The Evaluation of Counter Diffusion CVD Silica Membrane Formation Process by In Situ Analysis of Diffusion Carrier Gas
Abstract
1. Introduction
2. Experimental
2.1. Preparation of γ-Alumina Substrate
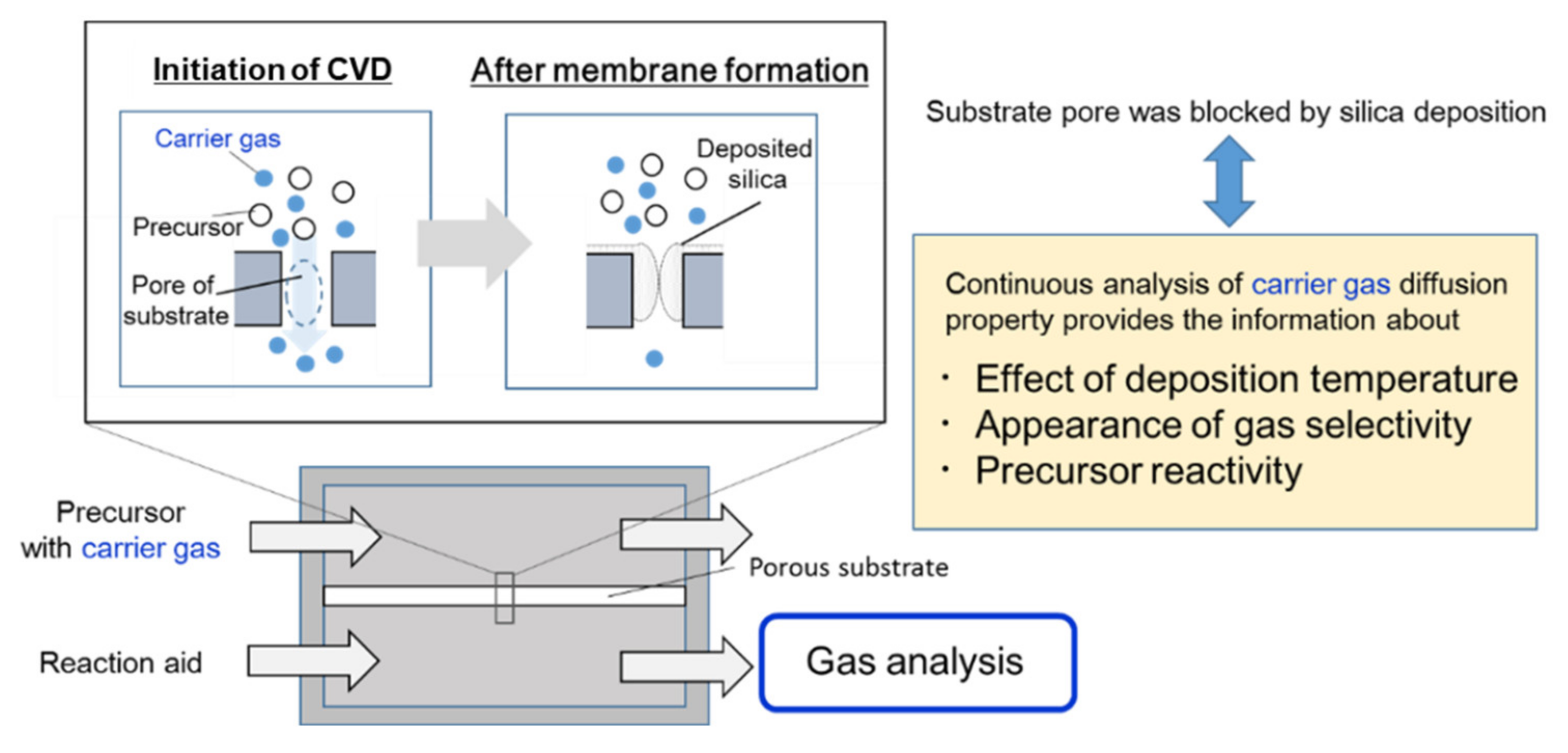
2.2. Counter Diffusion CVD
2.3. Characterization
3. Results and Discussion
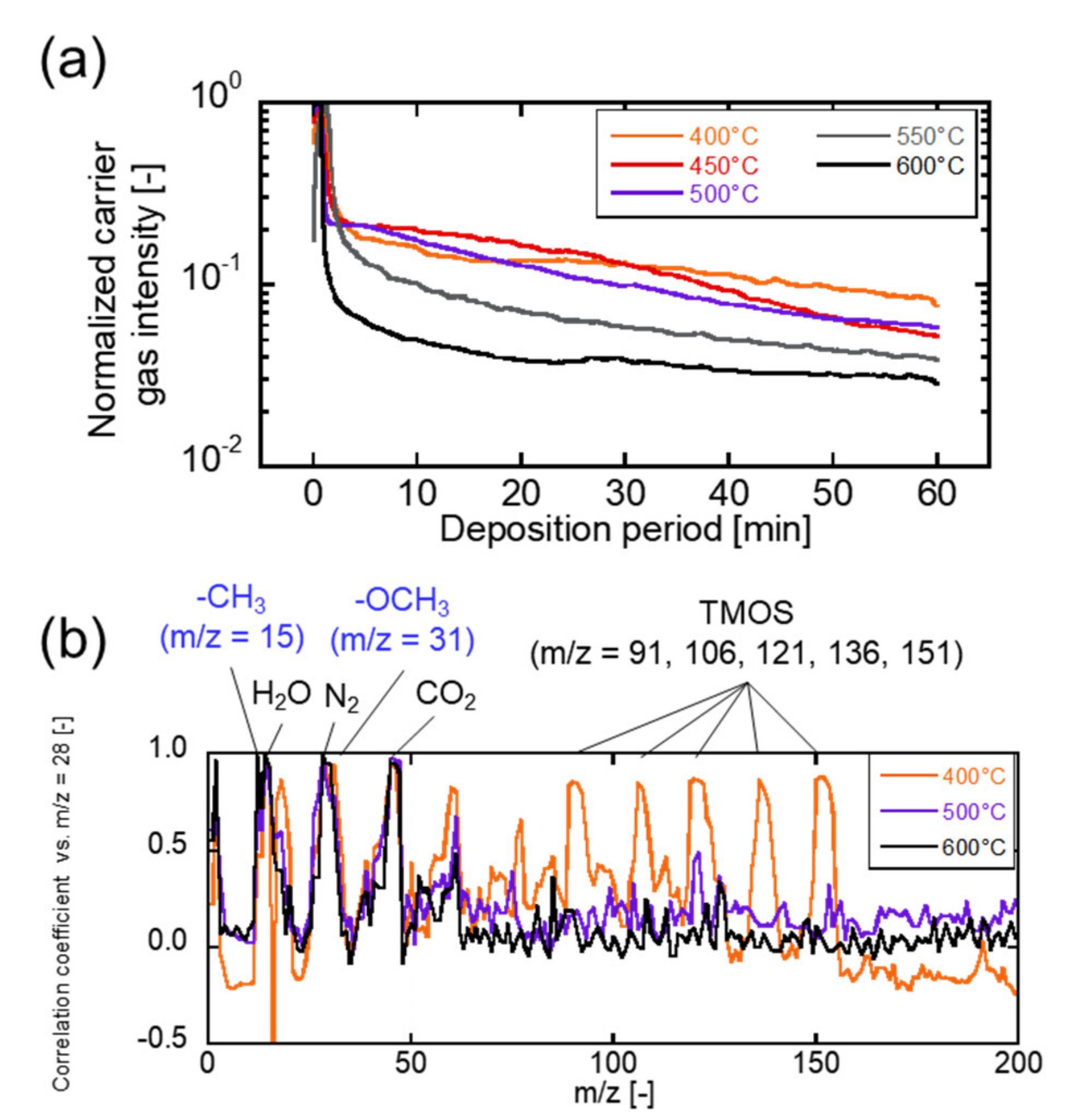
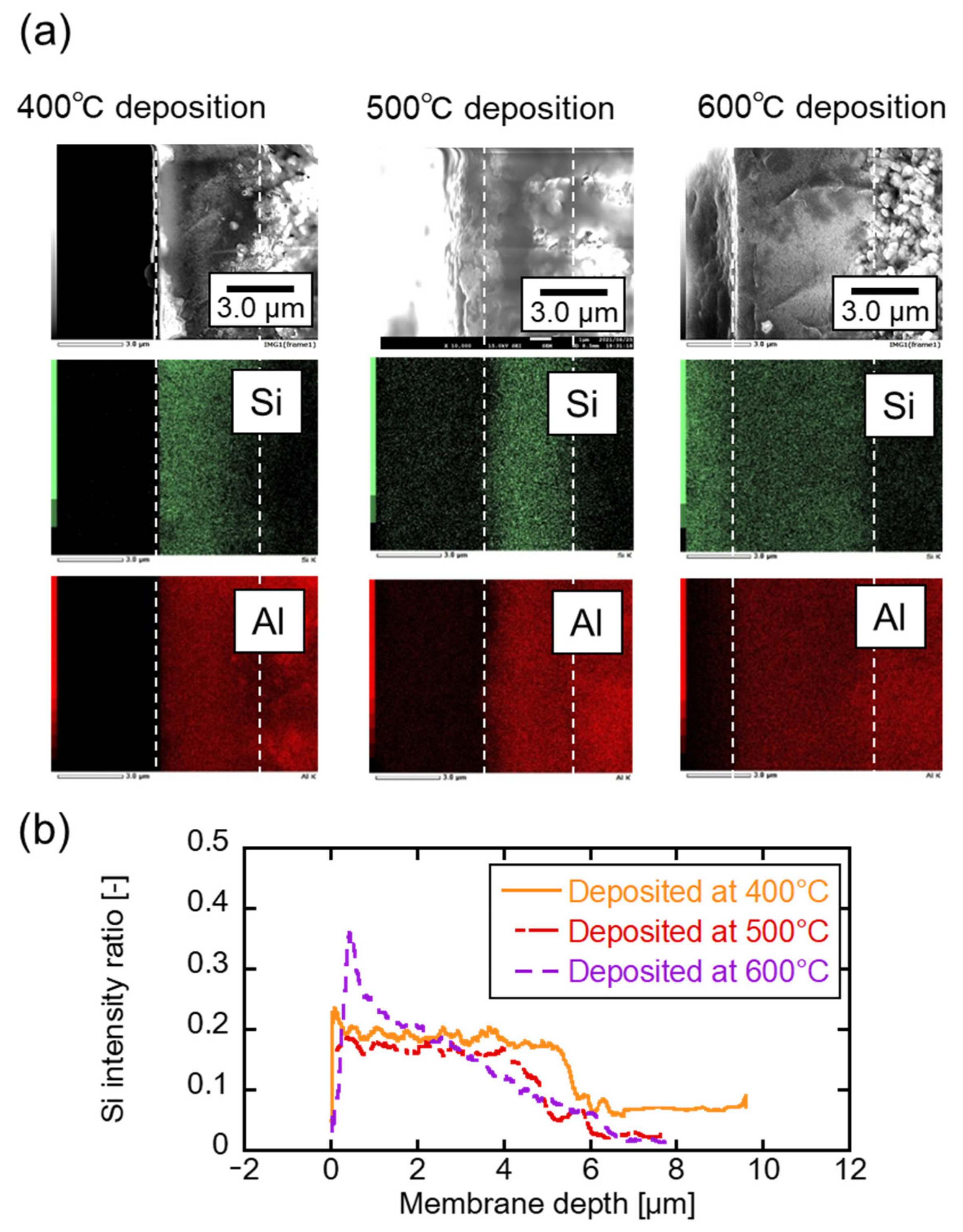
3.1. TMOS Deposition Analysis and Characterization
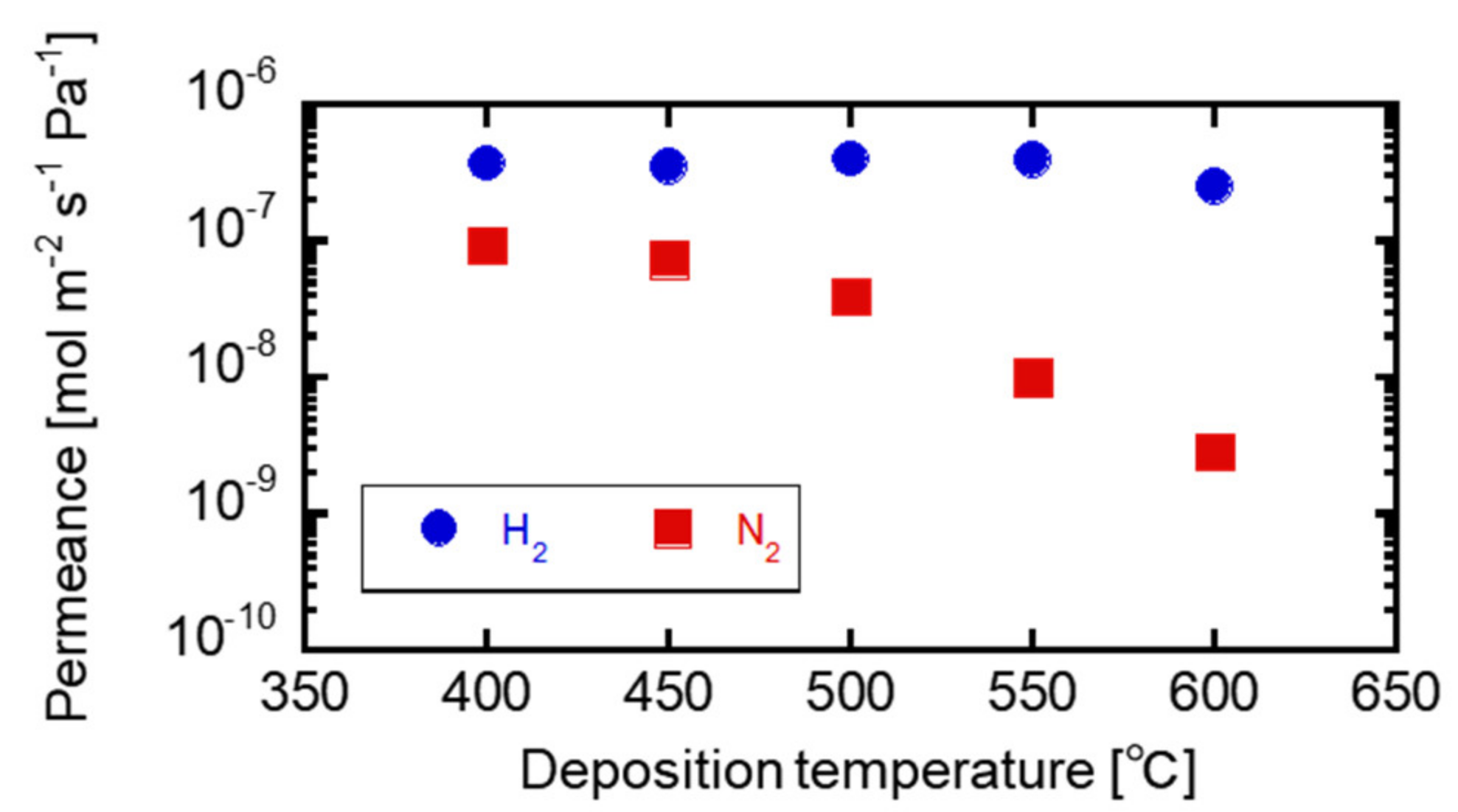
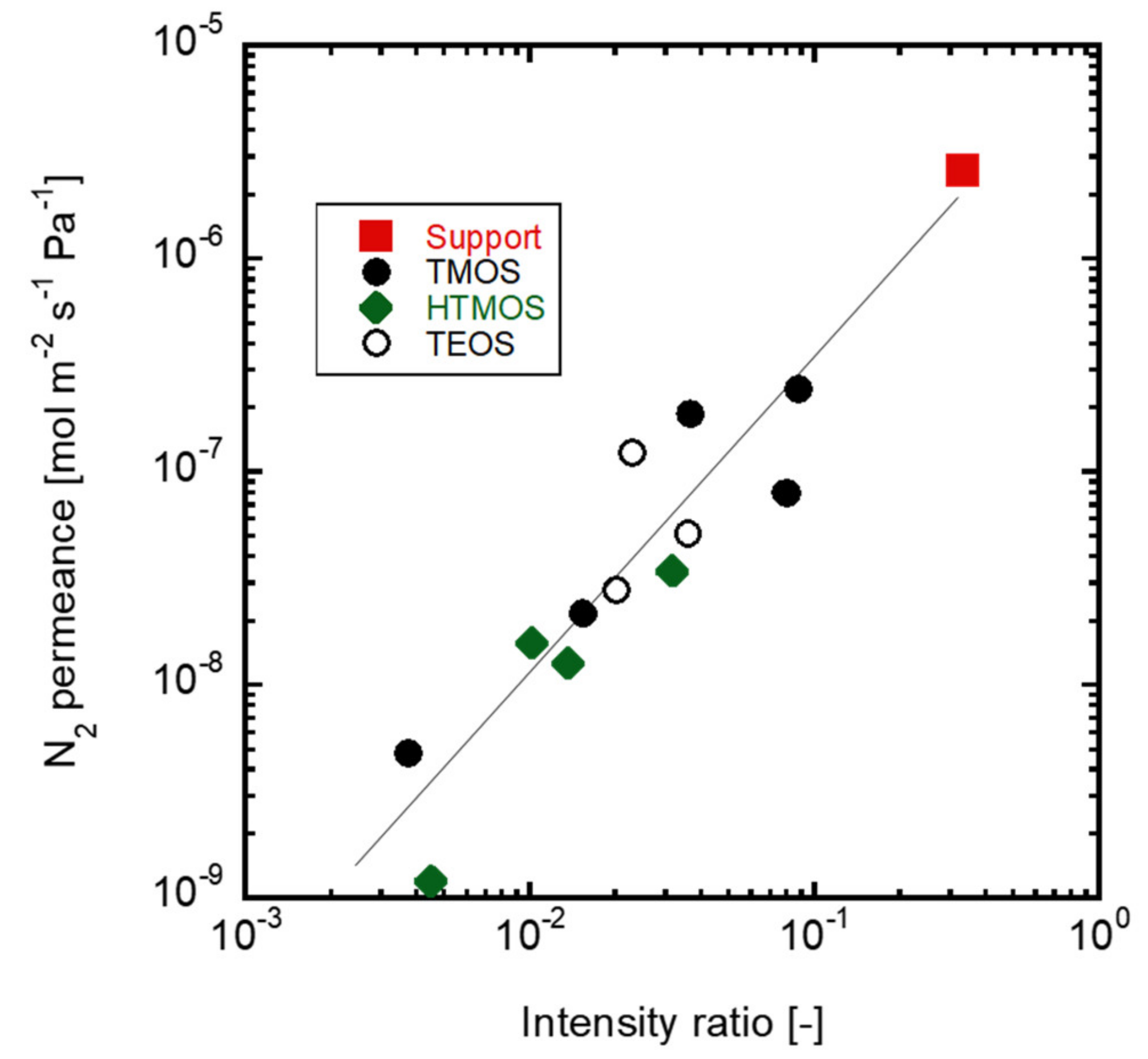
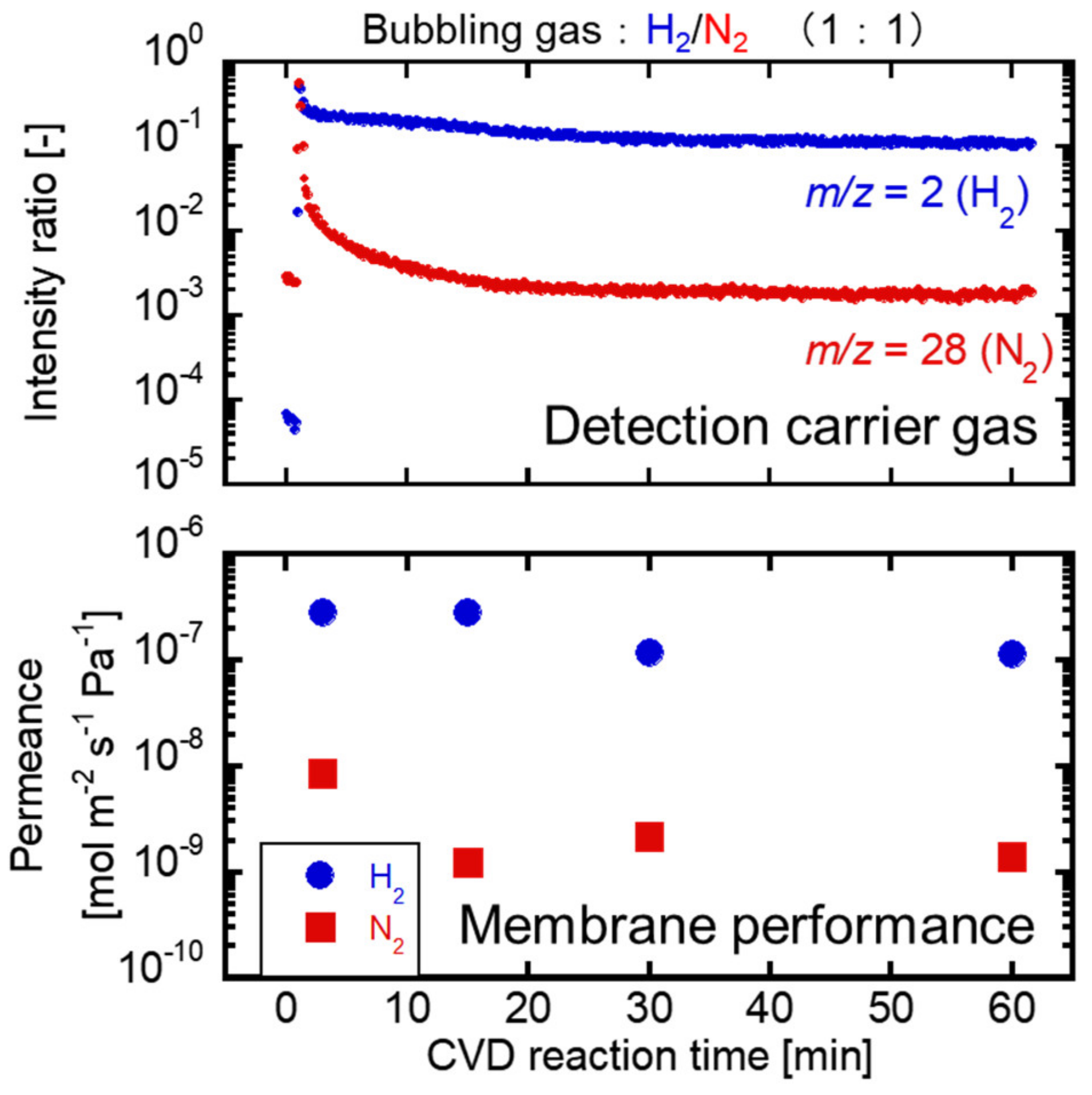
3.2. Correlation between the Membrane Perfomance and Carrier Gas Detection, and Applying Multicomponent Carrier to Evaluate Membrane Formation
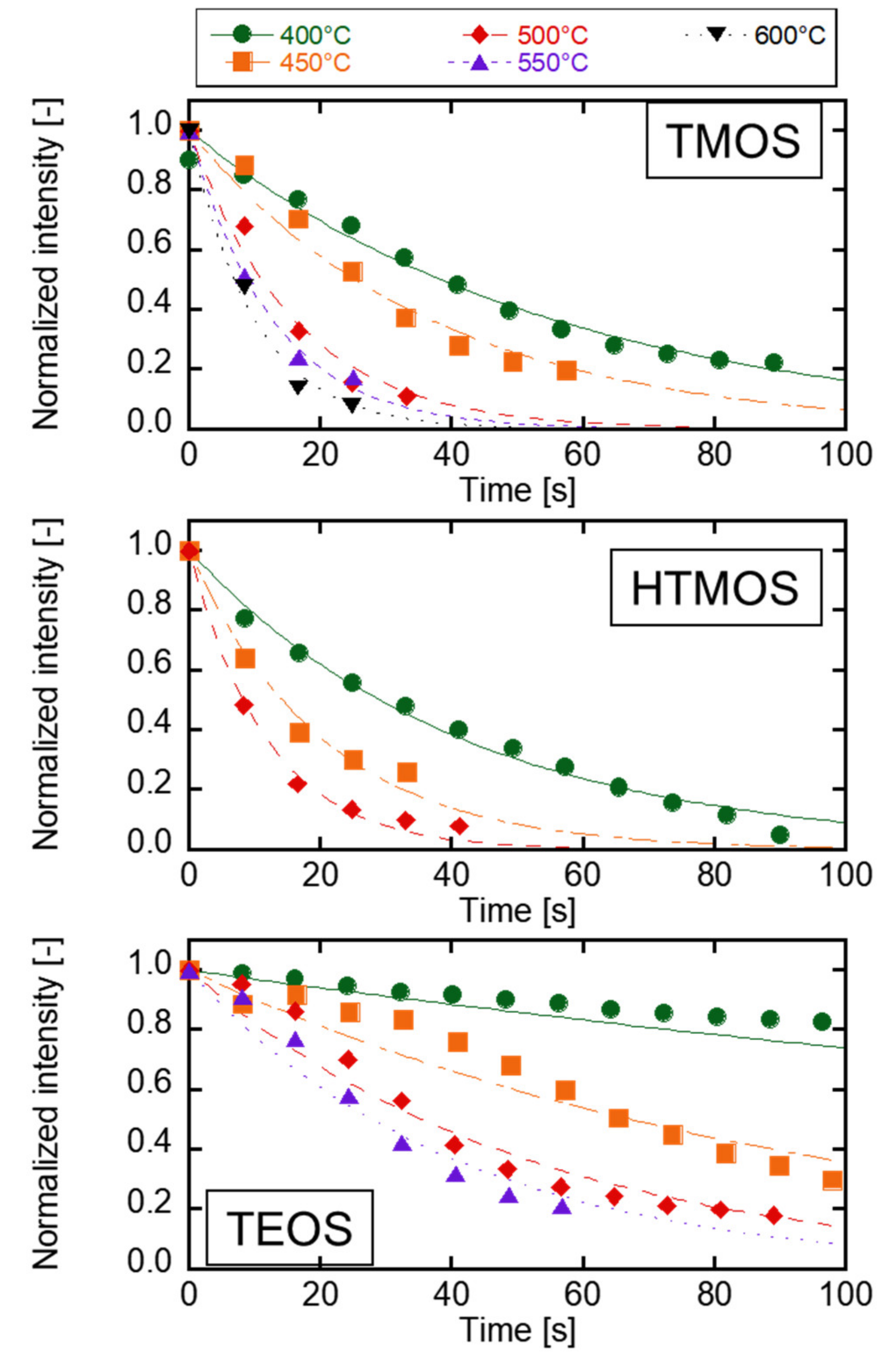
3.3. The Evaluation of the Kinetics of Precursor by Using Carrier Gas Diffusion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Myagmarjav, O.; Tanaka, N.; Nomura, M.; Kubo, S. Hydrogen production tests by hydrogen iodide decomposition membrane reactor equipped with silica-based ceramics membrane. Int. J. Hydrogen Energy 2017, 42, 29091–29100. [Google Scholar] [CrossRef]
- Myagmarjav, O.; Iwatsuki, J.; Tanaka, N.; Noguchi, H.; Kamiji, Y.; Ioka, I.; Kubo, S.; Nomura, M.; Yamaki, T.; Sawada, S.; et al. Research and development on membrane IS process for hydrogen production using solar heat. Int. J. Hydrogen Energy 2019, 44, 19141–19152. [Google Scholar] [CrossRef]
