Incorporating Carbon Nanotubes in Nanocomposite Mixed-Matrix Membranes for Gas Separation: A Review
Abstract
:1. Introduction
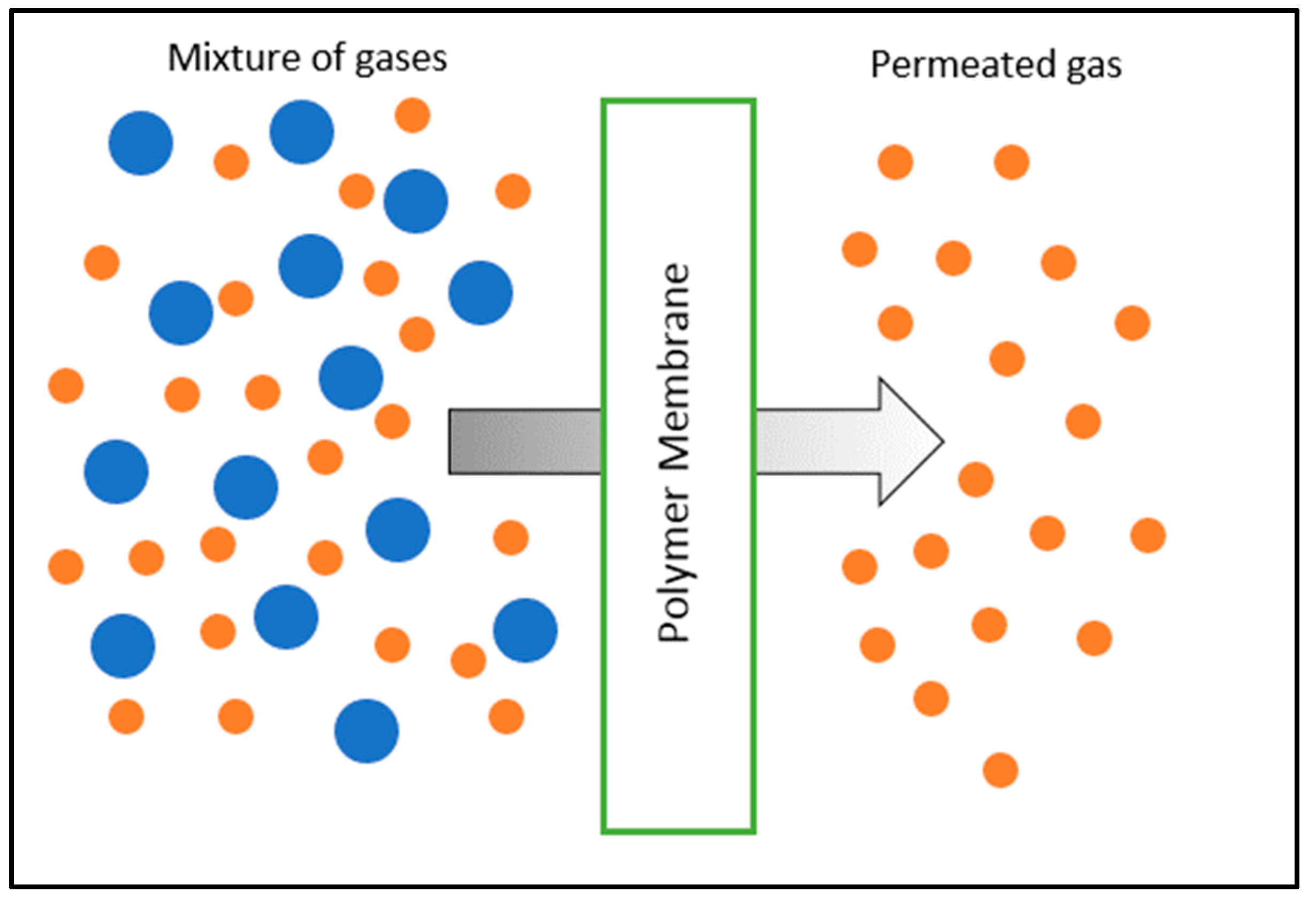
2. Polymeric Membranes
| Type of Polymer | Polymer Materials | Characteristics | Limitation | Refs. |
|---|---|---|---|---|
| Glassy Polymers | PSF | - high plasticization resistance (up to 50 bar) - good thermal, mechanical, and stability properties - excellent in separating CO2/CH4 because of its similar structure to sulfonyl groups | - moderate separation performance | [30,31] |
| Polyimide | - Low mobility of the polymer chain - Superior permeability/selectivity trade-off - High chemical resistance and thermal stability - High mechanical strength - Possesses intrinsic properties due to its imide structure and rigid aromatic moieties | - Has a high degree of polymer chain rigidity, resulting in strong intermolecular interactions - Poor economic viability - Ageing and plasticization issues for long-term uses | [33] | |
| Cellulose acetate | - Low cost - Ease of processability - Good fouling resistance - High CO2 solubility | - Low permeance | [34] | |
| PES | - Low cost - Long-term thermal stability chemical, and mechanical properties - The polymer’s ether unit provides an alternative mechanism for CO2 molecules to bind. | - Moderate plasticization resistance (around 28 bar) - Low permeance | [30,35] | |
