int. Rev. Neurobiol. 2015, 123, 1–26. [Google Scholar] [CrossRef]
- Niu, Y.; Tao, X.; Touhara, K.K.; Mackinnon, R. Cryo-Em Analysis of Pip2 Regulation in Mammalian Girk Channels. eLife 2020, 9, e60552. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Washiyama, K.; Ikeda, K. Inhibition of G Protein-Activated Inwardly Rectifying K+ Channels by Fluoxetine (Prozac). Br. J. Pharmacol. 2003, 138, 1119–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Washiyama, K.; Ikeda, K. Inhibition of G Protein-Activated Inwardly Rectifying K+ Channels by the Antidepressant Paroxetine. J. Pharmacol. Sci. 2006, 102, 278–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Washiyama, K.; Ikeda, K. Inhibition of G-Protein-Activated Inwardly Rectifying K+ Channels by the Selective Norepinephrine Reuptake Inhibitors Atomoxetine and Reboxetine. Neuropsychopharmacology 2010, 35, 1560–1569. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Chisari, M.; Raehal, K.M.; Kaltenbronn, K.M.; Bohn, L.M.; Mennerick, S.J.; Blumer, K.J. GIRK Channel Modulation by Assembly with Allosterically Regulated RGS Proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 19977–19982. [Google Scholar] [CrossRef] [Green Version]
- Doupnik, C.A. RGS Redundancy and Implications in GPCR-GIRK Signaling. Int. Rev. Neurobiol. 2015, 123, 87–116. [Google Scholar] [CrossRef]
- Cifelli, C.; Rose, R.A.; Zhang, H.; Voigtlaender-Bolz, J.; Bolz, S.S.; Backx, P.H.; Heximer, S.P. RGS4 Regulates Parasympathetic Signaling and Heart Rate Control in the Sinoatrial Node. Circ. Res. 2008, 103, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Huang, J.; Maity, B.; Gao, Z.; Lorca, R.A.; Gudmundsson, H.; Li, J.; Stewart, A.; Swaminathan, P.D.; Ibeawuchi, S.R.; et al. RGS6, a Modulator of Parasympathetic Activation in Heart. Circ. Res. 2010, 107, 1345–1349. [Google Scholar] [CrossRef]
- Posokhova, E.; Wydeven, N.; Allen, K.L.; Wickman, K.; Martemyanov, K.A. RGS6/Gβ5 Complex Accelerates IKACh Gating Kinetics in Atrial Myocytes and Modulates Parasympathetic Regulation of Heart Rate. Circ. Res. 2010, 107, 1350–1354. [Google Scholar] [CrossRef] [Green Version]
- Anderson, A.; Kulkarni, K.; Marron Fernandez De Velasco, E.; Carlblom, N.; Xia, Z.; Nakano, A.; Martemyanov, K.A.; Tolkacheva, E.G.; Wickman, K. Expression and Relevance of the G Protein-Gated K+ Channel in the Mouse Ventricle. Sci. Rep. 2018, 8, 1192. [Google Scholar] [CrossRef]
- DePaoli, A.M.; Bell, G.I.; Stoffel, M. G Protein-Activated Inwardly Rectifying Potassium Channel (GIRK1/KGA) MRNA in Adult Rat Heart and Brain by in Situ Hybridization Histochemistry. Mol. Cell Neurosci. 1994, 5, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Dobrzynski, H.; Janvier, N.C.; Leach, R.; Findlay, J.B.C.; Boyett, M.R. Effects of ACh and Adenosine Mediated by Kir3.1 and Kir3.4 on Ferret Ventricular Cells. Am. J. Physiol. Hear. Circ. Physiol. 2002, 283, H615–H630. [Google Scholar] [CrossRef] [Green Version]
- Liang, B.; Nissen, J.D.; Laursen, M.; Wang, X.; Skibsbye, L.; Hearing, M.C.; Andersen, M.N.; Rasmussen, H.B.; Wickman, K.; Grunnet, M.; et al. G-Protein-Coupled Inward Rectifier Potassium Current Contributes to Ventricular Repolarization. Cardiovasc. Res. 2014, 101, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, N.; Grunnet, M.; Olesen, S.P. Cardiac Potassium Channel Subtypes: New Roles in Repolarization and Arrhythmia. Physiol. Rev. 2014, 94, 609–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, H.; Maehara, K.; Onuki, N.; Saito, T.; Maruyama, Y. Decreased Contractility of the Left Ventricle Is Induced by the Neurotransmitter Acetylcholine, but Not by Vagal Stimulation in Rats. Jpn. Heart J. 2003, 44, 257–270. [Google Scholar] [CrossRef] [Green Version]
