Effect of Self-Made TiO2 Nanoparticle Size on the Performance of the PVDF Composite Membrane in MBR for Landfill Leachate Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Preparation of TiO2
2.1.2. Preparation of PVDF-X
2.2. Characterization of Samples
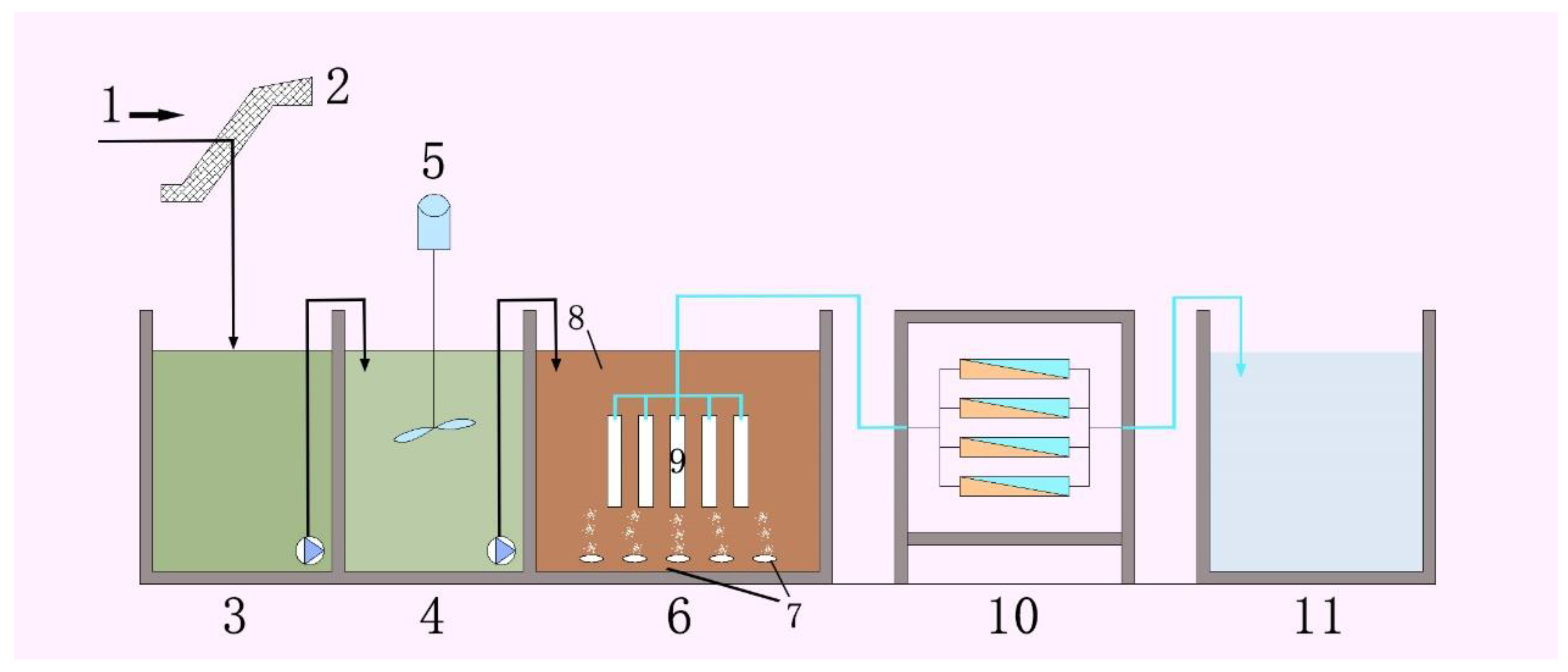
2.3. MBR and RO Setup and Operation
- (1)
- The effluent peristaltic pump was operated intermittently for 10 min and stopped for two minutes;
- (2)
- The leachate in the anaerobic tank entered the aerobic tank of the A/O-MBR reactor through siphonage;
- (3)
- During the experiment, DO, HRT, pH, and the other parameters in the system were strictly controlled, and the content of COD, ammonia nitrogen, and total nitrogen in the inlet and outlet water of the system was monitored in real time.
2.4. Pure Water Flux Measurement
2.5. Bovine Serum Albumin (BSA) Retention Rate
2.6. Antifouling Performance of Membranes
3. Results and Discussion
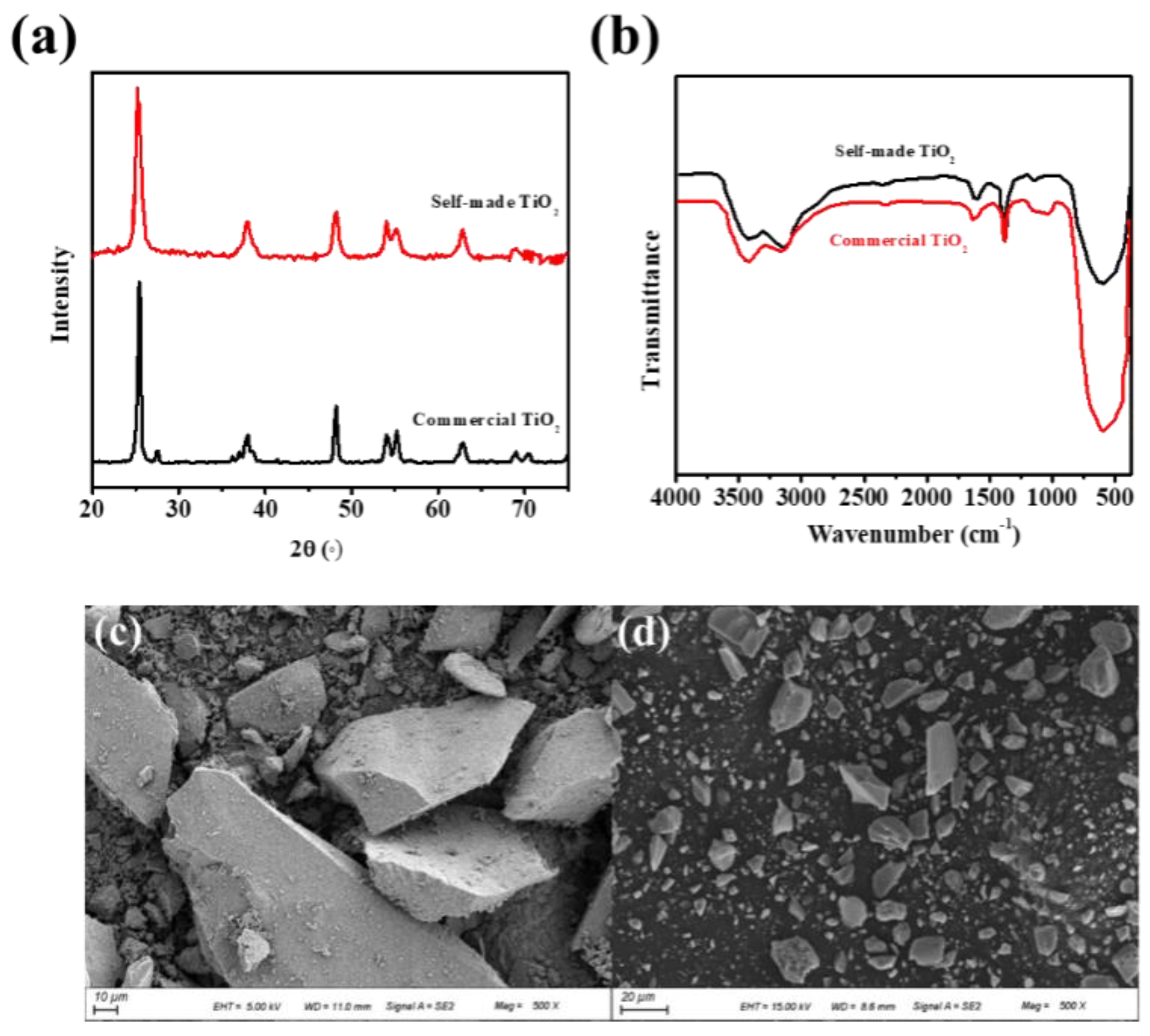
3.1. Structure and Morphology of Self-Made TiO2
3.2. Characterization of Composite Membranes
3.2.1. Water Flux, Contact Angle, and Porosity
3.2.2. Phase Separation Rate
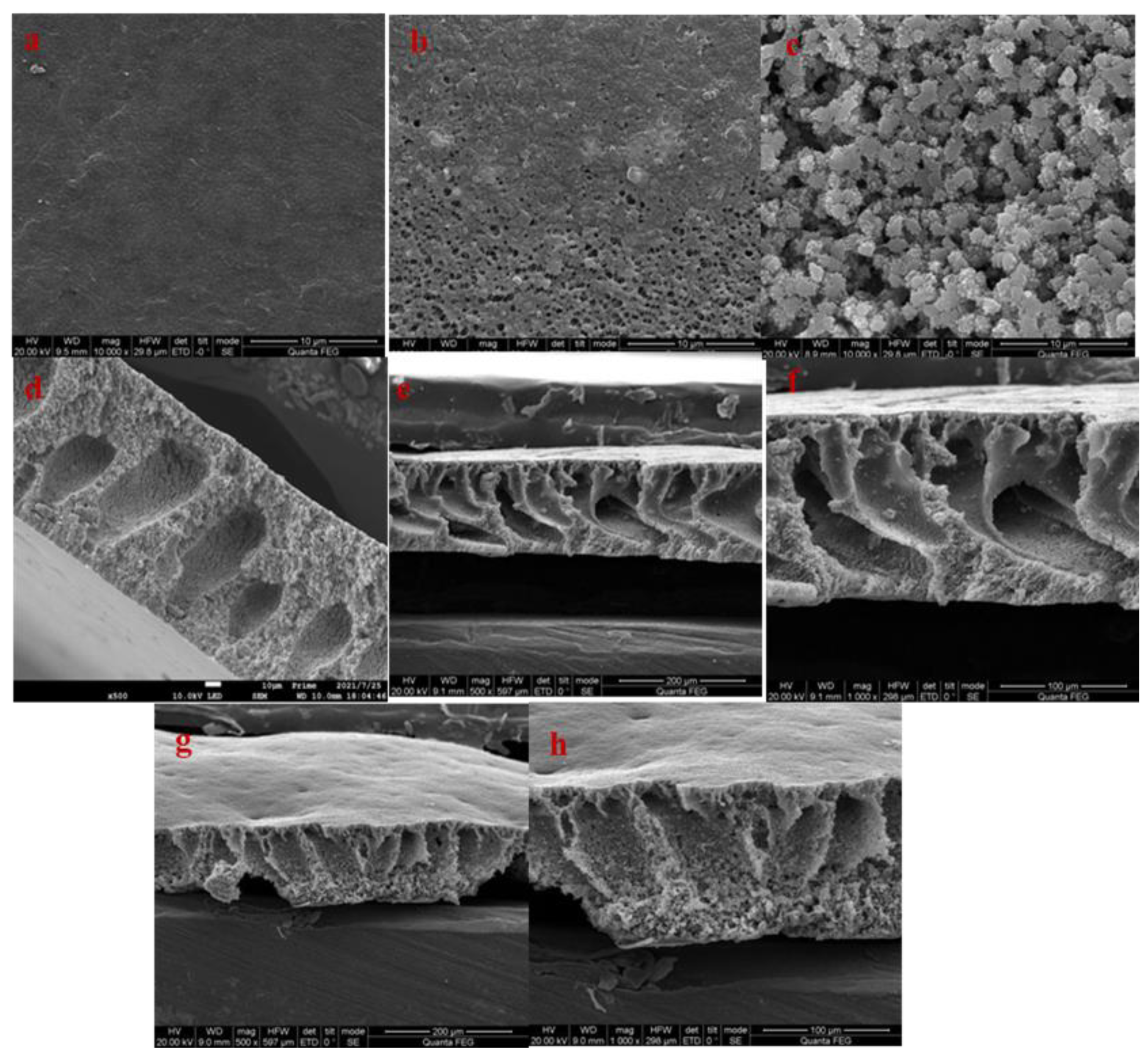
3.2.3. Scanning Electron Microscopy
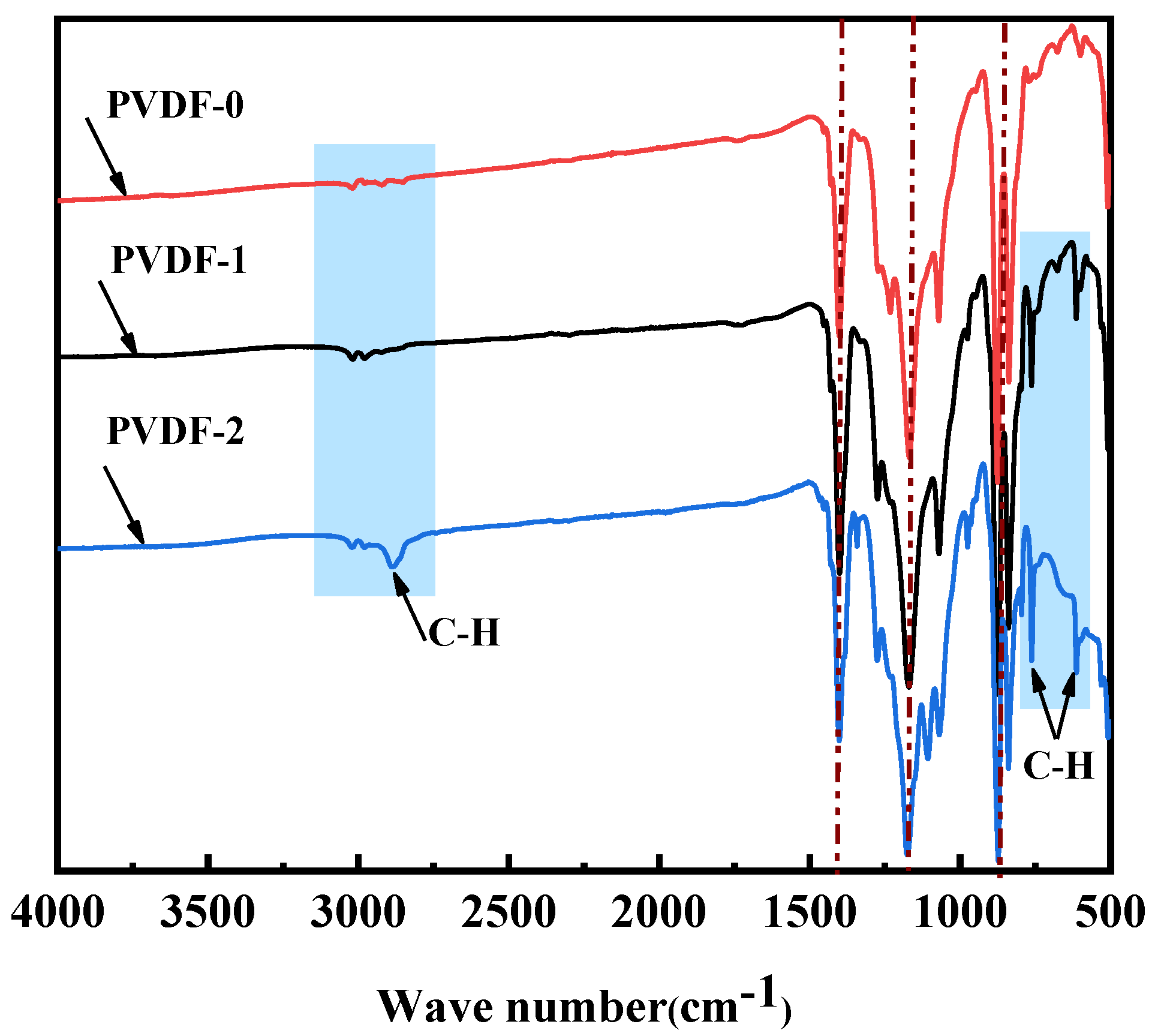
3.2.4. FTIR Spectroscopy
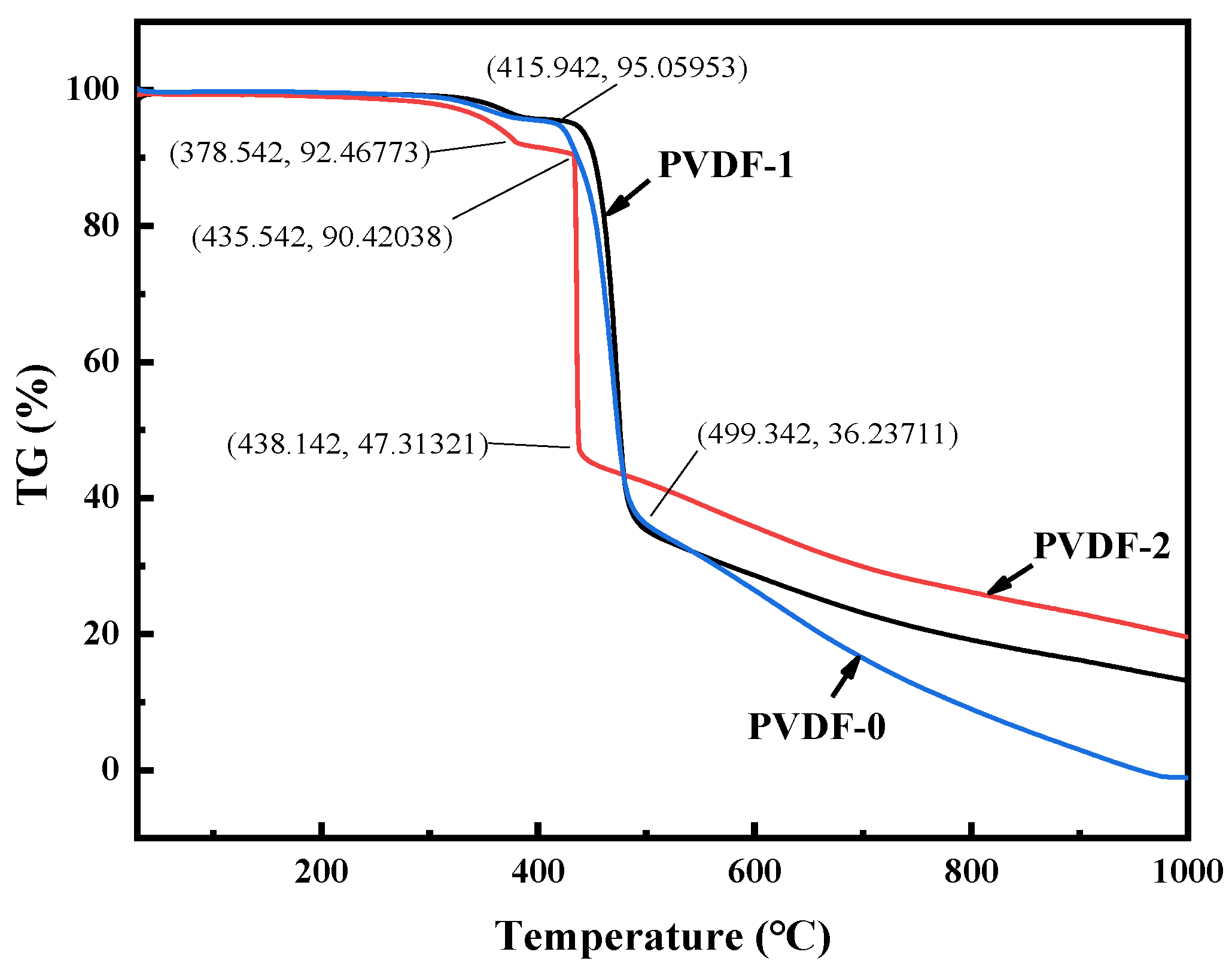
3.2.5. TGA
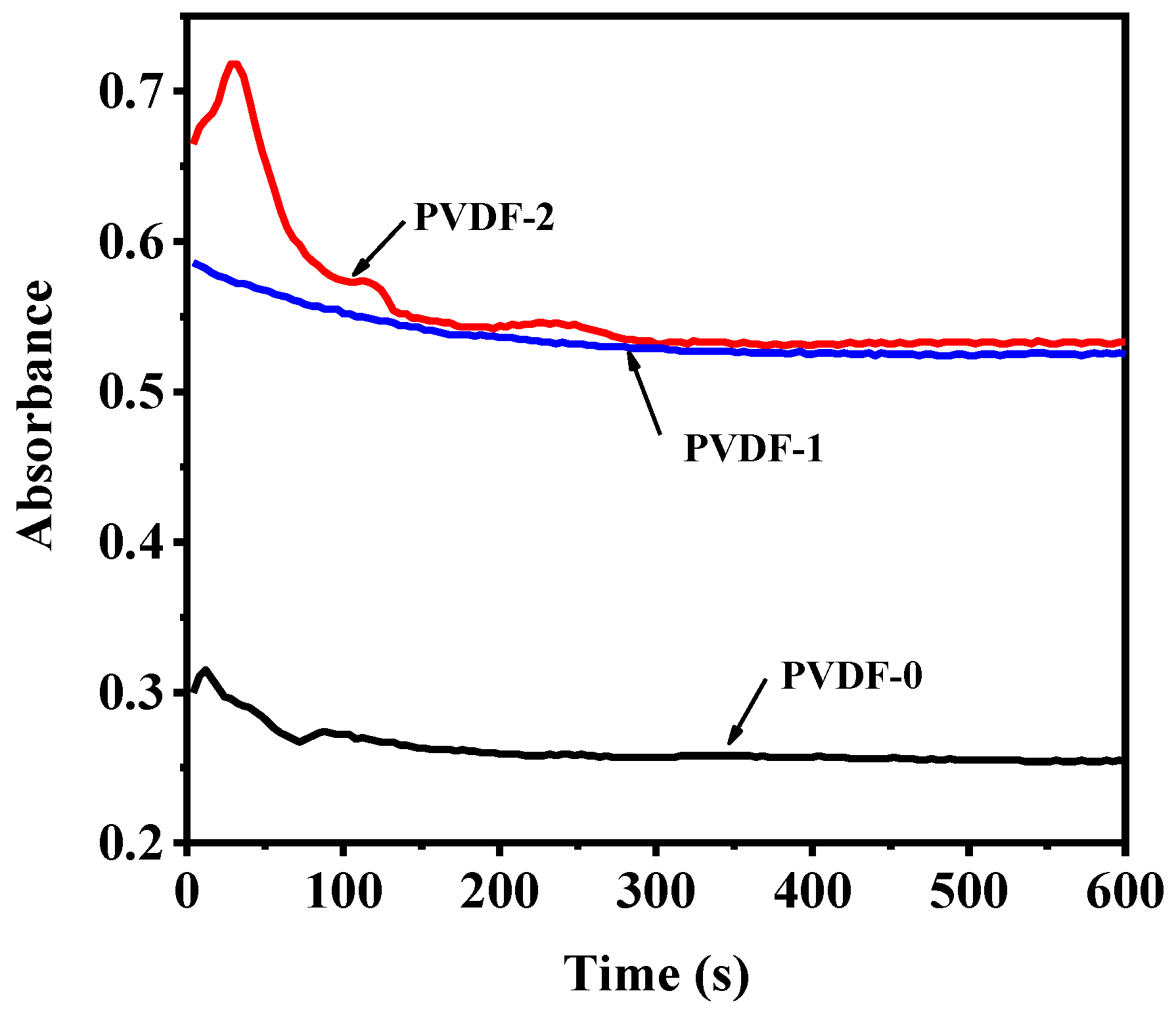
3.2.6. Antifouling Performance
3.3. A/O-MBR Treatment for Landfill Leachate
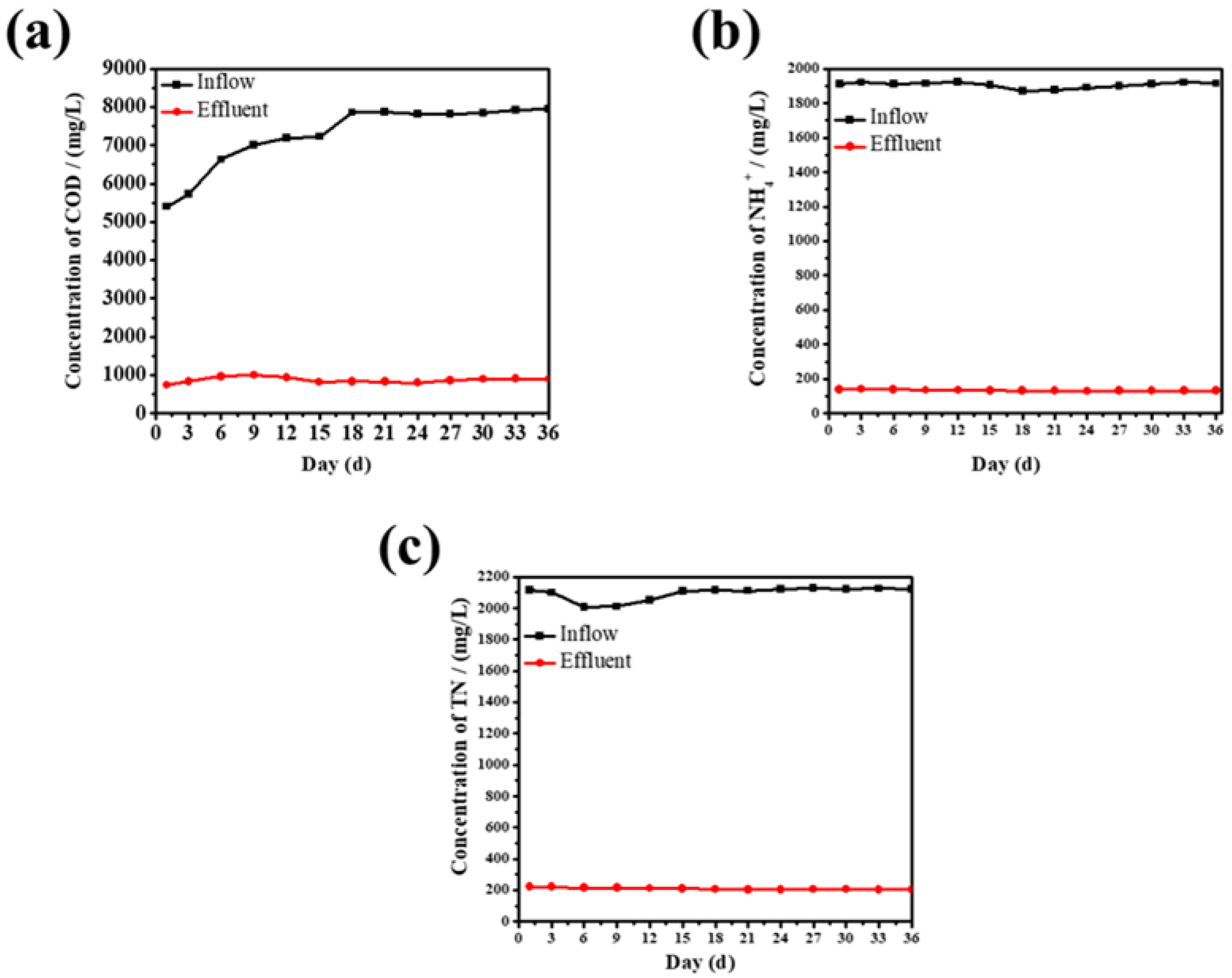
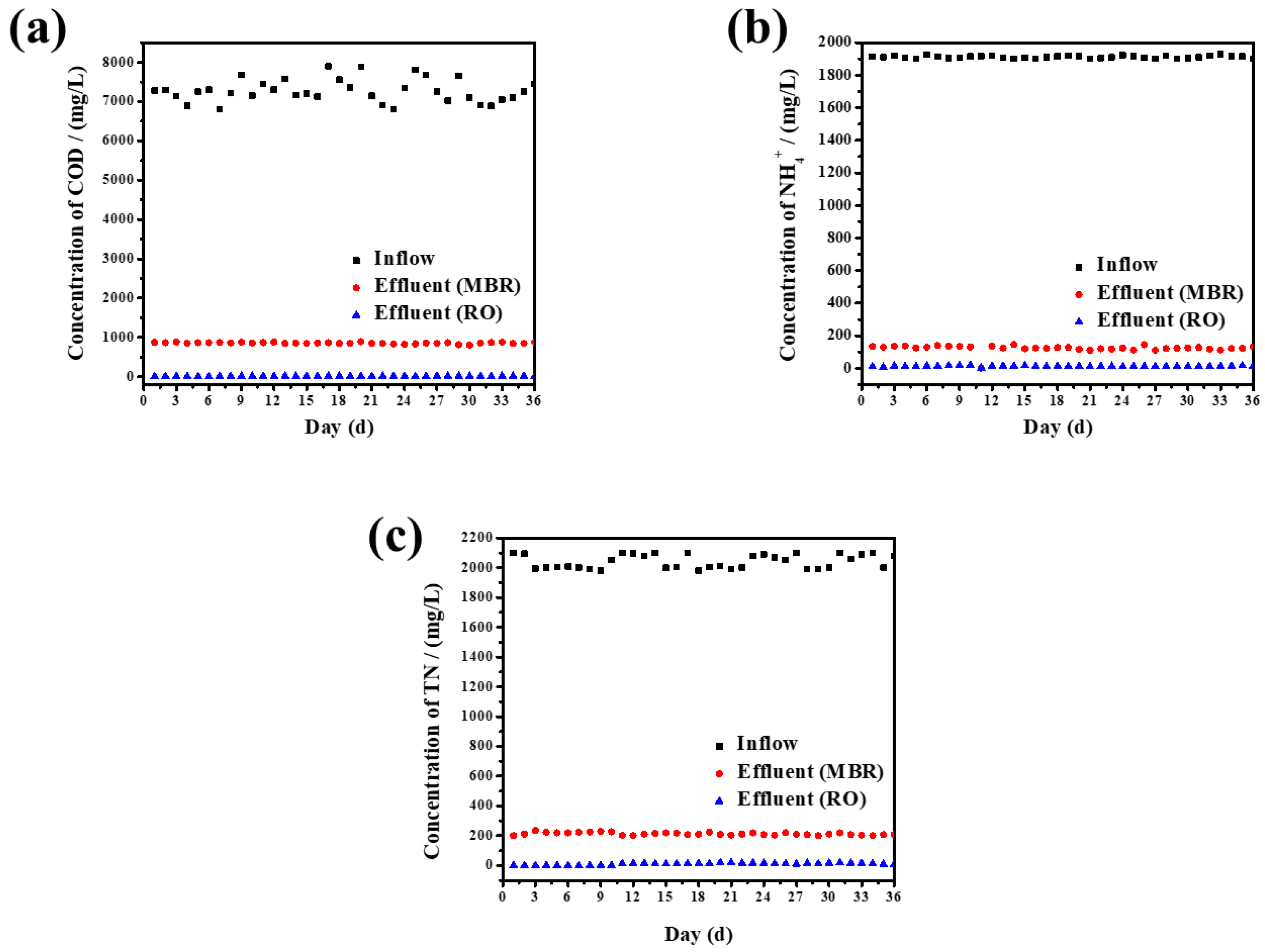
3.3.1. The Chemical Oxygen Demand (COD) Removal Characteristics of MBR
3.3.2. The Removal of Ammonia Nitrogen and Total Nitrogen by MBR
3.3.3. The Effect of Influential Factors on the Removal Efficiency of the Process
The Effect of Dissolved Oxygen (DO) on the COD, NH4+, and TN Removal
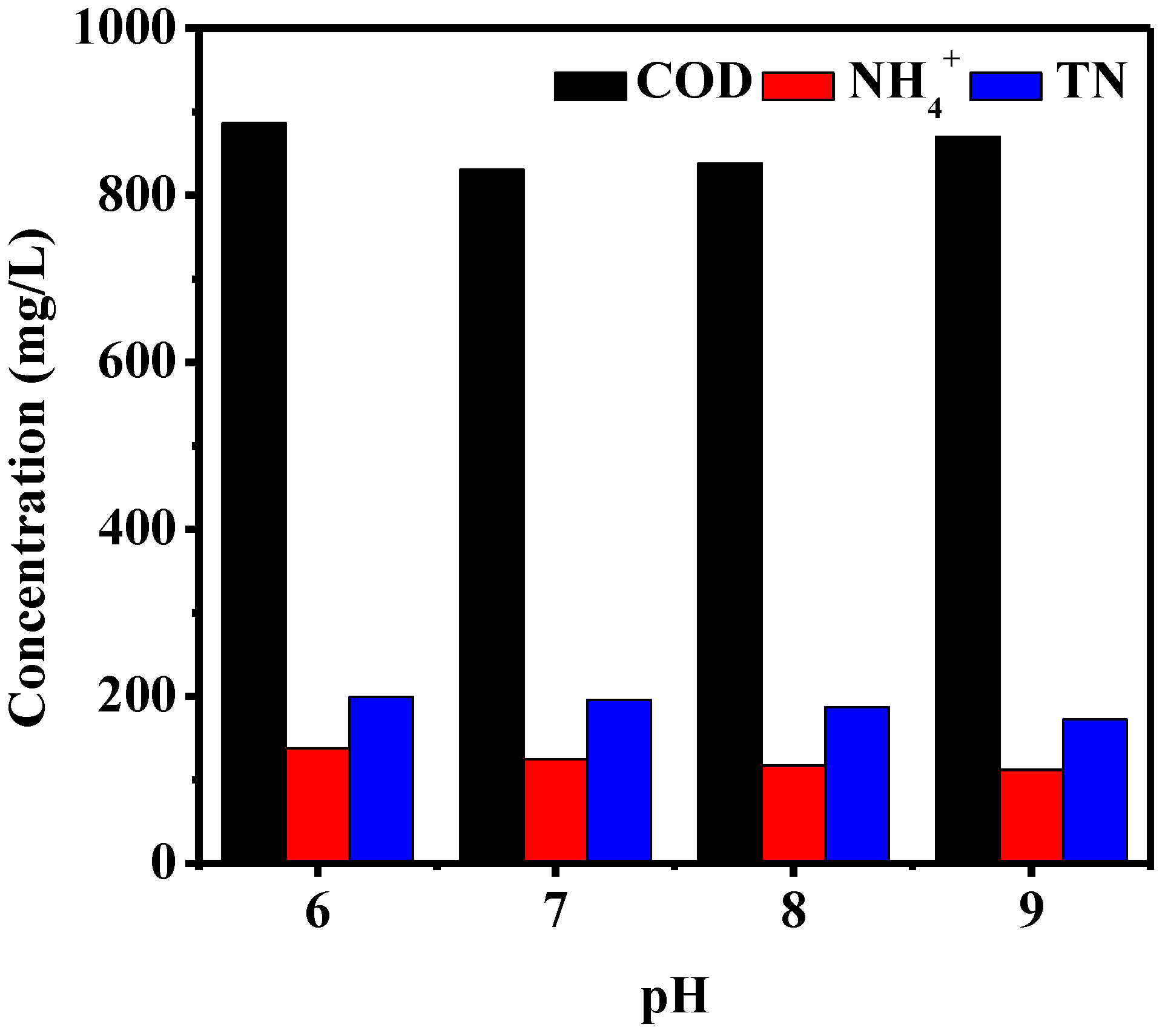
The Effect of pH on MBR Treatment COD, NH4+, and TN Removal
The Effect of Different Hydraulic Residence Times on MBR on COD, NH4+, and TN Removal Efficiency
3.4. Water Outlet of RO
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Full Name | Abbreviation |
