Membrane Applications in Autologous Cell Therapy
Abstract
:1. Introduction
- Autologous chondrocyte implantation (ACI) for cartilage repair [1].
- Autologous cell therapy for treatment of burns [2],
- Autologous stem cell transplantation for the treatment of multiple myeloma and multiple scoliosis [3], and
- Chimeric antigen receptor T-Cell (CAR-T) therapy for the treatment of blood cancers [4].
2. Hollow Fibre Membrane Bioreactors for CAR-T Immunotherapy
3. Membrane Bioreactor for Autologous Cell Expansion
4. Membrane applications in EV Production
5. Needs for Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| ACI | Autologous Chondrocyte Implantation |
| CAR | Chimeric Antigen Receptors |
| CAR-T | Chimeric Antigen Receptor T Cell |
| ECMO | Extracorporeal Membrane Oxygenation |
| ECS | Extra Capillary Space |
| ECs | Endothelial Cells |
| EVs | Extracellular Vesicles |
| GMP | Good Manufacture Practice |
| HFMBs | Hollow Fibre Membrane Bioreactors |
| HFs | Hollow Fibres |
| HSCs | Hematopoietic Stem Cells |
| IPSCs | Induced Pluripotent Stem Cells |
| mAb | Monoclonal Antibody |
| MF | Microfiltration |
| MSCs | Mesenchymal Stem Cells |
| MWCO | Molecular Weight Cut-Off |
| SEC | Size Exclusion Chromatography |
| TCRs | T Cell Receptors |
| TFF | Tangential Flow Filtration |
| TILs | Tumour-Infiltrating Lymphocytes |
| UF | Ultrafiltration |
References
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Wood, F.; Martin, L.; Lewis, D.; Rawlins, J.; McWilliams, T.; Burrows, S.; Rea, S. A Prospective Randomised Clinical Pilot Study to Compare the Effectiveness of Biobrane® Synthetic Wound Dressing, with or without Autologous Cell Suspension, to the Local Standard Treatment Regimen in Paediatric Scald Injuries. Burns 2012, 38, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Mancardi, G.; Saccardi, R. Autologous Haematopoietic Stem-Cell Transplantation in Multiple Sclerosis. Lancet. Neurol. 2008, 7, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Braendstrup, P.; Levine, B.L.; Ruella, M. The Long Road to the First FDA-Approved Gene Therapy: Chimeric Antigen Receptor T Cells Targeting CD19. Cytotherapy 2020, 22, 57–69. [Google Scholar] [CrossRef]
- Carticel. Available online: https://www.maci.com/patients/?carticel (accessed on 4 April 2022).
- Cost Effectiveness and Budget Impact of Tisagenlecleucel. PharmacoEconomics Outcomes News 2018, 817, 12. [CrossRef]
- Moutsatsou, P.; Ochs, J.; Schmitt, R.H.; Hewitt, C.J.; Hanga, M.P. Automation in Cell and Gene Therapy Manufacturing: From Past to Future. Biotechnol. Lett. 2019, 41, 1245–1253. [Google Scholar] [CrossRef] [Green Version]
- Lam, C.; Meinert, E.; Yang, A.; Cui, Z. Comparison between Centralized and Decentralized Supply Chains of Autologous Chimeric Antigen Receptor T-Cell Therapies: A UK Case Study Based on Discrete Event Simulation. Cytotherapy 2021, 23, 433–451. [Google Scholar] [CrossRef]
- Decker, W.K.; da Silva, R.F.; Sanabria, M.H.; Angelo, L.S.; Guimarães, F.; Burt, B.M.; Kheradmand, F.; Paust, S. Cancer Immunotherapy: Historical Perspective of a Clinical Revolution and Emerging Preclinical Animal Models. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Three Es of Cancer Immunoediting. Annu. Rev. Immunol. 2004, 22, 329–360. [Google Scholar] [CrossRef]
- Clemente, C.G.; Mihm, M.C.; Bufalino, R.; Zurrida, S.; Collini, P.; Cascinelli, N. Prognostic Value of Tumor Infiltrating Lymphocytes in the Vertical Growth Phase of Primary Cutaneous Melanoma. Cancer 1996, 77, 1303–1310. [Google Scholar] [CrossRef]
- Borst, J.; Ahrends, T.; Bąbała, N.; Melief, C.J.M.; Kastenmüller, W. CD4+ T Cell Help in Cancer Immunology and Immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef]
- Neefjes, J.; Jongsma, M.L.M.; Paul, P.; Bakke, O. Towards a Systems Understanding of MHC Class I and MHC Class II Antigen Presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef]
- Kalergis, A.M.; Ono, T.; Wang, F.; DiLorenzo, T.P.; Honda, S.; Nathenson, S.G. Single Amino Acid Replacements in an Antigenic Peptide Are Sufficient to Alter the TCR V Beta Repertoire of the Responding CD8+ Cytotoxic Lymphocyte Population. J. Immunol. 1999, 162, 7263–7270. [Google Scholar]
- Egerton, M.; Scollay, R.; Shortman, K. Kinetics of Mature T-Cell Development in the Thymus. Proc. Natl. Acad. Sci. USA 1990, 87, 2579–2582. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, S.A.; Spiess, P.; Lafreniere, R. A New Approach to the Adoptive Immunotherapy of Cancer with Tumor-Infiltrating Lymphocytes. Science 1986, 233, 1318–1321. [Google Scholar] [CrossRef]