| Rubbery Polymers | Pebax | - High mechanical strength and flexibility - Favorable selectivity for acid gas treatment and polar–nonpolar gases such CO2/CH4 - Increased CO2 permeability as a result of the PEO segment’s high affinity for the polar CO2 molecule - Has high chain mobility, which results in good interaction with fillers | - Low selectivity | [33,34,35] |
| Polyvinyl acetate (PVAc) | - Low cost - Has a strong affinity for CO2 and can result in a high solubility of CO2 as a result of the polar groups of acetate in its backbone | - Low gas permeance compared to another rubbery polymer - Difficult processability | [36] | |
| Polyethylene glycol (PEG) | - Due to the high quadruple moment of CO2 and the dipole moment of polar ether segments, this material exhibits good CO2 permeation characteristics. | - Poor mechanical and thermal properties | [37] | |
| Polydimethylsiloxane (PDMS) | - Possesses a dense cross-linked network structure and great chain mobility - Low material cost, high thermal and chemical stability | - Favors greater gas transport | [38] |
3. Inorganic Membranes

| Inorganic Fillers | Characteristics | Refs. |
|---|---|---|
| Zeolite | - Excellent mechanical and thermal stability, as well as resistance to chemicals - Separate gases based on their kinetic diameters - Enhanced separation at lower temperatures due to preferential adsorption | [60,61] |
| Carbon Molecular Sieve | - High CO2/CH4 selectivity - Better affinity to glassy polymer | [62] |
