Ti-40Al-10Nb-10Cr Porous Microfiltration Membrane with Hierarchical Pore Structure for Particulate Matter Capturing from High-Temperature Flue Gas
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instruments
2.3. Preparation Process of TiAl-Based Porous Materials
2.4. High-Temperature Oxidation of TiAl-Based Porous Materials
2.5. High-Temperature Filtration Performance of Ti-40Al-10Nb-10Cr Porous Alloy Tests
3. Results and Discussion
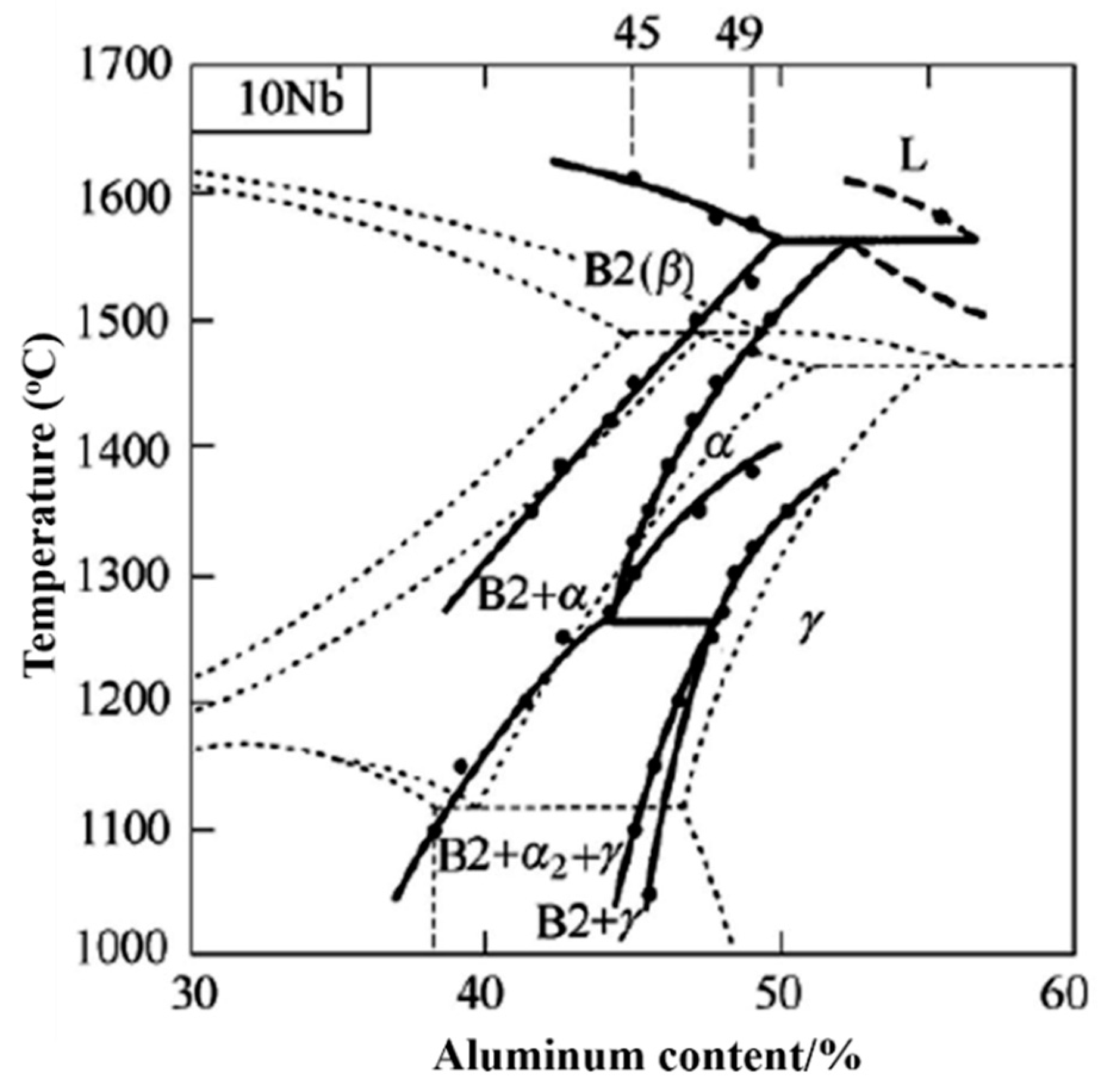
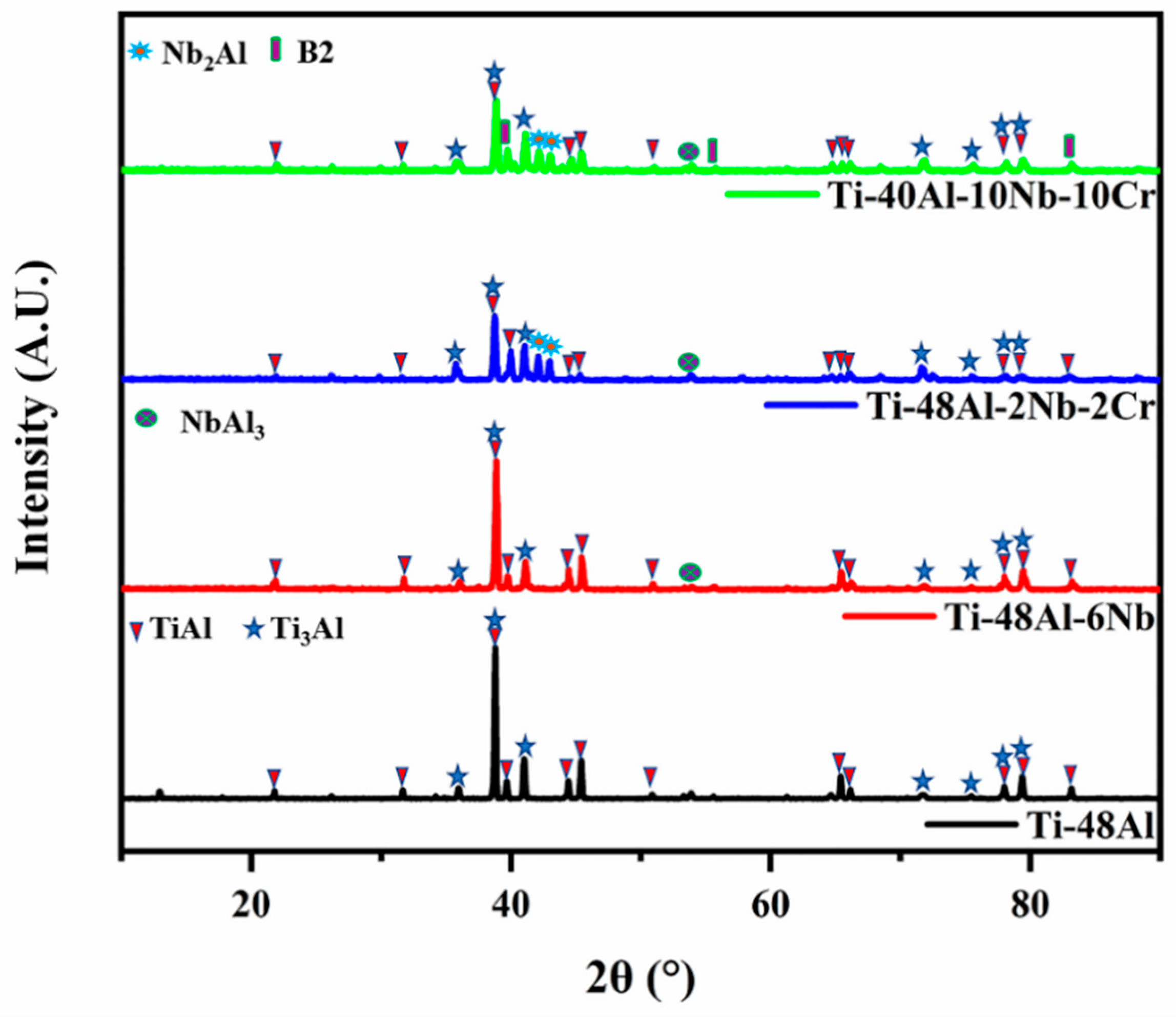
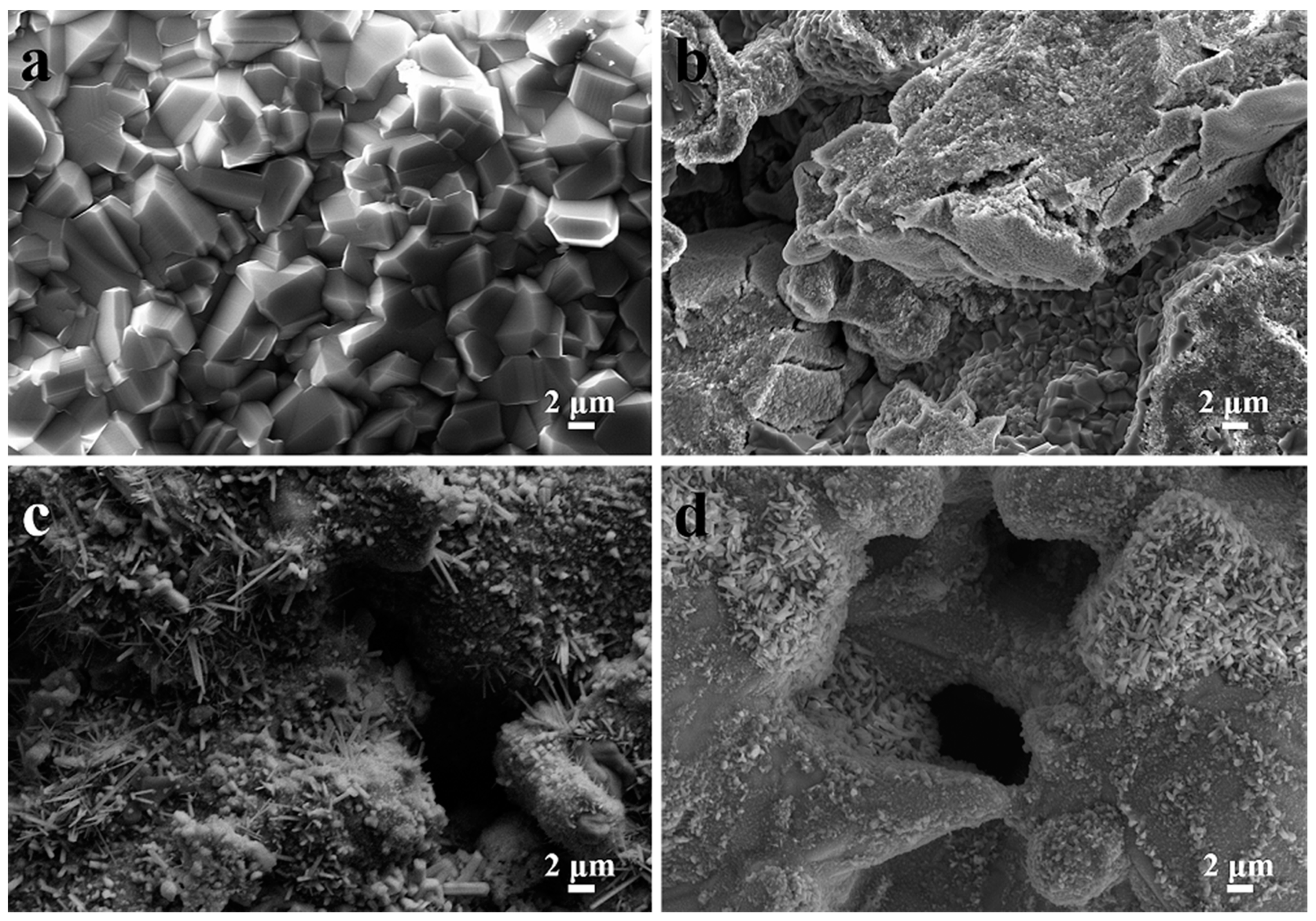
3.1. Phase Composition and Microstructure of TiAl-Based Porous Materials
3.2. Pore Parameters of TiAl-Based Porous Materials
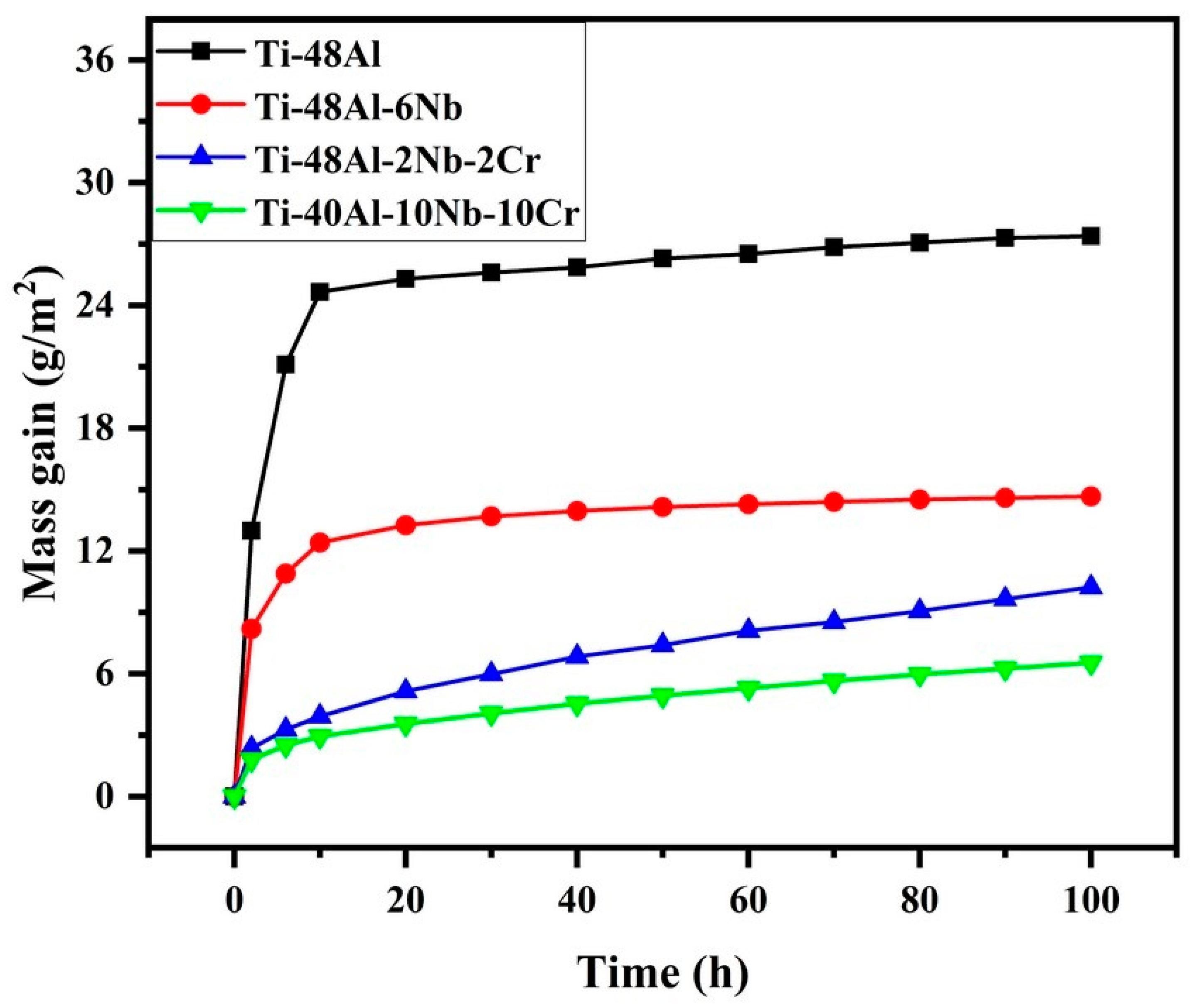
3.3. High-Temperature Oxidation Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.-Y.; Chen, L.-H.; Li, Y.; Rooke, J.C.; Sanchez, C.; Su, B.-L. Hierarchically porous materials: Synthesis strategies and structure design. Chem. Soc. Rev. 2017, 46, 481–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Guan, B.Y.; Gao, S.; Lou, X.W. A general dual-templating approach to biomass-derived hierarchically porous heteroatom-doped carbon materials for enhanced electrocatalytic oxygen reduction. Energy Environ. Sci. 2019, 12, 648–655. [Google Scholar] [CrossRef]
- Liu, J.; Ren, B.; Chen, Y.; Lu, Y.; Zhang, S.; Rong, Y.; Yang, J. Novel design of alumina foams with three-dimensional reticular architecture for effective high-temperature particulate matter capture. J. Am. Ceram. Soc. 2019, 102, 5576–5586. [Google Scholar] [CrossRef]