- Kusakabe, K.; Kuroda, T.; Morooka, S. Separation of carbon dioxide from nitrogen using ion-exchanged faujasite-type zeolite membranes formed on porous support tubes. J. Memb. Sci. 1998, 148, 13–23. [Google Scholar] [CrossRef]
- Sakai, M.; Sasaki, Y.; Tomono, T.; Seshimo, M.; Matsukata, M. Olefin Selective ag-exchanged X-type zeolite membrane for propylene/propane and ethylene/ethane separation. ACS Appl. Mater. Interfaces 2019, 11, 4145–4151. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, K.; Sakamoto, S.; Saie, T.; Morooka, S. Pore structure of silica membranes formed by a sol-gel technique using tetraethoxysilane and alkyltriethoxysilanes. Sep. Purif. Technol. 1999, 16, 139–146. [Google Scholar] [CrossRef]
- Kanezashi, M.; Yada, K.; Yoshioka, T.; Tsuru, T. Organic-inorganic hybrid silica membranes with controlled silica network size: Preparation and gas permeation characteristics. J. Memb. Sci. 2010, 348, 310–318. [Google Scholar] [CrossRef]
- Hwang, G. Hydrogen separation in H2–H2O–HI gaseous mixture using the silica membrane prepared by chemical vapor deposition. J. Memb. Sci. 1999, 162, 83–90. [Google Scholar] [CrossRef]
- Gavalas, G.R.; Megiris, C.E.; Nam, S.W. Deposition of H2-permselective SiO2 films. Chem. Eng. Sci. 1989, 44, 1829–1835. [Google Scholar] [CrossRef]