- Wang, S.; Sun, J.; Chen, K.; Ma, P.; Lei, Q.; Xing, S.; Cao, Z.; Sun, S.; Yu, Z.; Liu, Y.; et al. Perspectives of Tumor-Infiltrating Lymphocyte Treatment in Solid Tumors. BMC Med. 2021, 19, 140. [Google Scholar] [CrossRef]
- Eshhar, Z.; Waks, T.; Gross, G.; Schindler, D.G. Specific Activation and Targeting of Cytotoxic Lymphocytes through Chimeric Single Chains Consisting of Antibody-Binding Domains and the Gamma or Zeta Subunits of the Immunoglobulin and T-Cell Receptors. Proc. Natl. Acad. Sci. USA 1993, 90, 720–724. [Google Scholar] [CrossRef] [Green Version]
- Kalos, M.; Levine, B.L.; Porter, D.L.; Katz, S.; Grupp, S.A.; Bagg, A.; June, C.H. T Cells with Chimeric Antigen Receptors Have Potent Antitumor Effects and Can Establish Memory in Patients with Advanced Leukemia. Sci. Transl. Med. 2011, 3, 95ra73. [Google Scholar] [CrossRef] [Green Version]
- Hollyman, D.; Stefanski, J.; Przybylowski, M.; Bartido, S.; Borquez-Ojeda, O.; Taylor, C.; Yeh, R.; Capacio, V.; Olszewska, M.; Hosey, J.; et al. Manufacturing Validation of Biologically Functional T Cells Targeted to CD19 Antigen for Autologous Adoptive Cell Therapy. J. Immunother. 2009, 32, 169–180. [Google Scholar] [CrossRef] [Green Version]
- FDA Okays Second CAR-T for Kite. Nat. Biotechnol. 2020, 38, 1012. [CrossRef]
- Mullard, A. FDA Approves First CAR T Therapy. Nat. Rev. Drug Discov. 2017, 16, 669. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Second Anticancer CAR T Therapy Receives FDA Approval. Nat. Rev. Drug Discov. 2017, 16, 818. [Google Scholar] [CrossRef]
- Mullard, A. FDA Approves Fourth CAR-T Cell Therapy. Nat. Rev. Drug Discov. 2021, 20, 166. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. FDA Approves First BCMA-Targeted CAR-T Cell Therapy. Nat. Rev. Drug Discov. 2021, 20, 332. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. FDA Approves Second BCMA-Targeted CAR-T Cell Therapy. Nat. Rev. Drug Discov. 2022, 21, 249. [Google Scholar] [CrossRef]
- Rafiq, S.; Hackett, C.S.; Brentjens, R.J. Engineering Strategies to Overcome the Current Roadblocks in CAR T Cell Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 147–167. [Google Scholar] [CrossRef]
- Lyman, G.H.; Nguyen, A.; Snyder, S.; Gitlin, M.; Chung, K.C. Economic Evaluation of Chimeric Antigen Receptor T-Cell Therapy by Site of Care Among Patients With Relapsed or Refractory Large B-Cell Lymphoma. JAMA Netw. Open 2020, 3, e202072. [Google Scholar] [CrossRef]
- Karschnia, P.; Rejeski, K.; Winkelmann, M.; Schöberl, F.; Bücklein, V.L.; Blumenberg, V.; Schmidt, C.; Blobner, J.; von Bergwelt-Baildon, M.; Tonn, J.-C.; et al. Toxicities and Response Rates of Secondary CNS Lymphoma After Adoptive Immunotherapy With CD19-Directed Chimeric Antigen Receptor T Cells. Neurology 2022, 98, 884–889. [Google Scholar] [CrossRef]
- Mahal, H.; Branton, H.; Farid, S.S. End-to-end Continuous Bioprocessing: Impact on Facility Design, Cost of Goods, and Cost of Development for Monoclonal Antibodies. Biotechnol. Bioeng. 2021, 118, 3468–3485. [Google Scholar] [CrossRef]
- Jiang, J.; Ahuja, S. Addressing Patient to Patient Variability for Autologous CAR T Therapies. J. Pharm. Sci. 2021, 110, 1871–1876. [Google Scholar] [CrossRef]
- Castella, M.; Caballero-Baños, M.; Ortiz-Maldonado, V.; González-Navarro, E.A.; Suñé, G.; Antoñana-Vidósola, A.; Boronat, A.; Marzal, B.; Millán, L.; Martín-Antonio, B.; et al. Point-Of-Care CAR T-Cell Production (ARI-0001) Using a Closed Semi-Automatic Bioreactor: Experience From an Academic Phase I Clinical Trial. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Fernández, L.; Fernández, A.; Mirones, I.; Escudero, A.; Cardoso, L.; Vela, M.; Lanzarot, D.; de Paz, R.; Leivas, A.; Gallardo, M.; et al. GMP-Compliant Manufacturing of NKG2D CAR Memory T Cells Using CliniMACS Prodigy. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Jackson, Z.; Roe, A.; Sharma, A.A.; Lopes, F.B.T.P.; Talla, A.; Kleinsorge-Block, S.; Zamborsky, K.; Schiavone, J.; Manjappa, S.; Schauner, R.; et al. Automated Manufacture of Autologous CD19 CAR-T Cells for Treatment of Non-Hodgkin Lymphoma. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Smith, T.A. CAR-T Cell Expansion in a Xuri Cell Expansion System W25; Humana: New York, NY, USA, 2020; pp. 151–163. ISBN 978-1-0716-0145-7. [Google Scholar]
- Cytiva Xuri Cell Expansion System. Available online: https://www.cytivalifesciences.com/en/us/shop/cell-therapy/systems/xuri-cell-expansion-system-w25-p-06192 (accessed on 24 October 2022).
- Biotec, M. CliniMACS Prodigy. Available online: https://www.miltenyibiotec.com/GB-en/products/clinimacs-prodigy.html#200-075-301 (accessed on 24 October 2022).
- Lonza The Cocoon. Available online: https://demo-pharma.lonza.com/technologies-products/cocoon-platform (accessed on 24 October 2022).