- Mohanta, K.; Kumar, A.; Parkash, O.; Kumar, D. Low Cost Porous Alumina with Tailored Microstructure and Thermal Conductivity Prepared using Rice Husk and Sucrose. J. Am. Ceram. Soc. 2014, 97, 1708–1719. [Google Scholar] [CrossRef]
- Gui, W.Y.; Liang, Y.F.; Hao, G.J.; Lin, J.P.; Sun, D.Y.; Liu, M.D.; Liu, C.; Zhang, H. High Nb-TiAl-based porous composite with hierarchical micro-pore structure for high temperature applications. J. Alloys Compd. 2018, 744, 263. [Google Scholar] [CrossRef]
- Sun, M.-H.; Huang, S.-Z.; Chen, L.-H.; Li, Y.; Yang, X.-Y.; Yuan, Z.-Y.; Su, B.-L. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 2016, 45, 3479–3563. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lou, L.; Song, W.; Zhang, Q.; Huang, G.; Hua, Y.; Zhang, H.-T.; Xiao, J.; Wen, B.; Zhang, X. Controllably Manipulating Three-Dimensional Hybrid Nanostructures for Bulk Nanocomposites with Large Energy Products. Nano Lett. 2017, 17, 2985–2993. [Google Scholar] [CrossRef]
- Zhang, G.-H.; Zhu, Q.-H.; Zhang, L.; Yong, F.; Zhang, Z.; Wang, S.-L.; Wang, Y.; He, L.; Tao, G.-H. High-performance particulate matter including nanoscale particle removal by a self-powered air filter. Nat. Commun. 2020, 11, 1653. [Google Scholar] [CrossRef] [Green Version]