- Okubo, T.; Inoue, H. Introduction of specific gas selectivity to porous glass membranes by treatment with tetraethoxysilane. J. Memb. Sci. 1989, 42, 109–117. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Ying, X.; Tokimasa, Y.; Nair, B.N.; Sugawara, T.; Nakao, Q. Reaction control of tetraethyl orthosilicate (TEOS)/O3 and tetramethyl orthosilicate (TMOS)/O3 counterdiffusion chemical vapour deposition for preparation of molecular-sieve membranes. Phys. Chem. Chem. Phys. 2000, 2, 4465–4469. [Google Scholar] [CrossRef]
- Nagasawa, H.; Shigemoto, H.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Characterization and gas permeation properties of amorphous silica membranes prepared via plasma enhanced chemical vapor deposition. J. Memb. Sci. 2013, 441, 45–53. [Google Scholar] [CrossRef]
- Nagasawa, H.; Yamamoto, Y.; Tsuda, N.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Atmospheric-pressure plasma-enhanced chemical vapor deposition of microporous silica membranes for gas separation. J. Memb. Sci. 2017, 524, 644–651. [Google Scholar] [CrossRef]
- Lee, D.; Oyama, S.T. Gas permeation characteristics of a hydrogen selective supported silica membrane. J. Memb. Sci. 2002, 210, 291–306. [Google Scholar] [CrossRef]
- Nomura, M.; Ono, K.; Gopalakrishnan, S.; Sugawara, T.; Nakao, S.I. Preparation of a stable silica membrane by a counterdiffusion chemical vapor deposition method. J. Memb. Sci. 2005, 251, 151–158. [Google Scholar] [CrossRef]
