On-Site Application of Solar-Activated Membrane (Cr–Mn-Doped TiO2@Graphene Oxide) for the Rapid Degradation of Toxic Textile Effluents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of Graphene Oxide (GO) and TiO2 Nanowire (NW)
2.3. Synthesis of Cr–Mn-Doped TiO2/Graphene Oxide Aerogels
2.4. Characterization of Cr–Mn-Doped TiO2/Graphene Oxide Aerogels
2.5. Statistical Analysis
2.6. Photocatalytic Degradation Potential of Acid Black Dye by Cr–Mn-Doped TiO2 NW @GO Aerogels
3. Results and Discussion
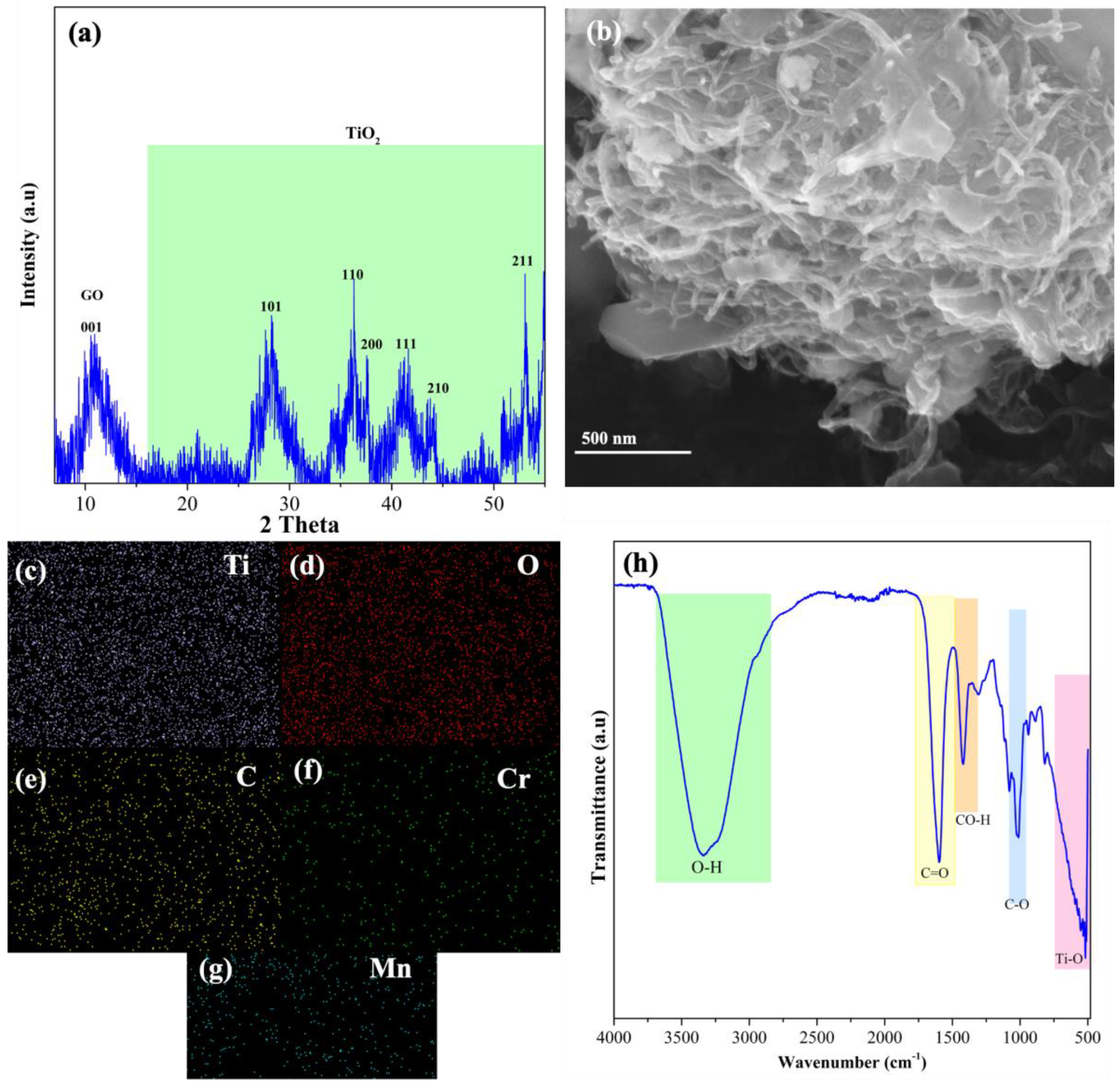
3.1. Characterization of Cr–Mn-Doped TiO2 NW @GO Aerogel
3.2. Statistical Evaluation of Photo Catalytic Activity of Cr–Mn-Doped TiO2@GO Aerogel
3.3. Evaluation of Extent of AB 1 Dye Degradation by Cr–Mn-Doped TiO2@GO Aerogel
3.4. Reusability of Cr–Mn-Doped TiO2@GO Aerogel Photocatalytic Membrane
3.5. Mechanism of Degradation of AB 1 Dye by Cr–Mn-Doped TiO2@GO Aerogel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aslam, M.M.; Kuo, H.-W.; Den, W.; Usman, M.; Sultan, M.; Ashraf, H. Functionalized Carbon Nanotubes (CNTs) for Water and Wastewater Treatment: Preparation to Application. Sustainability 2021, 13, 5717. [Google Scholar] [CrossRef]
- Usman, M.; Waseem, M.; Mani, N.; Andiego, G. Optimization of Soil Aquifer Treatment by Chemical Oxidation with Hydrogen Peroxide Addition. Pollution 2018, 4, 369–379. [Google Scholar]
- Benettayeb, A.; Ghosh, S.; Usman, M.; Seihoub, F.Z.; Sohoo, I.; Chia, C.H.; Sillanpää, M. Some Well-Known Alginate and Chitosan Modifications Used in Adsorption: A Review. Water 2022, 14, 1353. [Google Scholar] [CrossRef]
- Benettayeb, A.; Usman, M.; Tinashe, C.C.; Adam, T.; Haddou, B. A Critical Review with Emphasis on Recent Pieces of Evidence of Moringa Oleifera Biosorption in Water and Wastewater Treatment. Environ. Sci. Pollut. Res. 2022, 29, 48185–48209. [Google Scholar] [CrossRef]
- Khan, S.U.; Farooqi, I.H.; Usman, M.; Basheer, F. Energy Efficient Rapid Removal of Arsenic in an Electrocoagulation Reactor with Hybrid Fe/Al Electrodes: Process Optimization Using CCD and Kinetic Modeling. Water 2020, 12, 2876. [Google Scholar] [CrossRef]
- Alam, R.; Khan, S.U.; Usman, M.; Asif, M.; Farooqi, I.H. A Critical Review on Treatment of Saline Wastewater with Emphasis on Electrochemical Based Approaches. Process Saf. Environ. Prot. 2022, 158, 625–643. [Google Scholar] [CrossRef]