- Gui, W.Y.; Lin, J.P.; Liang, J.R.; Qu, Y.H.; Guo, Y.C.; Lv, K.; Zhang, H. Micro-/nano-dual-scale porous composite membranes for the separation of nanopollutants from water. ACS Appl. Nano Mater. 2019, 2, 806. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.J.; Kim, D.I.; Lee, S.; Kwon, O.S.; Kim, I.K. Moisture effect on particulate matter filtration performance using electro-spun nanofbers including density functional theory analysis. Sci. Rep. 2019, 9, 7015. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Yadav, P.; Ghosh, C.; Singh, B. Heavy metal capture from the suspended particulate matter by Morus alba and evidence of foliar uptake and translocation of PM associated zinc using radiotracer (65Zn). Chemosphere 2020, 254, 126863. [Google Scholar] [CrossRef]
- Aydoğmuş, T.; Palani, D.K.H.; Kelen, F. Processing of porous β-type Ti74Nb26 alloys for biomedical applications. J. Alloys Compd. 2021, 872, 159737. [Google Scholar] [CrossRef]
- Petriev, I.; Pushankina, P.; Bolotin, S.; Lutsenko, I.; Kukueva, E.; Baryshev, M. The influence of modifying nanoflower and nanostar type Pd coatings on low temperature hydrogen permeability through Pd-containing membranes. J. Membr. Sci. 2021, 620, 118894. [Google Scholar] [CrossRef]
- Banhart, J. Manufacture, characterisation and application of cellular metals and metal foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Jiang, Y.; Gao, L.; Yu, L.; Lin, N.; He, Y.; Liu, C. Direct separation of arsenic and antimony oxides by high-temperature filtration with porous FeAl intermetallic. J. Hazard. Mater. 2017, 338, 364–371. [Google Scholar] [CrossRef]