| Anoxic–Aerobic Membrane Bioreactor | A/O-MBR |
| Upflow Anerobic Sludge Bed Membrane Bioreactor | UASB-MBR |
| Reverse osmosis | RO |
| Ultrafiltration membranes | UF |
| Titanium dioxide | TiO2 |
| Sludge retention time | SRT |
| Membrane bioreactor | MBR |
| Poly(vinylidene fluoride) | PVDF |
| Hydrochloric acid | HCl |
| Rejection rate | R |
| Scanning electron microscopy | SEM |
| Fourier transform infrared spectroscopy | FT-IR |
| Thermogravimetric analysis | TGA |
| Chemical oxygen demand | COD |
| Ammonia nitrogen | NH4+ |
| Total nitrogen | TN |
| Dissolved oxygen | DO |
| Mixed liquor suspended solids | MLSS |
| Hydraulic retention time | HRT |
References
- Gao, J.L.; Oloibiri, V.; Chys, M.; Audenaert, W.; Decostere, B.; He, Y.L.; Langenhove, H.V.; Demeestere, K.; Van Hulle, S.W.H. The present status of landfill leachate treatment and its development trend from a technological point of view. Rev. Environ. Sci. Bio-Technol. 2015, 14, 93–122. [Google Scholar] [CrossRef]
- Xi, B.D.; Liu, D.M.; Li, M.X.; Meng, F.H. Innovation Development of Solid Waste Recycling Technology. J. Environ. Prot. 2017, 45, 16–19. [Google Scholar]
- Yin, J.; Roso, M.; Boaretti, C.; Lorenzetti, A.; Martucci, A.; Modesti, M. PVDF-TiO2 core-shell fibrous membranes by microwave-hydrothermal method: Preparation, characterization, and photocatalytic activity. J. Environ. Chem. Eng. 2021, 9, 106250. [Google Scholar] [CrossRef]
- Lee, K.W.; Seo, B.K.; Nam, S.T.; Han, M.J. Trade-off between thermodynamic enhancement and kinetic hindrance during phase inversion n the preparation of polysulfone membranes. Desalination 2003, 159, 289–296. [Google Scholar] [CrossRef]
- Han, M.J.; Nam, S.T. Themodyanmic and rheological variation in polysulfone solution by PVP and its effect in the preparation of phase inversiion membrane. J. Membr. Sci. 2002, 202, 56–61. [Google Scholar] [CrossRef]