- Bai, B.; Bai, F.; Li, X.; Nie, Q.; Jia, X.; Wu, H. The Remediation Efficiency of Heavy Metal Pollutants in Water by Industrial Red Mud Particle Waste. Environ. Technol. Innov. 2022, 28, 102944. [Google Scholar] [CrossRef]
- Shen, G.; Pan, L.; Lü, Z.; Wang, C.; Aleem, F.; Zhang, X.; Zou, J.-J. Fe-TiO2 and Fe2O3 Quantum Dots Co-Loaded on MCM-41 for Removing Aqueous Rose Bengal by Combined Adsorption/Photocatalysis. Chinese J. Catal. 2018, 39, 920–928. [Google Scholar] [CrossRef]
- Ahmad, M.; Yousaf, M.; Nasir, A.; Bhatti, I.A.; Mahmood, A.; Fang, X.; Jian, X.; Kalantar-Zadeh, K.; Mahmood, N. Porous Eleocharis@MnPE Layered Hybrid for Synergistic Adsorption and Catalytic Biodegradation of Toxic Azo Dyes from Industrial Wastewater. Environ. Sci. Technol. 2019, 53, 2161–2170. [Google Scholar] [CrossRef]
- Pathania, D.; Thakur, M.; Mishra, A.K. Alginate-Zr (IV) Phosphate Nanocomposite Ion Exchanger: Binary Separation of Heavy Metals, Photocatalysis and Antimicrobial Activity. J. Alloys Compd. 2017, 701, 153–162. [Google Scholar] [CrossRef]
- Almeida, E.J.R.; Corso, C.R. Decolorization and Removal of Toxicity of Textile Azo Dyes Using Fungal Biomass Pelletized. Int. J. Environ. Sci. Technol. 2019, 16, 1319–1328. [Google Scholar] [CrossRef]
- Saeed, M.; Nadeem, R.; Yousaf, M. Removal of Industrial Pollutant (Reactive Orange 122 Dye) Using Environment-Friendly Sorbent Trapa Bispinosa’s Peel and Fruit. Int. J. Environ. Sci. Technol. 2015, 12, 1223–1234. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Ma, J.; Zhu, Y.; Li, X.; Yu, H.; Sun, Y. Biodegradation Performance and Anti-Fouling Mechanism of an ICME/Electro-Biocarriers-MBR System in Livestock Wastewater (Antibiotic-Containing) Treatment. J. Hazard. Mater. 2022, 426, 128064. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, S.; Cui, D.; Zhou, H.; Wu, D.; Pan, S.; Xu, F.; Wang, Z. Co-Hydrothermal Carbonization of Organic Solid Wastes to Hydrochar as Potential Fuel: A Review. Sci. Total Environ. 2022, 850, 158034. [Google Scholar] [CrossRef] [PubMed]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Bal, G.; Thakur, A. Distinct Approaches of Removal of Dyes from Wastewater: A Review. Mater. Today Proc. 2022, 50, 1575–1579. [Google Scholar] [CrossRef]
- Liu, W.; Huang, F.; Liao, Y.; Zhang, J.; Ren, G.; Zhuang, Z.; Zhen, J.; Lin, Z.; Wang, C. Treatment of CrVI-Containing Mg(OH)2 Nanowaste. Angew. Chem. 2008, 120, 5701–5704. [Google Scholar] [CrossRef]