- Kitaoka, S.; Matsushima, Y.; Chen, C.; Awaji, H. Thermal Cyclic Fatigue Behavior of Porous Ceramics for Gas Cleaning. J. Am. Ceram. Soc. 2004, 87, 906–913. [Google Scholar] [CrossRef]
- Voigt, C.; Zienert, T.; Schubert, P.; Aneziris, C.G.; Hubálková, J. Reticulated Porous Foam Ceramics with Different Surface Chemistries. J. Am. Ceram. Soc. 2014, 97, 2046–2053. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Li, Y.; Sang, S.; Li, S. Effects of pore structure on thermal conductivity and strength of alumina porous ceramics using carbon black as pore-forming agent. Ceram. Int. 2016, 42, 8221–8228. [Google Scholar] [CrossRef]
- Han, L.; Li, F.; Deng, X.; Wang, J.; Zhang, H.; Zhang, S. Foam-gelcasting preparation, microstructure and thermal insulation performance of porous diatomite ceramics with hierarchical pore structures. J. Eur. Ceram. Soc. 2017, 37, 2717–2725. [Google Scholar] [CrossRef]
- He, Y.H.; Jiang, Y.; Xu, N.P.; Zou, J.; Huang, B.Y.; Liu, C.T.; Liaw, P.K. Fabrication of Ti–Al Micro/ Nanometer-Sized Porous Alloys through the Kirkendall Effect. Adv. Mater. 2007, 19, 2102–2106. [Google Scholar] [CrossRef]
- Gui, W.; Qu, Y.; Liang, Y.; Wang, Y.; Zhang, H.; Luan, B.; Lin, J. High efficiency hierarchical porous composite microfiltration membrane for high-temperature particulate matter capturing. NPJ Mater. Degrad. 2021, 5, 1. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, J.; Li, B.; Fan, Y. Preparation of ceramic membranes on porous Ti–Al alloy supports by an in-situ oxidation method. J. Membr. Sci. 2015, 476, 554–560. [Google Scholar] [CrossRef]