- Witte, P.V.D.; Dijkstra, P.J.; Berg, J.W.A.V.D.; Feijen, J. Phase separation precesses in polymer solutions in relation to membrane formation. J. Membr. Sci. 1996, 117, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Taweesan, A.; Koottatep, T.; Polprasert, C. Effective Measures for Municipal Solid Waste Management for Cities in Some Asian Countries. Expo. Health 2017, 9, 125–133. [Google Scholar] [CrossRef]
- Lombardi, L.; Carnevale, E.A.; Corti, A. Comparison of different biological treatment scenarios for the organic fraction of municipal solid waste. Int. J. Environ. Sci. Technol. 2015, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.F.; Ren, J.Z.; Goodsite, M.E.; Xu, G.Y. Waste-to-energy, municipal solid waste treatment and best available technology: Comprehensive evaluation by an interval-valued fuzzy multi-criteria decision making method. J. Clean. Prod. 2018, 172, 887–899. [Google Scholar] [CrossRef]
- Kebria, D.Y.; Ghavami, M.; Javadi, S.; Goharimanesh, M. Combining an experimental study and ANFIS modeling to predict landfill leachate transport in underlying soil-a case study in north of Iran. Environ. Monit. Assess. 2018, 190, 26. [Google Scholar] [CrossRef]
- Klauck, C.R.; Giacobbo, A.; Oliveira, E.D.L.d.; Silva, L.B.d.; Rodrigues, M.A.S. Evaluation of acute toxicity, cytotoxicity and genotoxicity of landfill leachate treated by biological lagoon and advanced oxidation processes. J. Environ. Chem. Eng. 2017, 5, 6188–6193. [Google Scholar] [CrossRef]
- Roy, D.; Azais, A.; Benkaraache, S.; Drogui, P.; Tyagi, R.D. Composting leachate: Characterization treatment, and future perspectives. Rev. Environ. Sci. Bio/Technol. 2018, 17, 323–349. [Google Scholar] [CrossRef] [Green Version]
- Hur, J.M.; Park, J.A.; Son, B.S.; Jang, B.G.; Kim, S.H. Mature landfill leachate treatment from an abandoned municipal waste disposal site. Korean J. Chem. Eng. 2001, 18, 233–239. [Google Scholar] [CrossRef]
- Ren, Y.; Ferraz, F.M.; Yuan, Q. Biological leachate treatment using anaerobic/aerobic process: Suspended growth-activated sludge versus aerobic granular sluge. Int. J. Environ. Sci. Technol. 2018, 15, 2295–2302. [Google Scholar] [CrossRef]
- Omar, H.; Rohani, S. Treatment of landfill waste, leachate and landfill gas: A review. Front. Chem. Sci. Eng. 2015, 9, 15–32. [Google Scholar] [CrossRef]