- Nakao, S.I.; Suzuki, T.; Sugawara, T.; Tsuru, T.; Kimura, S. Preparation of microporous membranes by TEOS/O3 CVD in the opposing reactants geometry. Microporous Mesoporous Mater. 2000, 37, 145–152. [Google Scholar] [CrossRef]
- Nomura, M.; Seshimo, M.; Aida, H.; Nakatani, K.; Gopalakrishnan, S.; Sugawara, T.; Ishikawa, T.; Kawamura, M.; Nakao, S.I. Preparation of a catalyst composite silica membrane reactor for steam reforming reaction by using a counterdiffusion CVD method. Ind. Eng. Chem. Res. 2006, 45, 3950–3954. [Google Scholar] [CrossRef]
- Ahn, S.J.; Yun, G.N.; Takagaki, A.; Kikuchi, R.; Oyama, S.T. Effects of pressure, contact time, permeance, and selectivity in membrane reactors: The case of the dehydrogenation of ethane. Sep. Purif. Technol. 2018, 194, 197–206. [Google Scholar] [CrossRef]
- Ishii, K.; Nagataki, Y.; Yoshiura, J.; Saito, Y.; Nagataki, T.; Nomura, M. Development of hydrogen permselective membranes for propylene production. J. Chem. Eng. Jpn. 2021, 54, 260–265. [Google Scholar] [CrossRef]
- Oda, K.; Akamatsu, K.; Sugawara, T.; Kikuchi, R.; Segawa, A.; Nakao, S.I. Dehydrogenation of methylcyclohexane to produce high-purity hydrogen using membrane reactors with amorphous silica membranes. Ind. Eng. Chem. Res. 2010, 49, 11287–11293. [Google Scholar] [CrossRef]
- Sea, B.K.; Kusakabe, K.; Morooka, S. Pore size control and gas permeation kinetics of silica membranes by pyrolysis of phenyl-substituted ethoxysilanes with cross-flow through a porous support wall. J. Memb. Sci. 1997, 130, 41–52. [Google Scholar] [CrossRef]