- Wang, F.; Liang, Y.F.; Shang, S.L.; Liu, Z.K.; Lin, J.P. Phase transformation in Ti-Al-Nb porous alloys and its influence on pore properties. Mater. Des. 2015, 83, 508. [Google Scholar] [CrossRef]
- Kim, D.; Seo, D.; Huang, X.; Sawatzky, T.; Saari, H.; Hong, J.; Kim, Y.-W. Oxidation behaviour of gamma titanium aluminides with or without protective coatings. Int. Mater. Rev. 2014, 59, 297. [Google Scholar]
- Zhao, L.; Li, G.; Zhang, L.; Lin, J.; Song, X.; Ye, F.; Chen, G. Influence of Y addition on the long time oxidation behaviors of high Nb containing TiAl alloys at 900 °C. Intermetallics 2010, 18, 1586–1596. [Google Scholar] [CrossRef]
- Ping, F.-P.; Hu, Q.-M.; Bakulin, A.; Kulkova, S.E.; Yang, R. Alloying effects on properties of Al2O3 and TiO2 in connection with oxidation resistance of TiAl. Intermetallics 2016, 68, 57–62. [Google Scholar] [CrossRef]
- Umakoshi, Y.; Yamaguchi, M.; Sakagami, T.; Yamane, T. Oxidation resistance of intermetallic compounds Al3Ti and TiAl. J. Mater. Sci. 1989, 24, 1599–1603. [Google Scholar] [CrossRef]
- Lin, J.; Zhao, L.; Li, G.; Zhang, L.; Song, X.; Ye, F.; Chen, G. Effect of Nb on oxidation behavior of high Nb containing TiAl alloys. Intermetallics 2011, 19, 131–136. [Google Scholar] [CrossRef]
- Fang, H.; Chen, R.; Chen, X.; Yang, Y.; Su, Y.; Ding, H.; Guo, J. Effect of Ta element on microstructure formation and mechanical properties of high-Nb TiAl alloys. Intermetallics 2019, 104, 43–51. [Google Scholar] [CrossRef]