- Kamaruddin, M.A.; Yusoff, M.S.; Rui, L.M.; Isa, A.M.; Zawawi, M.H.; Alrozi, R. An overview of municipal solid waste management and landfill leachate treatment: Malaysia and Asian perspectives. Environ. Sci. Pollut. Res. 2017, 24, 26988–27020. [Google Scholar] [CrossRef]
- Serdarevic, A. Landfill Leachate Management-Control and Treatment. In Proceedings of the International Symposium on Innovative and Interdisciplinary Applications of Advanced Technologies, Teslic, Bosnia and Herzegovina, 25–28 May 2017; volume 28, pp. 618–632. [Google Scholar]
- Segundo, I.D.B.; Gomes, A.I.; Souza-Chaves, B.M.; Park, M.; dos Santos, A.B.; Boaventura, R.A.; Moreira, F.C.; Silva, T.F.C.V.; Vilar, V.J. Incorporation of ozone-driven processes in a treatment line for a leachate from a hazardous industrial waste landfill: Impact on the bio-refractory character and dissolved organic matter distribution. J. Environ. Chem. Eng. 2021, 9, 105554. [Google Scholar] [CrossRef]
- Kamaruddin, M.A.; Suffian, Y.M.; Aziz, H.A.; Hung, Y.T. Sustainable treatment of landfill leachate. Appl. Water Sci. 2015, 5, 113–126. [Google Scholar] [CrossRef]
- Pelkonen, M.; Wang, Y. Leachate direct-discharge limits and incentives related to landfill aftercare costs. J. Mater. Cycles Waste Manag. 2017, 19, 413–422. [Google Scholar] [CrossRef]
- Wu, L.N.; Yan, Z.B.; Huang, S.; Li, J.; Su, B.Y.; Wang, C.Y.; Peng, Y.Z. Rapid start-up and stable maintenance of partial nitrification-anaerobic ammonium oxidation treatment of landfill leachate at low temperatures. Environ. Res. 2020, 191, 110131. [Google Scholar] [CrossRef]
- Gkotsis, P.; Tsilogeorgis, J.; Zouboulis, A. Hydraulic performance and fouling characteristics of a membrane sequencing batch reactor(MSBR) for landfill leachate treatment under various operating conditions. Environ. Sci. Pollut. Res. Int. 2018, 25, 12274–12283. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Zhao, W.T.; Chai, X.L. Treatment of Landfill Leachate Using Membrane Biorea-Mctors: Research Evolution. Nonferrous Met. Eng. Res. 2016, 37, 39–43. [Google Scholar] [CrossRef]