- Knazek, R.A.; Gullino, P.M.; Kohler, P.O.; Dedrick, R.L. Cell Culture on Artificial Capillaries: An Approach to Tissue Growth in Vitro. Science 1972, 178, 65–67. [Google Scholar] [CrossRef]
- Sohaib, Q.; Kalakech, C.; Charmette, C.; Cartier, J.; Lesage, G.; Mericq, J.-P. Hollow-Fiber Membrane Contactor for Biogas Recovery from Real Anaerobic Membrane Bioreactor Permeate. Membranes 2022, 12, 112. [Google Scholar] [CrossRef]
- Kleinstreuer, C.; Agarwal, S.S. Analysis and Simulation of Hollow-Fiber Bioreactor Dynamics. Biotechnol. Bioeng. 1986, 28, 1233–1240. [Google Scholar] [CrossRef]
- Tharakan, J.P.; Chau, P.C. A Radial Flow Hollow Fiber Bioreactor for the Large-Scale Culture of Mammalian Cells. Biotechnol. Bioeng. 1986, 28, 329–342. [Google Scholar] [CrossRef]
- Storm, M.P.; Sorrell, I.; Shipley, R.; Regan, S.; Luetchford, K.A.; Sathish, J.; Webb, S.; Ellis, M.J. Hollow Fiber Bioreactors for In Vivo-like Mammalian Tissue Culture. J. Vis. Exp. 2016, e53431. [Google Scholar] [CrossRef] [Green Version]
- Ladewig, B.; Al-Shaeli, M.N.Z. Fundamentals of Membrane Bioreactors. In Springer Transactions in Civil and Environmental Engineering; Springer: Singapore, 2017; ISBN 978-981-10-2013-1. [Google Scholar]
- FiberCellSystems. Available online: https://www.fibercellsystems.com/products/cartridges/ (accessed on 25 October 2022).
- Nold, P.; Brendel, C.; Neubauer, A.; Bein, G.; Hackstein, H. Good Manufacturing Practice-Compliant Animal-Free Expansion of Human Bone Marrow Derived Mesenchymal Stroma Cells in a Closed Hollow-Fiber-Based Bioreactor. Biochem. Biophys. Res. Commun. 2013, 430, 325–330. [Google Scholar] [CrossRef]
- Yoo, S.M.; Lau, V.W.C.; Aarts, C.; Bojovic, B.; Steinberg, G.; Hammill, J.A.; Dvorkin-Gheva, A.; Ghosh, R.; Bramson, J.L. Manufacturing T Cells in Hollow Fiber Membrane Bioreactors Changes Their Programming and Enhances Their Potency. Oncoimmunology 2021, 10. [Google Scholar] [CrossRef]
- Wahlig, S.; Peh, G.S.L.; Adnan, K.; Ang, H.-P.; Lwin, C.N.; Morales-Wong, F.; Ong, H.S.; Lovatt, M.; Mehta, J.S. Optimisation of Storage and Transportation Conditions of Cultured Corneal Endothelial Cells for Cell Replacement Therapy. Sci. Rep. 2020, 10, 1681. [Google Scholar] [CrossRef] [PubMed]
- Massie, I.; Selden, C.; Hodgson, H.; Fuller, B. Storage Temperatures for Cold-Chain Delivery in Cell Therapy: A Study of Alginate-Encapsulated Liver Cell Spheroids Stored at −80 °C or −170 °C for up to 1 Year. Tissue Eng. Part C. Methods 2013, 19, 189–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namdaroğlu, S.; Tekgündüz, E.; Bozdağ, S.C.; Durgun, G.; Sarıca, A.; Demiriz, I.Ş.; Koçubaba, Ş.; İskender, G.; Kayıkçı, Ö.; Altuntaş, F. Microbial Contamination of Hematopoietic Progenitor Cell Products. Transfus. Apher. Sci. 2013, 48, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska-Skrzypczak, M.; Bembnista, E.; Kubiak, A.; Matuszak, P.; Schneider, A.; Komarnicki, M. Microbial Contamination of Peripheral Blood and Bone Marrow Hematopoietic Cell Products and Environmental Contamination in a Stem Cell Bank: A Single-Center Report. Transplant. Proc. 2014, 46, 2873–2876. [Google Scholar] [CrossRef] [PubMed]
- Bersenev, A. CAR-T Cell Manufacturing: Time to Put It in Gear. Transfusion 2017, 57, 1104–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Rivière, I. Clinical Manufacturing of CAR T Cells: Foundation of a Promising Therapy. Mol. Ther. Oncolytics 2016, 3, 16015. [Google Scholar] [CrossRef] [Green Version]
- Tirughana, R.; Metz, M.Z.; Li, Z.; Hall, C.; Hsu, D.; Beltzer, J.; Annala, A.J.; Oganesyan, D.; Gutova, M.; Aboody, K.S. GMP Production and Scale-Up of Adherent Neural Stem Cells with a Quantum Cell Expansion System. Mol. Ther. Methods Clin. Dev. 2018, 10, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Ballermann, B.J.; Ott, M.J. Adhesion and Differentiation of Endothelial Cells by Exposure to Chronic Shear Stress: A Vascular Graft Model. Blood Purif. 1995, 13, 125–134. [Google Scholar] [CrossRef]
- Redmond, E.M.; Cahill, P.A.; Sitzmann, J. V Perfused Transcapillary Smooth Muscle and Endothelial Cell Co-Culture—A Novel in Vitro Model. In Vitro Cell. Dev. Biol. Anim. 1995, 31, 601–609. [Google Scholar] [CrossRef]