- Ohta, Y.; Akamatsu, K.; Sugawara, T.; Nakao, A.; Miyoshi, A.; Nakao, S.I. Development of pore size-controlled silica membranes for gas separation by chemical vapor deposition. J. Memb. Sci. 2008, 315, 93–99. [Google Scholar] [CrossRef]
- Nomura, M.; Nagayo, T.; Monma, K. Pore size control of a molecular sieve silica membrane prepared by a counter diffusion CVD method. J. Chem. Eng. Jpn. 2007, 40, 1235–1241. [Google Scholar] [CrossRef]
- Ishii, K.; Shibata, A.; Takeuchi, T.; Yoshiura, J.; Urabe, T.; Kameda, Y.; Nomura, M. Development of silica membranes to improve dehydration reactions. J. Jpn. Pet. Inst. 2019, 62, 211–219. [Google Scholar] [CrossRef]
- Ikeda, A.; Nomura, M. Preparation of amorphous silica based membranes for separation of hydrocarbons. J. Jpn. Pet. Inst. 2016, 59, 259–265. [Google Scholar] [CrossRef][Green Version]
- Matsuyama, E.; Ikeda, A.; Komatsuzaki, M.; Sasaki, M.; Nomura, M. High-temperature propylene/propane separation through silica hybrid membranes. Sep. Purif. Technol. 2014, 128, 25–30. [Google Scholar] [CrossRef]
- Myagmarjav, O.; Ikeda, A.; Tanaka, N.; Kubo, S.; Nomura, M. Preparation of an H2-permselective silica membrane for the separation of H2 from the hydrogen iodide decomposition reaction in the iodine–sulfur process. Int. J. Hydrogen Energy 2017, 42, 6012–6023. [Google Scholar] [CrossRef]