- Gui, W.Y.; Lin, J.P.; Liu, M.D.; Qu, Y.H.; Wang, Y.C.; Liang, Y.F. Effects of nano-NiO addition on the microstructure and corrosion properties of high Nb-TiAl alloy. J. Alloys Compd. 2019, 782, 973. [Google Scholar] [CrossRef]
- Wu, Y.; Hagihara, K.; Umakoshi, Y. Influence of Y-addition on the oxidation behavior of Al-rich γ-TiAl alloys. Intermetallics 2004, 12, 519. [Google Scholar] [CrossRef]
- Gong, X.; Chen, R.R.; Fang, H.Z.; Ding, H.S.; Guo, J.J.; Su, Y.Q.; Fu, H.Z. Synergistic effect of B and Y on the isothermal oxidation behavior of TiAl-Nb-Cr-V alloy. Corros. Sci. 2018, 131, 376. [Google Scholar] [CrossRef]
- Klein, T.; Rashkova, B.; Holec, D.; Clemens, H.; Mayer, S. Silicon distribution and silicide precipitation during annealing in an advanced multi-phase γ-TiAl based alloy. Acta Mater. 2016, 110, 236. [Google Scholar] [CrossRef]
- Park, S.Y.; Seob, D.Y.; Kim, S.W.; Kim, S.E.; Hong, J.K.; Lee, D.B. High temperature oxidation of Ti-46Al-6Nb-0.5W-0.5Cr-0.3Si-0.1C alloy. Intermetallics 2016, 74, 8. [Google Scholar] [CrossRef]
- Zuo, F.L.; Zhang, S.H.; Liu, H.; Fong, H.; Yin, X.; Yu, J.Y.; Ding, B. Free-standing polyurethane nanofiber/nets air filters for effective PM capture. Small 2017, 13, 1702139. [Google Scholar] [CrossRef]
- Chen, G.L.; Zhang, W.J.; Liu, Z.C.; Li, S.J.; Kim, Y.-W. Microstructure and properties of high-Nb containing TiAl-base alloy. In Gamma Titanium Aluminides; Kim, Y.-W., Dimidulk, D.M., Loretto, M.H., Eds.; TMS: Warrendale, PA, USA, 1999; p. 371. [Google Scholar]