- Cucullo, L.; Hossain, M.; Tierney, W.; Janigro, D. A New Dynamic In Vitro Modular Capillaries-Venules Modular System: Cerebrovascular Physiology in a Box. BMC Neurosci. 2013, 14, 18. [Google Scholar] [CrossRef] [Green Version]
- Jossen, V.; Pörtner, R.; Kaiser, S.C.; Kraume, M.; Eibl, D.; Eibl, R. Mass Production of Mesenchymal Stem Cells—Impact of Bioreactor Design and Flow Conditions on Proliferation and Differentiation. In Cells and Biomaterials in Regenerative Medicine; InTech: London, UK, 2014. [Google Scholar]
- Knöspel, F.; Freyer, N.; Stecklum, M.; Gerlach, J.C.; Zeilinger, K. Periodic Harvesting of Embryonic Stem Cells from a Hollow-Fiber Membrane Based Four-Compartment Bioreactor. Biotechnol. Prog. 2016, 32, 141–151. [Google Scholar] [CrossRef]
- Matsushita, S.; Kajiwara, T.; Mizumoto, H. Expansion and Differentiation of Human IPS Cells in a Three-Dimensional Culture Using Hollow Fibers and Separation of the Specific Population by Magnetic-Activated Cell Sorting. J. Biosci. Bioeng. 2019, 128, 480–486. [Google Scholar] [CrossRef]
- Stachelscheid, H.; Wulf-Goldenberg, A.; Eckert, K.; Jensen, J.; Edsbagge, J.; Björquist, P.; Rivero, M.; Strehl, R.; Jozefczuk, J.; Prigione, A.; et al. Teratoma Formation of Human Embryonic Stem Cells in Three-Dimensional Perfusion Culture Bioreactors. J. Tissue Eng. Regen. Med. 2013, 7, 729–741. [Google Scholar] [CrossRef] [Green Version]
- Mizukami, A.; Pereira Chilima, T.D.; Orellana, M.D.; Neto, M.A.; Covas, D.T.; Farid, S.S.; Swiech, K. Technologies for Large-Scale Umbilical Cord-Derived MSC Expansion: Experimental Performance and Cost of Goods Analysis. Biochem. Eng. J. 2018, 135, 36–48. [Google Scholar] [CrossRef]
- Frank, N.D.; Jones, M.E.; Vang, B.; Coeshott, C. Evaluation of Reagents Used to Coat the Hollow-Fiber Bioreactor Membrane of the Quantum® Cell Expansion System for the Culture of Human Mesenchymal Stem Cells. Mater. Sci. Eng. C 2019, 96, 77–85. [Google Scholar] [CrossRef]
- Vymetalova, L.; Kucirkova, T.; Knopfova, L.; Pospisilova, V.; Kasko, T.; Lejdarova, H.; Makaturova, E.; Kuglik, P.; Oralova, V.; Matalova, E.; et al. Large-Scale Automated Hollow-Fiber Bioreactor Expansion of Umbilical Cord-Derived Human Mesenchymal Stromal Cells for Neurological Disorders. Neurochem. Res. 2020, 45, 204–214. [Google Scholar] [CrossRef]
- De Napoli, I.E.; Scaglione, S.; Giannoni, P.; Quarto, R.; Catapano, G. Mesenchymal Stem Cell Culture in Convection-Enhanced Hollow Fibre Membrane Bioreactors for Bone Tissue Engineering. J. Memb. Sci. 2011, 379, 341–352. [Google Scholar] [CrossRef]
- Allen, A.; Vaninov, N.; Li, M.; Nguyen, S.; Singh, M.; Igo, P.; Tilles, A.W.; O’Rourke, B.; Miller, B.L.K.; Parekkadan, B.; et al. Mesenchymal Stromal Cell Bioreactor for Ex Vivo Reprogramming of Human Immune Cells. Sci. Rep. 2020, 10, 10142. [Google Scholar] [CrossRef]
- Xue, C.; Kwek, K.Y.C.; Chan, J.K.Y.; Chen, Q.; Lim, M. The Hollow Fiber Bioreactor as a Stroma-Supported, Serum-Free Ex Vivo Expansion Platform for Human Umbilical Cord Blood Cells. Biotechnol. J. 2014, 9, 980–989. [Google Scholar] [CrossRef]
- Allenby, M.C.; Tahlawi, A.; Morais, J.C.F.; Li, K.; Panoskaltsis, N.; Mantalaris, A. Ceramic Hollow Fibre Constructs for Continuous Perfusion and Cell Harvest from 3D Hematopoietic Organoids. Stem Cells Int. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Allenby, M.C.; Panoskaltsis, N.; Tahlawi, A.; Dos Santos, S.B.; Mantalaris, A. Dynamic Human Erythropoiesis in a Three-Dimensional Perfusion Bone Marrow Biomimicry. Biomaterials 2019, 188, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Misener, R.; Allenby, M.C.; Fuentes-Garí, M.; Gupta, K.; Wiggins, T.; Panoskaltsis, N.; Pistikopoulos, E.N.; Mantalaris, A. Stem Cell Biomanufacturing under Uncertainty: A Case Study in Optimizing Red Blood Cell Production. AIChE J. 2018, 64, 3011–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The CellCultureCompany. Available online: https://cellculturecompany.com/ (accessed on 4 April 2022).
- Flocel. Available online: https://www.flocel.com/ (accessed on 10 April 2022).
- Terumo. Terumo Quantum. Available online: https://www.terumobct.com/quantum/ (accessed on 31 March 2022).
- Oxford Mestar Ltd. Bioreactor Systems by Oxford Mestar. Available online: http://www.oxford-mestar.com/ (accessed on 31 March 2022).