- Kochling, T.; Sanz, J.L.; Gavazza, S.; Florencio, A. Analysis of microbial community structure and composition in leachates from a young landfill by 454 pyrosequencing. Appl. Microbiol. Biotechol. 2015, 99, 5657–5668. [Google Scholar] [CrossRef] [PubMed]
- Rizkallah, M.; El-Fadel, M.; Saikaly, P.; Ayoub, G.; Darwiche, N.; Hashisho, J. Hollow-fiber membrane bioreactor for the treatment of high-strength landfill leachate. Waste Manag. Res. 2013, 31, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ji, M.; Li, Z.; Li, J.; Fu, H.P.; Ma, W.J. Municipal Landfill Leachate Treatment by Using Combined UASB and MBR System and Its Problems. Urban Environ. Urban Ecol. 2003, 16, 215–217. [Google Scholar]
- Wang, F.; Zhou, G.M. Research on iron-carbon micro-electrolytic method for pretreating aged-landfill leachate. Tech. Equip. Environ. Pollut. Control 2004, 5, 63–65. [Google Scholar]
- Diamadopoulos, E. Characterization and treatment of recirculation stabilized leachate. Water Res. 1994, 28, 2439–2445. [Google Scholar] [CrossRef]
- Yang, Y.J.; Li, T.G.; Du, G.C.; Shen, Z.S.; Chen, J.; Zhang, G.P. Study on Electrolytic Oxidation Process for Preliminary Treatment of Landfill Leachates. Res. Environ. Sci. 2003, 16, 53–56. [Google Scholar]
- Li, P.; Wei, C.H.; Wu, C.F.; Liang, S.Z. Study on the Treatment of Landfill Leachate by Combining Anaerobic/Aerobic Biological Fluidized Bed. J. Chem. Eng. Chin. Univ. 2002, 16, 345–350. [Google Scholar]
- Tang, S.J.; Wang, Z.W.; Wu, Z.C.; Zhou, Q. Role of dissolved organic matter (DOM) in membrane fouling of membrane bioreactors for municipal wastewater treatment. J. Hazard. Mater. 2010, 178, 377–384. [Google Scholar] [CrossRef]
- Hashisho, J.; El-Fadel, M. Membrane bioreactor technology for leachate treatment at solid waste landfill. Rev. Environ. Sci. Bio/Technol. 2016, 15, 441–463. [Google Scholar] [CrossRef]
- Petros, G.; Dimitra, B.; Efrosini, N.P.; Zouboulis, A.; Samaras, P. Fouling Issues in Membrane Bioreactors (MBRs) for Wastewater Treatment: Major Mechanisms, Prevention and Control Strategies. Processes 2014, 2, 795–866. [Google Scholar]