- Shabir, M.; Yasin, M.; Hussain, M.; Shafiq, I.; Akhter, P.; Nizami, A.-S.; Jeon, B.-H.; Park, Y.-K. A Review on Recent Advances in the Treatment of Dye-Polluted Wastewater. J. Ind. Eng. Chem. 2022, 112, 1–19. [Google Scholar] [CrossRef]
- Chang, S.-H.; Wang, K.-S.; Li, H.-C.; Wey, M.-Y.; Chou, J.-D. Enhancement of Rhodamine B Removal by Low-Cost Fly Ash Sorption with Fenton Pre-Oxidation. J. Hazard. Mater. 2009, 172, 1131–1136. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, J.; Ou, X.; Liu, X.; Song, Y.; Tian, C.; Rong, W.; Shi, Z.; Dang, Z.; Lin, Z. Effective Extraction of Cr(VI) from Hazardous Gypsum Sludge via Controlling the Phase Transformation and Chromium Species. Environ. Sci. Technol. 2018, 52, 13336–13342. [Google Scholar] [CrossRef]
- Singh, R.; Rattan, G.; Singh, M.; Manne, R.; Oberoi, S.K.; Kaur, N. Advanced Oxidation Processes for Wastewater Treatment: Perspective Through Nanomaterials. In Environmental Science and Engineering, Proceedings of the International Conference on Chemical, Bio and Environmental Engineering, Jalandhar, India, 20–22 August 2021; Springer: Berlin/Heidelberg, Germany, 2022; pp. 57–68. [Google Scholar]
- Alemi, A.; Hanifehpour, Y.; Woo Joo, S.; Khandar, A.; Morsali, A.; Min, B.-K. Co-Reduction Synthesis of New LnxSb2−xS3 (Ln: Nd3+, Lu3+, Ho3+) Nanomaterials and Investigation of Their Physical Properties. Phys. B Condens. Matter 2011, 406, 2801–2806. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, T.; Liu, Y.; Liu, P.; Zhang, J.; Fang, L.; Sun, D. Enhancement of Desulfurization by Hydroxyl Ammonium Ionic Liquid Supported on Active Carbon. Environ. Res. 2022, 213, 113637. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liu, J.; Shangguan, W. A Review on Photocatalysis in Antibiotic Wastewater: Pollutant Degradation and Hydrogen Production. Chinese J. Catal. 2020, 41, 1440–1450. [Google Scholar] [CrossRef]
- Zhang, Y.; Shaad, K.; Vollmer, D.; Ma, C. Treatment of Textile Wastewater Using Advanced Oxidation Processes—A Critical Review. Water 2021, 13, 3515. [Google Scholar] [CrossRef]
- Shahab-ud-Din; Ahmad, M.Z.; Qureshi, K.; Bhatti, I.A.; Zahid, M.; Nisar, J.; Iqbal, M.; Abbas, M. Hydrothermal Synthesis of Molybdenum Trioxide, Characterization and Photocatalytic Activity. Mater. Res. Bull. 2018, 100, 120–130. [Google Scholar] [CrossRef]
- Ahmad, M.; Yousaf, M.; Cai, W.; Zhao, Z.-P. Formulation of Heterometallic ZIF-8@Cu/Ni/ZnO@CNTs Heterostructure Photocatalyst for Ultra-Deep Desulphurization of Coal and Fuels. Chem. Eng. J. 2023, 453, 139846. [Google Scholar] [CrossRef]