- Myagmarjav, O.; Tanaka, N.; Nomura, M.; Kubo, S. Module design of silica membrane reactor for hydrogen production via thermochemical IS process. Int. J. Hydrogen Energy 2019, 44, 10207–10217. [Google Scholar] [CrossRef]
- Ikeda, A.; Matsuyama, E.; Komatsuzaki, M.; Sasaki, M.; Nomura, M. Development of inorganic silica reverse osmosis membranes by using a counter diffusion chemical vapor deposition method. J. Chem. Eng. Jpn. 2014, 47, 574–578. [Google Scholar] [CrossRef]
- Ishii, K.; Ikeda, A.; Takeuchi, T.; Yoshiura, J.; Nomura, M. Silica-based ro membranes for separation of acidic solution. Membranes 2019, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Yoshiura, J.; Ishii, K.; Saito, Y.; Nagataki, T.; Nagataki, Y.; Ikeda, A.; Nomura, M. Permeation properties of ions through inorganic silica-based membranes. Membranes 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.S.; Shimogaki, Y.; Komjyama, H. Macro/microcavity method and its application in modeling chemical vapor deposition reaction systems. Thin Solid Films 2000, 365, 176–188. [Google Scholar] [CrossRef]
- Ponton, S.; Vergnes, H.; Samelor, D.; Sadowski, D.; Vahlas, C.; Caussat, B. Development of a kinetic model for the moderate temperature chemical vapor deposition of SiO2 films from tetraethyl orthosilicate and oxygen. AIChE J. 2018, 64, 3958–3966. [Google Scholar] [CrossRef]