- Zhang, J.; Xiao, K.; Huang, X. Full-scale MBR applications for leachate treatment in China: Practical, technical, and economic features. J. Hazard. Mater. 2020, 389, 122138. [Google Scholar] [CrossRef] [PubMed]
- Mavukkandy, M.O.; Bilad, M.R.; Giwa, A.; Hasan, S.W.; Arafat, H.A. Leaching of PVP from PVDF/PVP blend membranes: Impacts on membrane structure and fouling in membrane bioreactor. J. Mater. Sci. 2016, 51, 4328–4341. [Google Scholar] [CrossRef]
- Bet-Moushoul, E.; Mansourpanah, Y.; Farhadi, K.; Tabatabaei, M. TiO2 nanocomposite based polymeric membranes: A review on performance improvement for various applications in chemical engineering processes. Chem. Eng. J. 2016, 283, 29–46. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, N.; Lee, Y.T. Preparation and characterization of PVDF/TiO2 organic–inorganic composite membranes for fouling resistance improvement. J. Membr. Sci. 2009, 345, 13–20. [Google Scholar] [CrossRef]
- Qin, A.; Li, X.; Zhao, X.; Liu, D.; He, C. Engineering a highly hydrophilic PVDF membrane via binding TiO2 nanoparticles and a PVA layer onto a membrane surface. ACS Appl. Mater. Interfaces 2015, 7, 8427–8436. [Google Scholar] [CrossRef] [PubMed]
- Diaz, O.; Vera, L.; Garcia, E.; Garcia, E.; Rodriguez-Sevilla, J. Effect of sludge characteristics on membrane fouling during start-up of a tertiary submerged membrane bioreactor. Environ. Sci. Pollut. Res. 2016, 23, 8951–8962. [Google Scholar] [CrossRef]
- Sheng, X.; Liu, Z.H.; Zhang, X. Study on anti-fouling performance of PVDF-TiO2 nano blending membrane supported by nylon fabric in MBR. Technol. Water Treat. 2012, 38, 41–44. [Google Scholar]
- Chu, G.; Zhao, S.G.; Zhang, J.Y. Study on the Velocity and Distribution of Dissolved Oxygen and Nitrogen and Phosphorus Removal of A2/O-MBR. Environ. Eng. 2018, 36, 21–25. [Google Scholar]
- Wyffels, S.; Van Hulle, S.W.H.; Boeckx, P.; Volcke, E.I.P.; Cleemput, O.V.; Vanrolleghem, P.A.; Verstraete, W. Modeling and simulation of oxygen-limited partial nitritation in a membrane-assisted bioreactor (MBR). Biotechnol. Bioeng. 2004, 86, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z.; He, Y.L.; Ohandja, D.G.; Ji, J.; Li, J.F.; Zhou, T. Simultaneous nitrification–denitrification achieved by an innovative internal-loop airlift MBR: Comparative study. Bioresour. Technol. 2008, 99, 5867–5872. [Google Scholar] [CrossRef] [PubMed]