- Tang, Z.; Shemet, V.; Niewolak, L. Effect of Cr addition on oxidation behavior of Ti-48Al-2Ag alloys. Intermetallics 2003, 11, 1. [Google Scholar] [CrossRef]
- Gui, W.; Liu, J.; Song, X.; Zhang, H.; Lin, J.; Luan, B. A new microfiltration membrane with three-dimensional reticular architecture for Nano-pollutants removal from wastewater. Prog. Nat. Sci. 2021, 31, 414–419. [Google Scholar] [CrossRef]
- Rat’Ko, A.I.; Ivanets, A.I.; Azarov, S.M. Effect of additives on the pore structure of ceramics based on crystalline SiO2. Inorg. Mater. 2008, 44, 778–784. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, C.; Hsu, P.-C.; Zhang, C.; Liu, N.; Zhang, J.; Lee, H.R.; Lu, Y.; Qiu, Y.; Chu, S.; et al. Nanofiber Air Filters with High-Temperature Stability for Efficient PM2.5 Removal from the Pollution Sources. Nano Lett. 2016, 16, 3642–3649. [Google Scholar] [CrossRef] [PubMed]



| Samples | Total Pore Volume (cm3/g) | Total Pore Area (m2/g) | Bulk Density (g/cm3) | Porosity (%) |
|---|---|---|---|---|
| Ti-48Al | 0.113 ± 0.008 | 0.055 ± 0.007 | 3.683 ± 0.122 | 29.445 ± 0.054 |
| Ti-48Al-6Nb | 0.118 ± 0.013 | 0.052 ± 0.011 | 3.749 ± 0.134 | 30.577 ± 0.069 |
| Ti-48Al-2Nb-2Cr | 0.082 ± 0.005 | 0.038 ± 0.007 | 4.644 ± 0.095 | 27.551 ± 0.038 |
| Ti-40Al-10Nb-10Cr | 0.097 ± 0.003 | 0.040 ± 0.001 | 4.358 ± 0.037 | 29.805 ± 0.003 |
| Samples | Ti | Al | Nb | Cr | O |
|---|---|---|---|---|---|