- Coeshott, C.; Vang, B.; Jones, M.; Nankervis, B. Large-Scale Expansion and Characterization of CD3+ T-Cells in the Quantum® Cell Expansion System. J. Transl. Med. 2019, 17, 258. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Nankervis, B.; Roballo, K.S.; Pham, H.; Bushman, J.; Coeshott, C. A Comparison of Automated Perfusion- and Manual Diffusion-Based Human Regulatory T Cell Expansion and Functionality Using a Soluble Activator Complex. Cell Transplant. 2020, 29, 096368972092357. [Google Scholar] [CrossRef] [PubMed]
- Hanley, P.J.; Mei, Z.; Durett, A.G.; da Graca Cabreira-Harrison, M.; Klis, M.; Li, W.; Zhao, Y.; Yang, B.; Parsha, K.; Mir, O.; et al. Efficient Manufacturing of Therapeutic Mesenchymal Stromal Cells with the Use of the Quantum Cell Expansion System. Cytotherapy 2014, 16, 1048–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haack-Sørensen, M.; Follin, B.; Juhl, M.; Brorsen, S.K.; Søndergaard, R.H.; Kastrup, J.; Ekblond, A. Culture Expansion of Adipose Derived Stromal Cells. A Closed Automated Quantum Cell Expansion System Compared with Manual Flask-Based Culture. J. Transl. Med. 2016, 14, 319. [Google Scholar] [CrossRef] [Green Version]
- Paccola Mesquita, F.C.; Hochman-Mendez, C.; Morrissey, J.; Sampaio, L.C.; Taylor, D.A. Laminin as a Potent Substrate for Large-Scale Expansion of Human Induced Pluripotent Stem Cells in a Closed Cell Expansion System. Stem Cells Int. 2019, 2019, 9704945. [Google Scholar] [CrossRef] [Green Version]
- Gobin, J.; Muradia, G.; Mehic, J.; Westwood, C.; Couvrette, L.; Stalker, A.; Bigelow, S.; Luebbert, C.C.; Bissonnette, F.S.-D.; Johnston, M.J.W.; et al. Hollow-Fiber Bioreactor Production of Extracellular Vesicles from Human Bone Marrow Mesenchymal Stromal Cells Yields Nanovesicles That Mirrors the Immuno-Modulatory Antigenic Signature of the Producer Cell. Stem Cell Res. Ther. 2021, 12, 127. [Google Scholar] [CrossRef]
- Usuludin, S.B.M.; Cao, X.; Lim, M. Co-Culture of Stromal and Erythroleukemia Cells in a Perfused Hollow Fiber Bioreactor System as an in Vitro Bone Marrow Model for Myeloid Leukemia. Biotechnol. Bioeng. 2012, 109, 1248–1258. [Google Scholar] [CrossRef]
- Blaber, E.; Sato, K.; Almeida, E.A.C. Stem Cell Health and Tissue Regeneration in Microgravity. Stem Cells Dev. 2014, 23, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A Review of Therapeutic Effects of Mesenchymal Stem Cell Secretions and Induction of Secretory Modification by Different Culture Methods. J. Transl. Med. 2014, 12, 260. [Google Scholar] [CrossRef]
- Sagaradze, G.; Grigorieva, O.; Nimiritsky, P.; Basalova, N.; Kalinina, N.; Akopyan, Z.; Efimenko, A. Conditioned Medium from Human Mesenchymal Stromal Cells: Towards the Clinical Translation. Int. J. Mol. Sci. 2019, 20, 1656. [Google Scholar] [CrossRef] [Green Version]
- Shojaei, F.; Rahmati, S.; Banitalebi Dehkordi, M. A Review on Different Methods to Increase the Efficiency of Mesenchymal Stem Cell-based Wound Therapy. Wound Repair Regen. 2019, 27, 661–671. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal Stem Cells Enhance Wound Healing Through Differentiation and Angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Tredget, E.E.; Wu, P.Y.G.; Wu, Y. Paracrine Factors of Mesenchymal Stem Cells Recruit Macrophages and Endothelial Lineage Cells and Enhance Wound Healing. PLoS One 2008, 3, e1886. [Google Scholar] [CrossRef] [Green Version]
- Yew, T.-L.; Hung, Y.-T.; Li, H.-Y.; Chen, H.-W.; Chen, L.-L.; Tsai, K.-S.; Chiou, S.-H.; Chao, K.-C.; Huang, T.-F.; Chen, H.-L.; et al. Enhancement of Wound Healing by Human Multipotent Stromal Cell Conditioned Medium: The Paracrine Factors and P38 MAPK Activation. Cell Transplant. 2011, 20, 693–706. [Google Scholar] [CrossRef]
- Pawitan, J.A. Prospect of Stem Cell Conditioned Medium in Regenerative Medicine. Biomed Res. Int. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Phan, J.; Kumar, P.; Hao, D.; Gao, K.; Farmer, D.; Wang, A. Engineering Mesenchymal Stem Cells to Improve Their Exosome Efficacy and Yield for Cell-Free Therapy. J. Extracell. Vesicles 2018, 7, 1522236. [Google Scholar] [CrossRef]