- Holakoo, L.; Nakhla, G.; Bassi, A.; Yanful, E. Long term performance of MBR for biological nitrogen removal from synthetic municipal wastewater. Chemosphere 2007, 66, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Bukhari, Z.; Liu, Y.; Wen, J.F.; Lechevallier, M.W. Development of ultra-low DO operation at a full-scale MBR system and the corresponding impacts on microbial ecology. Proc. Water Environ. Fed. 2017, 3, 2752–2778. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Zheng, T.; Sun, Y.L.; Wang, P. Ozone-activated carbon combined technology used in depth treatment of landfill leachate MBR effluent. Chin. J. Environ. Eng. 2017, 11, 4535–4541. [Google Scholar]
- Song, X.Y.; Liu, R.; Chen, L.; Kawagishi, T. Comparative experiment on treating digested piggery wastewater with a biofilm MBR and conventional MBR: Simultaneous removal of nitrogen and antibiotics. Front. Environ. Sci. Eng. 2017, 2, 123–131. [Google Scholar] [CrossRef]
- Thien, V.N.T.; Hung, D.V.; Hoa, N.T.T. An A2O-MBR system for simultaneous nitrogen and phosphorus removal from brewery wastewater. Sci. Technol. Dev. J.-Sci. Earth Environ. 2019, 3, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Du, D.L.; Zhang, C.Y.; Zhao, K.X.; Sun, G.R.; Zou, S.Q.; Yuan, L.M.; He, S.L. Effect of different carbon sources on performance of an A 2 N-MBR process and its microbial community structure. Front. Environ. Sci. Eng. 2018, 12, 4. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, A.; Leyva-Diaz, J.C.; Calderon, K.; Poyatos, J.M.; Gonzalez-Lopez, J. Impact of Hydraulic Retention Time on MBR and Hybrid MBBR-MBR Systems through Microbiological Approach: TGGE and Enzyme Activities. Front. Wastewater Treat. Model. 2017, 4, 561–566. [Google Scholar]