| Graphene Nanosheets | - Large interfacial area - High degree of hydrophilicity - Interlayer spacing between the GNs sheets can be adjusted to optimize the transport of specific molecules. - high flexibility and mechanical strength | [29] |
| MOF | - High CO2 adsorption capacities - Great mechanical flexibility and structure tunability - Synthesized easily and rapidly at a low cost | [53] |
| Carbon Nanotubes | - Excellent mechanical strength - Inherent smoothness of MWCNTs, which allows rapid transport of gases | [63] |
| Alumina | - Economical and easily obtainable - Toxic-free substance with a high degree of resistance | [37] |
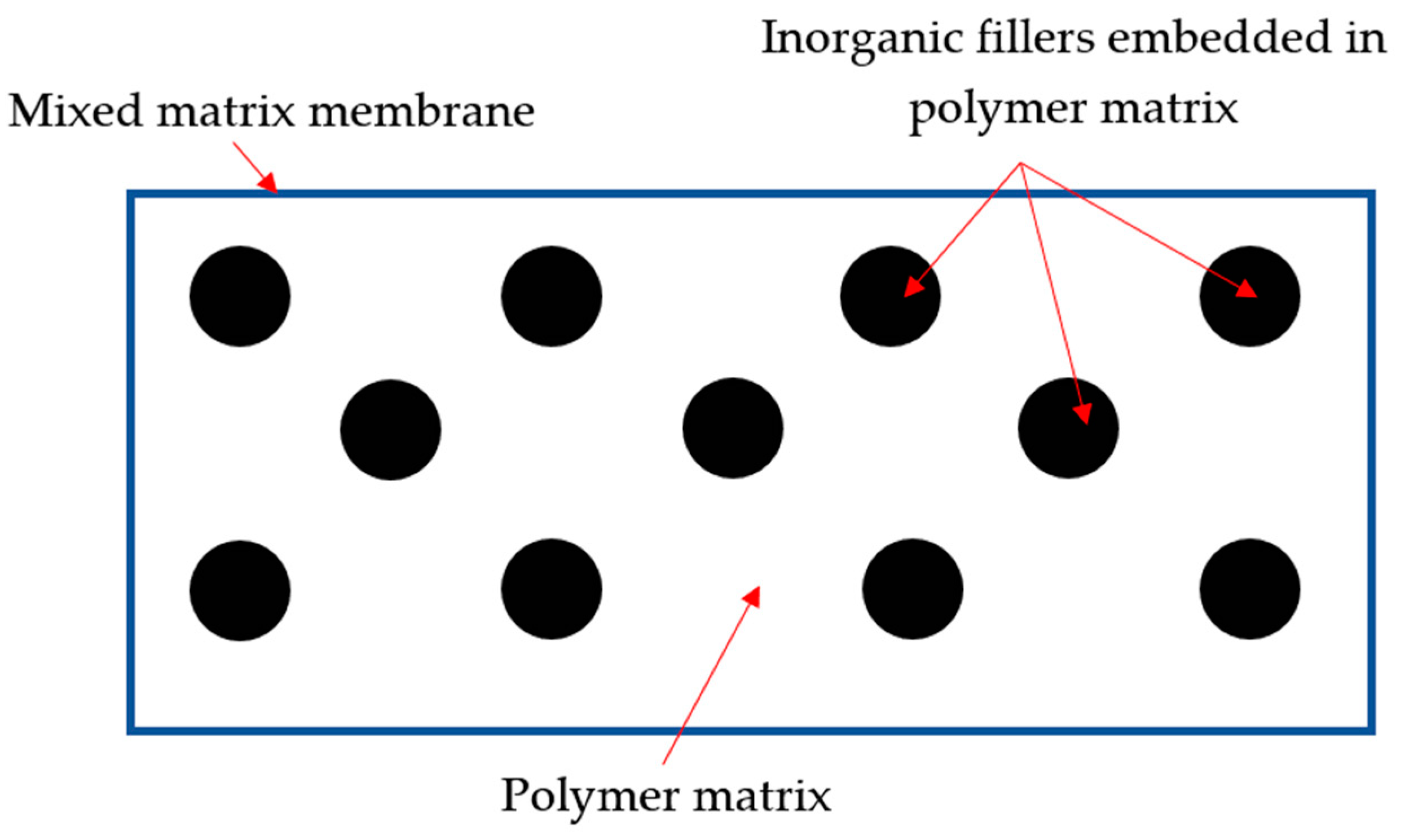
4. Mixed-Matrix Membranes (MMMs)
5. Carbon Nanotubes (CNTs)
6. CNT-Polymer Nanocomposites
6.1. Dispersion of CNTs
6.1.1. Covalent Functionalization
6.1.2. Non-Covalent Functionalization
6.2. CNT–Polymer Mixed-Matrix Membrane in Gas Separation
7. CNT MMMs Gas Separation Application
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, R.W.; Low, B.T. Gas separation membrane materials: A perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Nejad, M.N.; Asghari, M.; Afsari, M. Investigation of carbon nanotubes in mixed matrix membranes for gas separation: A review. ChemBioEng Rev. 2016, 3, 276–298. [Google Scholar] [CrossRef]
- Global Market Study on Membrane Technology: Revenues to Exceed US$ 10 Bn by 2019 End, North America to Remain Key Market. Persistence Market Research. Available online: https://www.persistencemarketresearch.com/market-research/membrane-technology-market.asp (accessed on 5 February 2022).