- Yousaf, M.; Ahmad, M.; Zhao, Z.-P. Rapid and Highly Selective Conversion of CO2 to Methanol by Heterometallic Porous ZIF-8. J. CO2 Util. 2022, 64, 102172. [Google Scholar] [CrossRef]
- Liu, P.; Li, S.; Zhang, L.; Yin, X.; Ma, Y. Shearing Bridge Bonds in Carbon Nitride Vesicles with Enhanced Hot Carrier Utilization for Photocatalytic Hydrogen Production. Catal. Sci. Technol. 2022, 12, 4193–4200. [Google Scholar] [CrossRef]
- Feng, C.; Chen, Z.; Li, W.; Zhang, F.; Li, X.; Xu, L.; Sun, M. First-Principle Calculation of the Electronic Structures and Optical Properties of the Metallic and Nonmetallic Elements-Doped ZnO on the Basis of Photocatalysis. Phys. B Condens. Matter 2019, 555, 53–60. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of Advanced Oxidation Processes for Water and Wastewater Treatment—A Critical Review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification Strategies of TiO2 for Potential Applications in Photocatalysis: A Critical Review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef]
- Li, G.; Zou, B.; Feng, S.; Shi, H.; Liao, K.; Wang, Y.; Wang, W.; Zhang, G. Synthesis of N-Doped TiO2 with Good Photocatalytic Property. Phys. B Condens. Matter 2020, 588, 412184. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Pankratova, V.; Popov, A.I.; Pankratov, V. Study of Phase Composition, Photocatalytic Activity, and Photoluminescence of TiO2 with Eu Additive Produced by the Extraction-Pyrolytic Method. J. Mater. Res. Technol. 2021, 13, 2350–2360. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and Mechanisms of Photocatalytic Dye Degradation on TiO2 Based Photocatalysts: A Comparative Overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Tian, B.; Li, C.; Zhang, J. One-Step Preparation, Characterization and Visible-Light Photocatalytic Activity of Cr-Doped TiO2 with Anatase and Rutile Bicrystalline Phases. Chem. Eng. J. 2012, 191, 402–409. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Co-Doping a Metal (Cr, Fe, Co, Ni, Cu, Zn, Ce, and Zr) on Mn/TiO2 Catalyst and Its Effect on the Selective Reduction of NO with NH3 at Low-Temperatures. Appl. Catal. B Environ. 2011, 110, 195–206. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Romanova, M.; Kotomin, E.A.; Popov, A.I. Extraction–Pyrolytic Method for TiO2 Polymorphs Production. Crystals 2021, 11, 431. [Google Scholar] [CrossRef]