- González-Pérez, D.M.; Pérez, J.I.; Nieto, M.Á.G. Carbamazepine behaviour and effects in an urban wastewater MBR working with high sludge and hydraulic retention time. J. Environ. Sci. Health Part A 2016, 51, 855–860. [Google Scholar] [CrossRef]
- Fudala-Ksiazek, S.; Pierpaoli, M.; Luczkiewicz, A. Efficiency of landfill leachate treatment in a MBR/UF system combined with NF, with a special focus on phthalates and bisphenol A removal. Waste Manag. 2018, 78, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.S.; Chen, S.S.; Chang, T.C.; Tzi-Chin, C.; Nhat-Thien, N.; Hsu-Hui, C.; Hung-Te, H. Fouling potential and reclamation feasibility for a closed landfill leachate treated by various pretreatment processes on membrane system. Desalin. Water Treat. 2015, 55, 3568–3575. [Google Scholar] [CrossRef]





| Name | Value |
|---|---|
| Inflow volume | 10 L/h |
| Membrane flux | 4.17 m3/h·dm2 |
| Membrane element area | 2.4 dm3 |
| Working pressure | 3~5 MPa |
| Clear liquid yield | 80% |
| Sample Name | Pure Water Flux/L·m−2·h−1 | Contact Angle/° | Porosity/% | R/% |
|---|---|---|---|---|
| PVDF-0 | 61.5 | 91.84 | 65.40 | 20.33 |
| PVDF-1 | 200.6 | 84.22 | 70.58 | 42.36 |
| PVDF-2 | 288.3 | 72.21 | 69.97 | 49.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Ding, K. Effect of Self-Made TiO2 Nanoparticle Size on the Performance of the PVDF Composite Membrane in MBR for Landfill Leachate Treatment. Membranes 2022, 12, 216. https://doi.org/10.3390/membranes12020216
Wang H, Ding K. Effect of Self-Made TiO2 Nanoparticle Size on the Performance of the PVDF Composite Membrane in MBR for Landfill Leachate Treatment. Membranes. 2022; 12(2):216. https://doi.org/10.3390/membranes12020216
Chicago/Turabian StyleWang, Huiya, and Keqiang Ding. 2022. "Effect of Self-Made TiO2 Nanoparticle Size on the Performance of the PVDF Composite Membrane in MBR for Landfill Leachate Treatment" Membranes 12, no. 2: 216. https://doi.org/10.3390/membranes12020216
APA StyleWang, H., & Ding, K. (2022). Effect of Self-Made TiO2 Nanoparticle Size on the Performance of the PVDF Composite Membrane in MBR for Landfill Leachate Treatment. Membranes, 12(2), 216. https://doi.org/10.3390/membranes12020216