- Hoover, D.B.; Ganote, C.E.; Ferguson, S.M.; Blakely, R.D.; Parsons, R.L. Localization of Cholinergic Innervation in Guinea Pig Heart by Immunohistochemistry for High-Affinity Choline Transporters. Cardiovasc. Res. 2004, 62, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, C.; Rinne, A.; Littwitz, C.; Mintert, E.; Bösche, L.I.; Kienitz, M.C.; Pott, L.; Bender, K. G Protein-Activated (GIRK) Current in Rat Ventricular Myocytes Is Masked by Constitutive Inward Rectifier Current (IK1). Cell. Physiol. Biochem. 2008, 21, 259–268. [Google Scholar] [CrossRef]
- Kovoor, P.; Wickman, K.; Maguire, C.T.; Pu, W.; Gehrmann, J.; Berul, C.I.; Clapham, D.E. Evaluation of the Role of IKAChin Atrial Fibrillation Using a Mouse Knockout Model. J. Am. Coll. Cardiol. 2001, 37, 2136–2143. [Google Scholar] [CrossRef] [Green Version]
- Long, V.P.; Bonilla, I.M.; Baine, S.; Glynn, P.; Kumar, S.; Schober, K.; Mowrey, K.; Weiss, R.; Lee, N.Y.; Mohler, P.J.; et al. Chronic Heart Failure Increases Negative Chronotropic Effects of Adenosine in Canine Sinoatrial Cells via A1R Stimulation and GIRK-Mediated IKado. Life Sci. 2020, 240, 117068. [Google Scholar] [CrossRef] [PubMed]
- Cha, T.J.; Ehrlich, J.R.; Chartier, D.; Qi, X.Y.; Xiao, L.; Nattel, S. Kir3-Based Inward Rectifier Potassium Current. Circulation 2006, 113, 1730–1737. [Google Scholar] [CrossRef]
- Ehrlich, J.R.; Cha, T.-J.; Zhang, L.; Chartier, D.; Villeneuve, L.; Hébert, T.E.; Nattel, S. Characterization of a Hyperpolarization-Activated Time-Dependent Potassium Current in Canine Cardiomyocytes from Pulmonary Vein Myocardial Sleeves and Left Atrium. J. Physiol. 2004, 557, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Chen, S.A.; Chen, Y.C.; Yeh, H.I.; Chan, P.; Chang, M.S.; Lin, C.I. Effects of Rapid Atrial Pacing on the Arrhythmogenic Activity of Single Cardiomyocytes from Pulmonary Veins: Implication in Initiation of Atrial Fibrillation. Circulation 2001, 104, 2849–2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pappone, C.; Rosanio, S.; Oreto, G.; Tocchi, M.; Gugliotta, F.; Vicedomini, G.; Salvati, A.; Dicandia, C.; Mazzone, P.; Santinelli, V.; et al. Circumferential Radiofrequency Ablation of Pulmonary Vein Ostia. Circulation 2000, 102, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Nolte, I.M.; Munoz, M.L.; Tragante, V.; Amare, A.T.; Jansen, R.; Vaez, A.; Von Der Heyde, B.; Avery, C.L.; Bis, J.C.; Dierckx, B.; et al. Genetic Loci Associated with Heart Rate Variability and Their Effects on Cardiac Disease Risk. Nat. Commun. 2017, 8, 15805. [Google Scholar] [CrossRef] [Green Version]
- Voigt, N.; Friedrich, A.; Bock, M.; Wettwer, E.; Christ, T.; Knaut, M.; Strasser, R.H.; Ravens, U.; Dobrev, D. Differential Phosphorylation-Dependent Regulation of Constitutively Active and Muscarinic Receptor-Activated IK,ACh Channels in Patients with Chronic Atrial Fibrillation. Cardiovasc. Res. 2007, 74, 426–437. [Google Scholar] [CrossRef] [Green Version]
- Dobrev, D.; Friedrich, A.; Voigt, N.; Jost, N.; Wettwer, E.; Christ, T.; Knaut, M.; Ravens, U. The G Protein-Gated Potassium Current I(K,ACh) Is Constitutively Active in Patients with Chronic Atrial Fibrillation. Circulation 2005, 112, 3697–3706. [Google Scholar] [CrossRef] [Green Version]
- Voigt, N.; Trausch, A.; Knaut, M.; Matschke, K.; Varró, A.; Van Wagoner, D.R.; Nattel, S.; Ravens, U.; Dobrev, D. Left-to-Right Atrial Inward Rectifier Potassium Current Gradients in Patients with Paroxysmal Versus Chronic Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2010, 3, 472–480. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Csepe, T.A.; Hansen, B.J.; Sul, L.V.; Kalyanasundaram, A.; Zakharkin, S.O.; Zhao, J.; Guha, A.; Van Wagoner, D.R.; Kilic, A.; et al. Adenosine-Induced Atrial Fibrillation. Circulation 2016, 134, 486–498. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yang, J.; Shang, F.; Hong, C.; Guo, W.; Wang, B.; Zheng, Q. Silencing GIRK4 Expression in Human Atrial Myocytes by Adenovirus-Delivered Small Hairpin RNA. Mol. Biol. Rep. 2009, 36, 1345–1352. [Google Scholar] [CrossRef]