- Tsebriienko, T.; Popov, A.I. Effect of Poly(Titanium Oxide) on the Viscoelastic and Thermophysical Properties of Interpenetrating Polymer Networks. Crystals 2021, 11, 794. [Google Scholar] [CrossRef]
- Khan, I.; Chu, X.; Liu, Y.; Khan, S.; Bai, L.; Jing, L. Synthesis of Ni2+ Cation Modified TS-1 Molecular Sieve Nanosheets as Effective Photocatalysts for Alcohol Oxidation and Pollutant Degradation. Chinese J. Catal. 2020, 41, 1589–1602. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent Advances in Graphene Based Polymer Composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qi, L.; Ran, J.; Yu, J.; Qiao, S.Z. Ternary NiS/ZnxCd1-XS/Reduced Graphene Oxide Nanocomposites for Enhanced Solar Photocatalytic H2-Production Activity. Adv. Energy Mater. 2014, 4, 1301925. [Google Scholar] [CrossRef]
- Wu, R.; Tan, Y.; Meng, F.; Zhang, Y.; Huang, Y.-X. PVDF/MAF-4 Composite Membrane for High Flux and Scaling-Resistant Membrane Distillation. Desalination 2022, 540, 116013. [Google Scholar] [CrossRef]
- Tan, Z.; Dong, B.; Xing, M.; Sun, X.; Xi, B.; Dai, W.; He, C.; Luo, Y.; Huang, Y. Electric Field Applications Enhance the Electron Transfer Capacity of Dissolved Organic Matter in Sludge Compost. Environ. Technol. 2022, 1–11. [Google Scholar] [CrossRef]
- Ge, D.; Yuan, H.; Xiao, J.; Zhu, N. Insight into the Enhanced Sludge Dewaterability by Tannic Acid Conditioning and PH Regulation. Sci. Total Environ. 2019, 679, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Lu, K.; Hardison, A.K.; Liu, Z.; Xu, X.; Gao, D.; Gong, J.; Gardner, W.S. Membrane Inlet Mass Spectrometry Method (REOX/MIMS) to Measure 15N-Nitrate in Isotope-Enrichment Experiments. Ecol. Indic. 2021, 126, 107639. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue Luminescent Graphene Quantum Dots and Graphene Oxide Prepared by Tuning the Carbonization Degree of Citric Acid. Carbon N. Y. 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Liu, B.; Chen, H.M.; Liu, C.; Andrews, S.C.; Hahn, C.; Yang, P. Large-Scale Synthesis of Transition-Metal-Doped TiO2 Nanowires with Controllable Overpotential. J. Am. Chem. Soc. 2013, 135, 9995–9998. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Ran, X.; Wu, X.; Zhang, D. Evaluation of Electro-Oxidation of Biologically Treated Landfill Leachate Using Response Surface Methodology. J. Hazard. Mater. 2011, 188, 261–268. [Google Scholar] [CrossRef]