- Colao, I.L.; Corteling, R.; Bracewell, D.; Wall, I. Manufacturing Exosomes: A Promising Therapeutic Platform. Trends Mol. Med. 2018, 24, 242–256. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.-T.; Chang, Y.-C.; Lin, L.-C.; Liao, P.-C. Collection of in Vivo-like Liver Cell Secretome with Alternative Sample Enrichment Method Using a Hollow Fiber Bioreactor Culture System Combined with Tangential Flow Filtration for Secretomics Analysis. Anal. Chim. Acta 2011, 684, 81–88. [Google Scholar] [CrossRef]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.-C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X.; et al. Generation and Testing of Clinical-Grade Exosomes for Pancreatic Cancer. JCI Insight 2018, 3, e99263. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.M.; Dennahy, I.S.; Bhatti, U.F.; Halaweish, I.; Xiong, Y.; Chang, P.; Nikolian, V.C.; Chtraklin, K.; Brown, J.; Zhang, Y.; et al. Mesenchymal Stem Cell-Derived Exosomes Provide Neuroprotection and Improve Long-Term Neurologic Outcomes in a Swine Model of Traumatic Brain Injury and Hemorrhagic Shock. J. Neurotrauma 2019, 36, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Potter, D.R.; Miyazawa, B.Y.; Gibb, S.L.; Deng, X.; Togaratti, P.P.; Croze, R.H.; Srivastava, A.K.; Trivedi, A.; Matthay, M.; Holcomb, J.B.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Pulmonary Vascular Permeability and Lung Injury Induced by Hemorrhagic Shock and Trauma. J. Trauma Acute Care Surg. 2018, 84, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Park, J.; Jung, J.-H.; Lee, R.; Park, J.-H.; Yuk, J.M.; Hwang, H.; Yeon, J.H. Cyclic Tangential Flow Filtration System for Isolation of Extracellular Vesicles. APL Bioeng. 2021, 5, 016103. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Peng, C.; Yi, J.; Zhang, D.; Xiang, X.; Peng, X.; Su, B.; Liu, B.; Shen, Y.; Qiao, L. Highly Efficient Exosome Purification from Human Plasma by Tangential Flow Filtration Based Microfluidic Chip. Sens. Actuators B Chem. 2021, 333, 129563. [Google Scholar] [CrossRef]
- Liang, L.-G.; Sheng, Y.-F.; Zhou, S.; Inci, F.; Li, L.; Demirci, U.; Wang, S. An Integrated Double-Filtration Microfluidic Device for Detection of Extracellular Vesicles from Urine for Bladder Cancer Diagnosis. In Extracellular Vesicles; Humana Press: New York, NY, USA, 2017; pp. 355–364. [Google Scholar]
- Liu, F.; Vermesh, O.; Mani, V.; Ge, T.J.; Madsen, S.J.; Sabour, A.; Hsu, E.-C.; Gowrishankar, G.; Kanada, M.; Jokerst, J.V.; et al. The Exosome Total Isolation Chip. ACS Nano 2017, 11, 10712–10723. [Google Scholar] [CrossRef]
- Woo, H.-K.; Sunkara, V.; Park, J.; Kim, T.-H.; Han, J.-R.; Kim, C.-J.; Choi, H.-I.; Kim, Y.-K.; Cho, Y.-K. Exodisc for Rapid, Size-Selective, and Efficient Isolation and Analysis of Nanoscale Extracellular Vesicles from Biological Samples. ACS Nano 2017, 11, 1360–1370. [Google Scholar] [CrossRef]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques Used for the Isolation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey. J. Extracell. Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef]
- Welton, J.L.; Webber, J.P.; Botos, L.-A.; Jones, M.; Clayton, A. Ready-Made Chromatography Columns for Extracellular Vesicle Isolation from Plasma. J. Extracell. Vesicles 2015, 4, 27269. [Google Scholar] [CrossRef]
- Vergauwen, G.; Dhondt, B.; Van Deun, J.; De Smedt, E.; Berx, G.; Timmerman, E.; Gevaert, K.; Miinalainen, I.; Cocquyt, V.; Braems, G.; et al. Confounding Factors of Ultrafiltration and Protein Analysis in Extracellular Vesicle Research. Sci. Rep. 2017, 7, 2704. [Google Scholar] [CrossRef]
- McNamara, R.P.; Caro-Vegas, C.P.; Costantini, L.M.; Landis, J.T.; Griffith, J.D.; Damania, B.A.; Dittmer, D.P. Large-Scale, Cross-Flow Based Isolation of Highly Pure and Endocytosis-Competent Extracellular Vesicles. J. Extracell. Vesicles 2018, 7, 1541396. [Google Scholar] [CrossRef] [Green Version]
- Busatto, S.; Vilanilam, G.; Ticer, T.; Lin, W.-L.; Dickson, D.; Shapiro, S.; Bergese, P.; Wolfram, J. Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid. Cells 2018, 7, 273. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.S.; Cho, W.L.; Choi, Y.J.; Kim, J.D.; Park, H.-A.; Kim, S.Y.; Park, J.H.; Jo, D.-G.; Cho, Y.W. Functional Recovery in Photo-Damaged Human Dermal Fibroblasts by Human Adipose-Derived Stem Cell Extracellular Vesicles. J. Extracell. Vesicles 2019, 8, 1565885. [Google Scholar] [CrossRef] [Green Version]
- Liangsupree, T.; Multia, E.; Riekkola, M.-L. Modern Isolation and Separation Techniques for Extracellular Vesicles. J. Chromatogr. A 2021, 1636, 461773. [Google Scholar] [CrossRef]
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N.E.; Timmers, L.; van Rijen, H.V.; Doevendans, P.A.; Pasterkamp, G.; et al. Mesenchymal Stem Cell-Derived Exosomes Increase ATP Levels, Decrease Oxidative Stress and Activate PI3K/Akt Pathway to Enhance Myocardial Viability and Prevent Adverse Remodeling after Myocardial Ischemia/Reperfusion Injury. Stem Cell Res. 2013, 10, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Musante, L.; Tataruch, D.; Gu, D.; Benito-Martin, A.; Calzaferri, G.; Aherne, S.; Holthofer, H. A Simplified Method to Recover Urinary Vesicles for Clinical Applications and Sample Banking. Sci. Rep. 2015, 4, 7532. [Google Scholar] [CrossRef] [Green Version]
- FDA Pyrogens, Still a Danger. Available online: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-technical-guides/pyrogens-still-danger (accessed on 27 August 2022).