- Holmegard, H.N.; Theilade, J.; Benn, M.; Duno, M.; Haunso, S.; Svendsen, J.H. Genetic Variation in the Inwardly Rectifying K+ Channel Subunits KCNJ3 (GIRK1) and KCNJ5 (GIRK4) in Patients with Sinus Node Dysfunction. Cardiology 2010, 115, 176–181. [Google Scholar] [CrossRef]
- Kuß, J.; Stallmeyer, B.; Goldstein, M.; Rinné, S.; Pees, C.; Zumhagen, S.; Seebohm, G.; Decher, N.; Pott, L.; Kienitz, M.C.; et al. Familial Sinus Node Disease Caused by a Gain of GIRK (G-Protein Activated Inwardly Rectifying K+ Channel) Channel Function. Circ. Genomic Precis. Med. 2019, 12, e002238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mighiu, A.S.; Heximer, S.P. Controlling Parasympathetic Regulation of Heart Rate: A Gatekeeper Role for RGS Proteins in the Sinoatrial Node. Front. Physiol. 2012, 3, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milnes, J.T.; Madge, D.J.; Ford, J.W. New Pharmacological Approaches to Atrial Fibrillation. Drug Discov. Today 2012, 17, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Hashimoto, N. A Multiple Ion Channel Blocker, NIP-142, for the Treatment of Atrial Fibrillation. Cardiovasc. Drug Rev. 2007, 25, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Yamashita, T.; Tsuruzoe, N. Characterization of In Vivo and In Vitro Electrophysiological and Antiarrhythmic Effects of a Novel IKACh Blocker, NIP-151: A Comparison with an IKr-Blocker Dofetilide. J. Cardiovasc. Pharmacol. 2008, 51, 162–169. [Google Scholar] [CrossRef]
- Sobota, V.; Gatta, G.; van Hunnik, A.; van Tuijn, I.; Kuiper, M.; Milnes, J.; Jespersen, T.; Schotten, U.; Verheule, S. The Acetylcholine-Activated Potassium Current Inhibitor XAF-1407 Terminates Persistent Atrial Fibrillation in Goats. Front. Pharmacol. 2021, 11, 608410. [Google Scholar] [CrossRef]
- Yamamoto, W.; Hashimoto, N.; Matsuura, J.; Machida, T.; Ogino, Y.; Kobayashi, T.; Yamanaka, Y.; Ishiwata, N.; Yamashita, T.; Tanimoto, K.; et al. Effects of the Selective KACh Channel Blocker NTC-801 on Atrial Fibrillation in a Canine Model of Atrial Tachypacing. J. Cardiovasc. Pharmacol. 2014, 63, 421–427. [Google Scholar] [CrossRef]

| Subunit | Species | Location in the Heart | Expression Determination | Reference |
|---|---|---|---|---|
| GIRK1 GIRK4 | Mouse | Atria Ventricles | mRNA expression | [80] |
| GIRK1 | Rat | Atria | Protein expression | [54,81] |
| GIRK1 GIRK4 | Guinea pig | Atria Ventricles | Protein expression | [54] |
| GIRK4 | Rat | Right atrium (intercalated discs and sarcolemma) Left ventricle (intercalated discs) | [83] | |
| GIRK1 GIRK4 | Ferret | Atria Ventricles | mRNA expression Protein expression | [81] |
| GIRK1 | Dog | Atria Ventricles | mRNA expression | [82] |
| GIRK4 | Human | Left ventricle (intercalated discs and t-tubules) | Protein expression | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos-Ríos, A.; Rueda-Ruzafa, L.; Lamas, J.A. The Relevance of GIRK Channels in Heart Function. Membranes 2022, 12, 1119. https://doi.org/10.3390/membranes12111119
Campos-Ríos A, Rueda-Ruzafa L, Lamas JA. The Relevance of GIRK Channels in Heart Function. Membranes. 2022; 12(11):1119. https://doi.org/10.3390/membranes12111119
Chicago/Turabian StyleCampos-Ríos, Ana, Lola Rueda-Ruzafa, and José Antonio Lamas. 2022. "The Relevance of GIRK Channels in Heart Function" Membranes 12, no. 11: 1119. https://doi.org/10.3390/membranes12111119