- Ponton, S.; Dhainaut, F.; Vergnes, H.; Samelor, D.; Sadowski, D.; Rouessac, V.; Lecoq, H.; Sauvage, T.; Caussat, B.; Vahlas, C. Investigation of the densification mechanisms and corrosion resistance of amorphous silica films. J. Non-Cryst. Solids 2019, 515, 34–41. [Google Scholar] [CrossRef]
- Kim, E.J.; Gill, W.N. Modeling of CVD of Silicon Dioxide Using TEOS and Ozone in a Single-Wafer Reactor. J. Electrochem. Soc. 1994, 141, 3462–3472. [Google Scholar] [CrossRef]
- Pavelescu, C.; Kleps, I. Activation energies in chemical vapour deposition kinetics of SiO2 films using TEOS chemistry. Thin Solid Films 1990, 190, 3–5. [Google Scholar] [CrossRef]
- Kim, E.J.; Gill, W.N. Analytical model for chemical vapor deposition of SiO2 films using tetraethoxysilane and ozone. J. Cryst. Growth 1994, 140, 315–326. [Google Scholar] [CrossRef]
- Kawahara, T.; Yuuki, A.; Matsui, Y. Reaction mechanism of chemical vapor deposition using tetraethylorthosilicate and ozone at atmospheric pressure. Jpn. J. Appl. Phys. 1992, 31, 2925–2930. [Google Scholar] [CrossRef]
- Sirakami, K.; Kobayashi, K.; Kikuchi, H.; Fuwa, A. Thermal decomposition of tetraethoxysilane studied by atmospheric mass-spectrometer. Nippon Kinzoku Gakkaishi 1996, 60, 751–756. [Google Scholar] [CrossRef][Green Version]
- Hasegawa, Y.; Kimura, K.; Nemoto, Y.; Nagase, T.; Kiyozumi, Y.; Nishide, T.; Mizukami, F. Real-time monitoring of permeation properties through polycrystalline MFI-type zeolite membranes during pervaporation using mass-spectrometry. Sep. Purif. Technol. 2008, 58, 386–392. [Google Scholar] [CrossRef]