| Ti-48Al | 32.96 | 0 | 0 | 0 | 67.04 |
| Ti-48Al-6Nb | 34.18 | 6.51 | 1.59 | 0 | 57.72 |
| Ti-48Al-2Nb-2Cr | 39.78 | 8.45 | 1.23 | 0.31 | 50.23 |
| Ti-40Al-10Nb-10Cr | 41.19 | 11.91 | 2.05 | 0.63 | 44.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gui, W.; Shi, Z.; Zhang, Y.; Liang, Y.; Qin, J.; Wang, Y.; Lin, J.; Luan, B. Ti-40Al-10Nb-10Cr Porous Microfiltration Membrane with Hierarchical Pore Structure for Particulate Matter Capturing from High-Temperature Flue Gas. Membranes 2022, 12, 104. https://doi.org/10.3390/membranes12020104
Gui W, Shi Z, Zhang Y, Liang Y, Qin J, Wang Y, Lin J, Luan B. Ti-40Al-10Nb-10Cr Porous Microfiltration Membrane with Hierarchical Pore Structure for Particulate Matter Capturing from High-Temperature Flue Gas. Membranes. 2022; 12(2):104. https://doi.org/10.3390/membranes12020104
Chicago/Turabian StyleGui, Wanyuan, Zhenjing Shi, Yin Zhang, Yongfeng Liang, Jingyan Qin, Yanli Wang, Junpin Lin, and Benli Luan. 2022. "Ti-40Al-10Nb-10Cr Porous Microfiltration Membrane with Hierarchical Pore Structure for Particulate Matter Capturing from High-Temperature Flue Gas" Membranes 12, no. 2: 104. https://doi.org/10.3390/membranes12020104
APA StyleGui, W., Shi, Z., Zhang, Y., Liang, Y., Qin, J., Wang, Y., Lin, J., & Luan, B. (2022). Ti-40Al-10Nb-10Cr Porous Microfiltration Membrane with Hierarchical Pore Structure for Particulate Matter Capturing from High-Temperature Flue Gas. Membranes, 12(2), 104. https://doi.org/10.3390/membranes12020104