- Eibl, R.; Werner, S.; Eibl, D. Bag Bioreactor Based on Wave-Induced Motion: Characteristics and Applications. Adv. Biochem. Eng. Biotechnol. 2009, 115, 55–87. [Google Scholar] [CrossRef]
- Baudequin, T.; Nyland, R.; Ye, H. Objectives, Benefits and Challenges of Bioreactor Systems for the Clinical-Scale Expansion of T Lymphocyte Cells. Biotechnol. Adv. 2021, 49, 107735. [Google Scholar] [CrossRef]
- Boubriak, O.A.; Urban, J.P.; Cui, Z. Monitoring of Metabolite Gradients in Tissue-Engineered Constructs. J. R. Soc. Interface 2006, 3, 637–648. [Google Scholar] [CrossRef] [Green Version]
- Allen, S.; Holena, D.; McCunn, M.; Kohl, B.; Sarani, B. A Review of the Fundamental Principles and Evidence Base in the Use of Extracorporeal Membrane Oxygenation (ECMO) in Critically Ill Adult Patients. J. Intensive Care Med. 2011, 26, 13–26. [Google Scholar] [CrossRef] [PubMed]

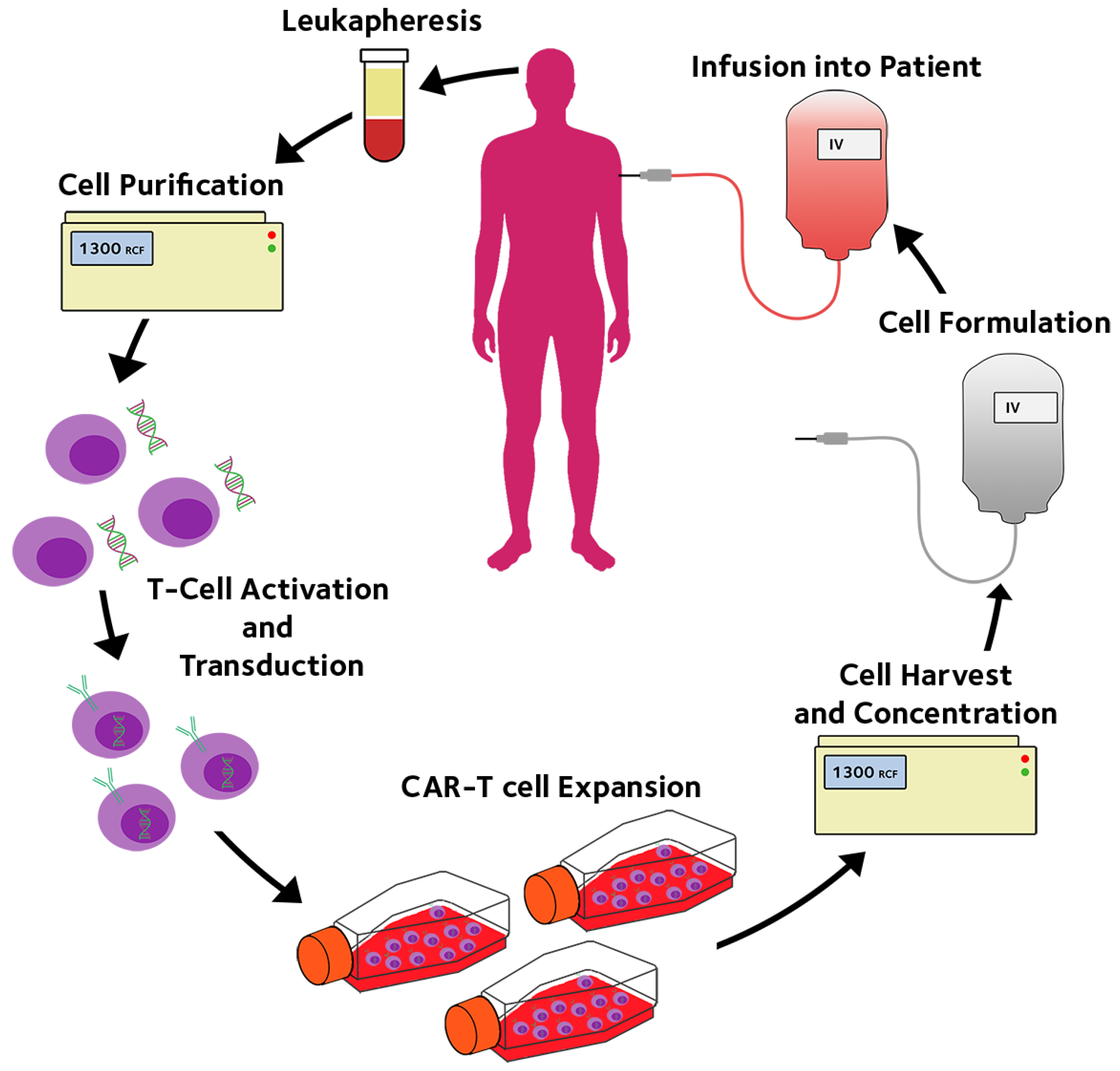

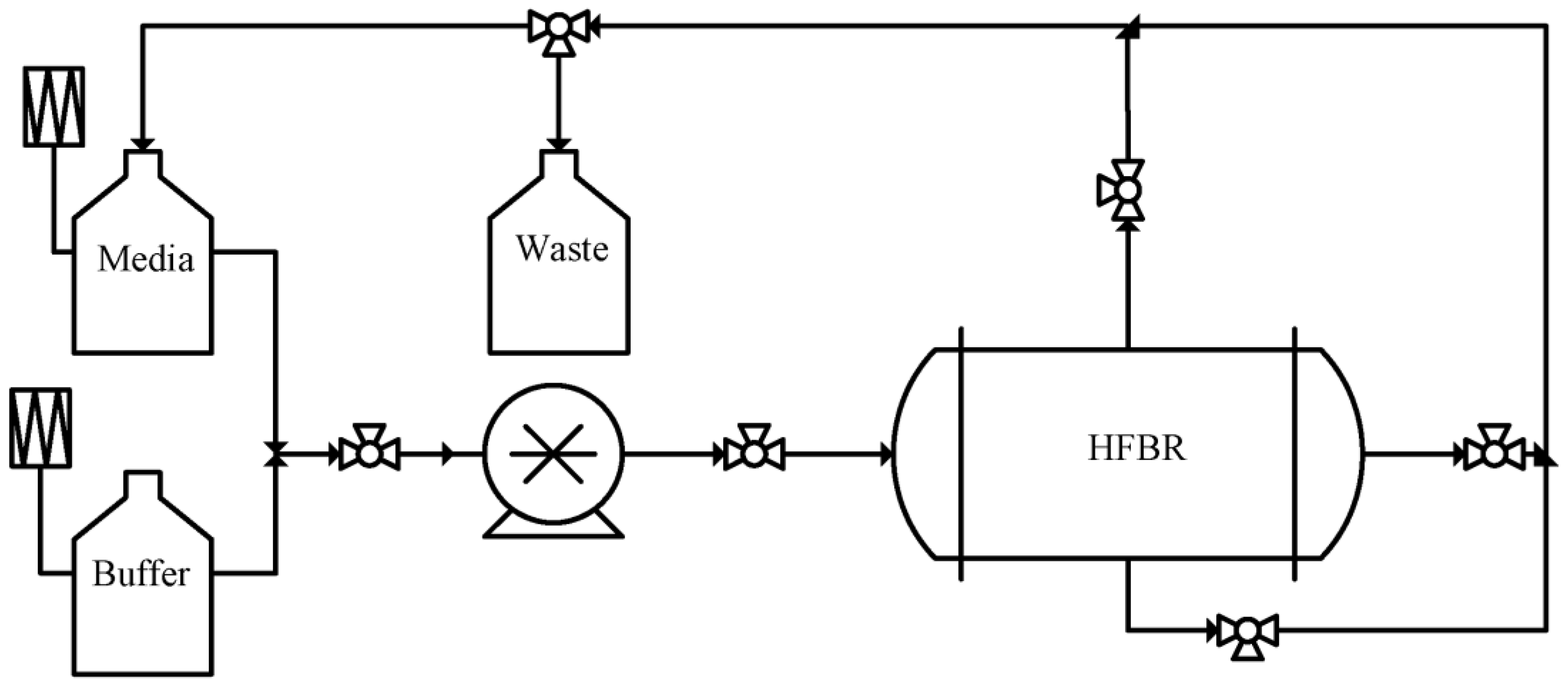
| Name | Company | Principle | Volume/Surface Area | Scalability | Gene Editing? | Temperature Control | Final formulation |
|---|---|---|---|---|---|---|---|
| CliniMACS Prodigy | Miltenyi Biotec | CentriCultUnit or External culture vessel | low/dependant on external vessel size | low | In place | Requires external temperature control when external vessel used. No reagent temperature control | Fill and finish capable |
| Cocoon | Lonza | Customizable cassette | 460 mL—low | medium | In place | Duel environmental control for reagents and cell growth | Fill and finish capable |
| Xuri cell | Cytiva | Wavebag | 0.3–25 L—medium | medium | In place | Integrated tray heater and sensors | External finishing required |
| Duet Pump | FibreCellSystems | Hollow fibre membrane | 80 cm2–1.2 m2 | medium | No | Requires CO2 Incubator | External finishing required |
| Quantum | TERUMO | Hollow fibre membrane | 1.7–2.1 m2 | High | No | Continuous control of temperature | External finishing required |
| HF Primer | CellCultureCompany | Hollow fibre membrane | 1.5 m2 | medium | No | Requires CO2 Incubator | Concentrates harvest but external finishing required |
| AutovaxID | CellCultureCompany | Hollow fibre membrane | 80–100 L equivalent | High | No | Automated control of temperature | Integrated refrigerator for continuous harvest |