- Araki, S.; Mohri, N.; Yoshimitsu, Y.; Miyake, Y. Synthesis, characterization and gas permeation properties of a silica membrane prepared by high-pressure chemical vapor deposition. J. Memb. Sci. 2007, 290, 138–145. [Google Scholar] [CrossRef]
- Park, H.M.; Lee, J.Y.; Jee, K.Y.; Nakao, S.i.; Lee, Y.T. Hydrocarbon separation properties of a CVD-deposited ceramic membrane under single gases and binary mixed gas. Sep. Purif. Technol. 2021, 254, 117642. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishii, K.; Nomura, M. The Evaluation of Counter Diffusion CVD Silica Membrane Formation Process by In Situ Analysis of Diffusion Carrier Gas. Membranes 2022, 12, 102. https://doi.org/10.3390/membranes12020102
Ishii K, Nomura M. The Evaluation of Counter Diffusion CVD Silica Membrane Formation Process by In Situ Analysis of Diffusion Carrier Gas. Membranes. 2022; 12(2):102. https://doi.org/10.3390/membranes12020102
Chicago/Turabian StyleIshii, Katsunori, and Mikihiro Nomura. 2022. "The Evaluation of Counter Diffusion CVD Silica Membrane Formation Process by In Situ Analysis of Diffusion Carrier Gas" Membranes 12, no. 2: 102. https://doi.org/10.3390/membranes12020102
APA StyleIshii, K., & Nomura, M. (2022). The Evaluation of Counter Diffusion CVD Silica Membrane Formation Process by In Situ Analysis of Diffusion Carrier Gas. Membranes, 12(2), 102. https://doi.org/10.3390/membranes12020102