- El-Desoky, M.M.; Morad, I.; Wasfy, M.H.; Mansour, A.F. Synthesis, Structural and Electrical Properties of PVA/TiO2 Nanocomposite Films with Different TiO2 Phases Prepared by Sol–Gel Technique. J. Mater. Sci. Mater. Electron. 2020, 31, 17574–17584. [Google Scholar] [CrossRef]
- Yasin, G.; Arif, M.; Shakeel, M.; Dun, Y.; Zuo, Y.; Khan, W.Q.; Tang, Y.; Khan, A.; Nadeem, M. Exploring the Nickel–Graphene Nanocomposite Coatings for Superior Corrosion Resistance: Manipulating the Effect of Deposition Current Density on Its Morphology, Mechanical Properties, and Erosion-Corrosion Performance. Adv. Eng. Mater. 2018, 20, 1701166. [Google Scholar] [CrossRef]
- Yousaf, M.; Ahmad, M.; Batool, A.; Zhao, Z.-P. Highly-Stable, Bifunctional, Binder-Free & Stand-Alone Photoelectrode (FexNi1-XO@a-CC) for Natural Waters Splitting into Hydrogen. Int. J. Hydrogen Energy 2022, 47, 36032–36045. [Google Scholar] [CrossRef]
- Mohsin, M.; Bhatti, I.A.; Ashar, A.; Khan, M.W.; Farooq, M.U.; Khan, H.; Hussain, M.T.; Loomba, S.; Mohiuddin, M.; Zavabeti, A.; et al. Iron-Doped Zinc Oxide for Photocatalyzed Degradation of Humic Acid from Municipal Wastewater. Appl. Mater. Today 2021, 23, 101047. [Google Scholar] [CrossRef]
- Tan, Z.; Zhu, H.; He, X.; Xi, B.; Tian, Y.; Sun, X.; Zhang, H.; Ouche, Q. Effect of Ventilation Quantity on Electron Transfer Capacity and Spectral Characteristics of Humic Substances during Sludge Composting. Environ. Sci. Pollut. Res. 2022, 29, 70269–70284. [Google Scholar] [CrossRef]
- Mbarek, W.B.; Azabou, M.; Pineda, E.; Fiol, N.; Escoda, L.; Suñol, J.J.; Khitouni, M. Rapid Degradation of Azo-Dye Using Mn–Al Powders Produced by Ball-Milling. RSC Adv. 2017, 7, 12620–12628. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Chen, C.; Ji, H.; Ma, W.; Zhao, J. Mechanism of TiO2-Assisted Photocatalytic Degradation of Dyes under Visible Irradiation: Photoelectrocatalytic Study by TiO2-Film Electrodes. J. Phys. Chem. B 2005, 109, 21900–21907. [Google Scholar] [CrossRef]
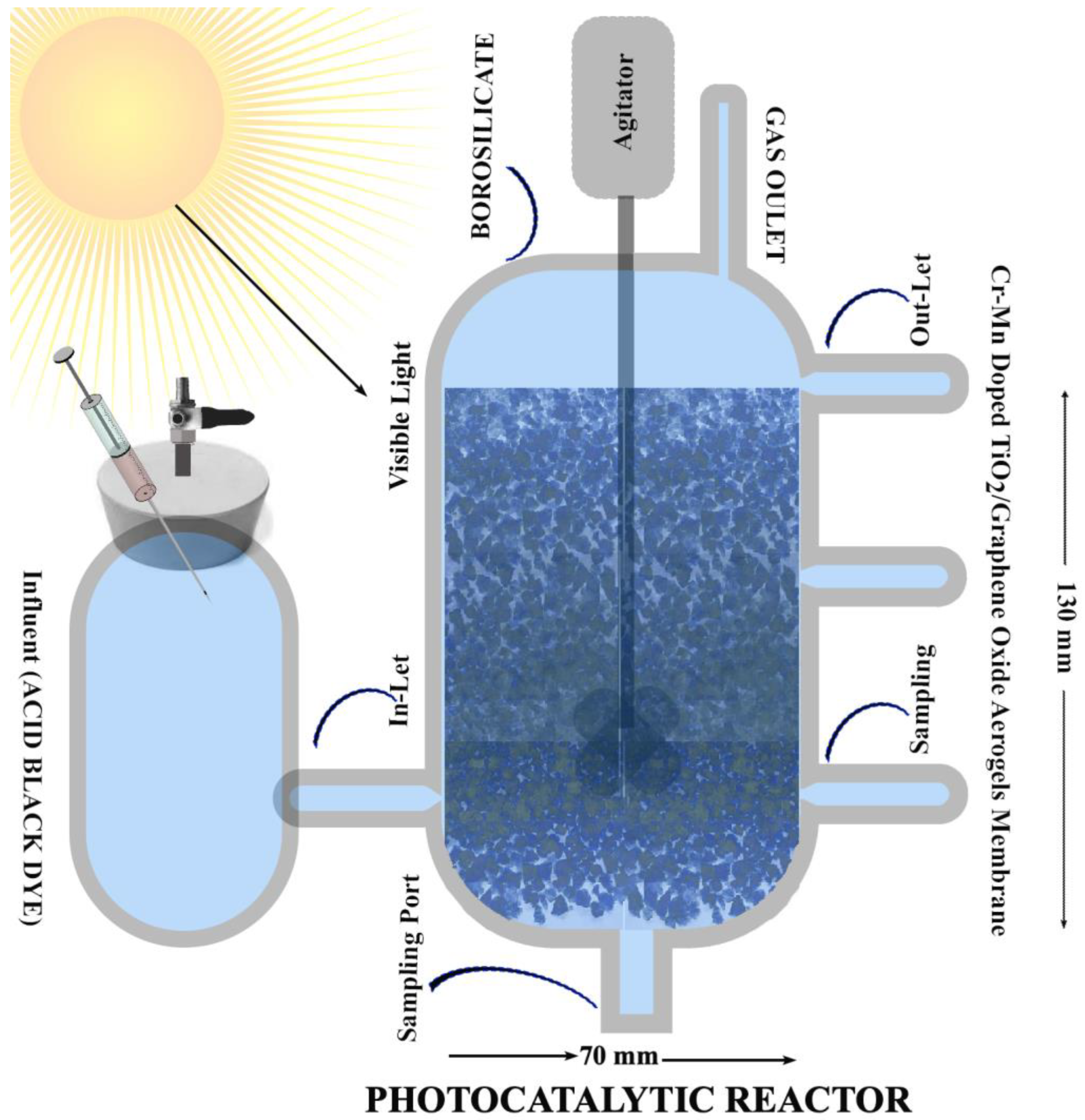





| Factor | Variables | Unit | Low Actual | High Actual |
|---|---|---|---|---|
| A | pH | 4 | 10 | |
| B | Oxidant concentration | mmol | 10 | 50 |
| C | Irradiation Time | min | 60 | 300 |
| D | Size of aerogels | mm | 10 | 15 |
| Sr. No | TiO2 NW | Cr–Mn-Doped TiO2 NW | Cr–Mn-Doped TiO2 NW @GO Aerogel | |
|---|---|---|---|---|
| 1 | SBET (m2/g) | 46.2 | 79.3 | 280.2 |
| 2 | Pore volume (cm3/g) | 0.135 | 0.216 | 0.35 |