| AcuSyst-Maximizer | CellCultureCompany | Hollow fibre membrane | 80–200 L equivalent | High | No | Automated control of temperature | Integrated refrigerator for continuous harvest and In-line filter for harvest clarification to reduce downstream processing |
| AcuSyst-Xcellerator | CellCultureCompany | Hollow fibre membrane | 500–2000 L equivalent | High | No | Automated control of temperature | Integrated refrigerator for continuous harvest and In-line filter for harvest clarification to reduce downstream processing |
| Method | Principle | Throughput | Scalability | Cost | Operation | Effects on EVs |
|---|---|---|---|---|---|---|
| Ultracentrifugation | Sequential centrifugation step, separated EVs based on size and density | Large | Low | High equipment cost | Manual labour intensive, time intensive, batch variability | Mechanical damage |
| Immunoaffinity | Capture EVs based on their surface markers | Low | Medium | High cost for antibodies | Require a pre-concentration step, time consuming | Reversible step required |
| Precipitation | Use precipitating agent to induce the pelleting of EVs | Low | Medium | Medium/Low | Further purification required to remove the precipitating agents | Introduction of synthetic precipitating agents to EVs |
| Size exclusion chromatography | Separated EVs based on size with a packed column of with fine, porous beads | Medium | Medium | Medium | Require concentration step before and after | Minimal detrimental effects on EVs |
| Membrane filtration | Separated EVs based on size with filters | Large | High | Medium/Low | Time-efficient | Less detrimental effects on EVs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, R.; Lei, R.; Zeng, Y.; Zhu, J.; Chang, H.; Ye, H.; Cui, Z. Membrane Applications in Autologous Cell Therapy. Membranes 2022, 12, 1182. https://doi.org/10.3390/membranes12121182
Martin R, Lei R, Zeng Y, Zhu J, Chang H, Ye H, Cui Z. Membrane Applications in Autologous Cell Therapy. Membranes. 2022; 12(12):1182. https://doi.org/10.3390/membranes12121182
Chicago/Turabian StyleMartin, Risto, Rui Lei, Yida Zeng, Jiachen Zhu, Hong Chang, Hua Ye, and Zhanfeng Cui. 2022. "Membrane Applications in Autologous Cell Therapy" Membranes 12, no. 12: 1182. https://doi.org/10.3390/membranes12121182
APA StyleMartin, R., Lei, R., Zeng, Y., Zhu, J., Chang, H., Ye, H., & Cui, Z. (2022). Membrane Applications in Autologous Cell Therapy. Membranes, 12(12), 1182. https://doi.org/10.3390/membranes12121182