| Run | A | B | C | D | Degradation (%) |
|---|---|---|---|---|---|
| 1 | 7 | 70 | 180 | 12.50 | 70 |
| 2 | 4 | 10 | 300 | 15 | 42 |
| 3 | 13 | 30 | 180 | 12.50 | 50 |
| 4 | 7 | 30 | 180 | 7.50 | 76 |
| 5 | 7 | 30 | 180 | 12.50 | 89 |
| 6 | 4 | 50 | 60 | 15 | 59 |
| 7 | 10 | 10 | 300 | 10 | 58 |
| 8 | 7 | 7 | 60 | 12.50 | 55 |
| 9 | 4 | 10 | 60 | 15 | 32 |
| 10 | 10 | 10 | 60 | 10 | 51 |
| 11 | 10 | 10 | 300 | 10 | 62 |
| 12 | 10 | 10 | 60 | 15 | 43 |
| 13 | 10 | 50 | 300 | 15 | 56 |
| 14 | 10 | 50 | 60 | 15 | 58 |
| 15 | 7 | 30 | 160 | 10 | 87 |
| 16 | 10 | 30 | 180 | 10 | 58 |
| 17 | 7 | 30 | 60 | 12.50 | 82 |
| 18 | 7 | 30 | 180 | 7 | 78 |
| 19 | 7 | 30 | 300 | 12.50 | 63 |
| 20 | 7 | 10 | 420 | 15 | 46 |
| 21 | 4 | 50 | 180 | 10 | 50 |
| 22 | 4 | 50 | 60 | 15 | 70 |
| 23 | 7 | 30 | 300 | 12.50 | 87 |
| 24 | 7 | 30 | 300 | 12.50 | 86 |
| 25 | 10 | 10 | 60 | 10 | 89 |
| 26 | 4 | 50 | 80 | 10 | 49 |
| 27 | 1 | 30 | 300 | 10 | 34 |
| 28 | 4 | 10 | 60 | 15 | 30.2 |
| 29 | 7 | 30 | 60 | 12.50 | 60.4 |
| 30 | 4 | 10 | 180 | 15.23 | 41.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousaf, M.; Akram, M.; Bhatti, I.A.; Ahmad, M.; Usman, M.; Khan, M.U.; Sarwar, A.; Sultan, M.; Sohoo, I. On-Site Application of Solar-Activated Membrane (Cr–Mn-Doped TiO2@Graphene Oxide) for the Rapid Degradation of Toxic Textile Effluents. Membranes 2022, 12, 1178. https://doi.org/10.3390/membranes12121178
Yousaf M, Akram M, Bhatti IA, Ahmad M, Usman M, Khan MU, Sarwar A, Sultan M, Sohoo I. On-Site Application of Solar-Activated Membrane (Cr–Mn-Doped TiO2@Graphene Oxide) for the Rapid Degradation of Toxic Textile Effluents. Membranes. 2022; 12(12):1178. https://doi.org/10.3390/membranes12121178
Chicago/Turabian StyleYousaf, Maryam, Mariam Akram, Ijaz Ahmad Bhatti, Muhammad Ahmad, Muhammad Usman, Muhammad Usman Khan, Abid Sarwar, Muhammad Sultan, and Ihsanullah Sohoo. 2022. "On-Site Application of Solar-Activated Membrane (Cr–Mn-Doped TiO2@Graphene Oxide) for the Rapid Degradation of Toxic Textile Effluents" Membranes 12, no. 12: 1178. https://doi.org/10.3390/membranes12121178
APA StyleYousaf, M., Akram, M., Bhatti, I. A., Ahmad, M., Usman, M., Khan, M. U., Sarwar, A., Sultan, M., & Sohoo, I. (2022). On-Site Application of Solar-Activated Membrane (Cr–Mn-Doped TiO2@Graphene Oxide) for the Rapid Degradation of Toxic Textile Effluents. Membranes, 12(12), 1178. https://doi.org/10.3390/membranes12121178