- Bernardo, P.; Clarizia, G. 30 Years of Membrane Technology for Gas Separation. Chem. Eng. Trans. 2013, 32, 1999–2004. [Google Scholar] [CrossRef]
- Siegelman, R.L.; Milner, P.J.; Kim, E.J.; Weston, S.C.; Long, J.R. Challenges and opportunities for adsorption-based CO2 capture from natural gas combined cycle emissions. Energy Environ. Sci. 2019, 12, 2161–2173. [Google Scholar] [CrossRef] [PubMed]
- Grande, C.A.; Roussanaly, S.; Anantharaman, R.; Lindqvist, K.; Singh, P.; Kemper, J. CO2 Capture in Natural Gas Production by Adsorption Processes. Energy Procedia 2017, 114, 2259–2264. [Google Scholar] [CrossRef]
- Aaron, D.; Tsouris, C. Separation of CO2 from flue gas: A review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Maqsood, K.; Mullick, A.; Ali, A.; Kargupta, K.; Ganguly, S. Cryogenic carbon dioxide separation from natural gas: A review based on conventional and novel emerging technologies. Rev. Chem. Eng. 2014, 30, 453–477. [Google Scholar] [CrossRef]
- Castel, C.; Bounaceur, R.; Favre, E. Engineering of membrane gas separation processes: State of the art and prospects. J. Membr. Sci. Res. 2020, 6, 295–303. [Google Scholar] [CrossRef]
- Alqaheem, Y.; Alomair, A.; Vinoba, M.; Pérez, A. Polymeric Gas-Separation Membranes for Petroleum Refining. Int. J. Polym. Sci. 2017, 2017, 4250927. [Google Scholar] [CrossRef]
- Pandey, P.; Chauhan, R.S. Membranes for gas separation. Prog. Polym. Sci. 2001, 26, 853–893. [Google Scholar] [CrossRef]
- Castro-muñoz, R.; Martin-gil, V.; Ahmad, M.Z. Matrimid® 5218 in preparation of membranes for gas separation: Current state-of-the-art. Chem. Eng. Commun. 2018, 205, 161–196. [Google Scholar] [CrossRef]
- Cong, H.; Radosz, M.; Towler, B.F.; Shen, Y. Polymer-inorganic nanocomposite membranes for gas separation. Sep. Purif. Technol. 2007, 55, 281–291. [Google Scholar] [CrossRef]
- Singh, A.; Koros, W.J. Significance of Entropic Selectivity for Advanced Gas Separation Membranes. Ind. Eng. Chem. Res. 1996, 35, 1231–1234. [Google Scholar] [CrossRef]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. Mixed matrix membranes using carbon molecular sieves: II. Modeling permeation behavior. J. Memb. Sci. 2003, 211, 335–348. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Z.; Meng, X.; Wang, R. MXene-engineered lithium-sulfur batteries. J. Mater. Chem. A 2019, 7, 22730–22743. [Google Scholar] [CrossRef]
- Hamid, M.R.A.; Jeong, H.K. Recent advances on mixed-matrix membranes for gas separation: Opportunities and engineering challenges. Korean J. Chem. Eng. 2018, 35, 1577–1600. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.F.; Matsuura, T.; Montazer-Rahmati, M.M. Performance studies of mixed matrix membranes for gas separation: A review. Sep. Purif. Technol. 2010, 75, 229–242. [Google Scholar] [CrossRef]
- Dai, H. Carbon nanotubes: Opportunities and challenges. Surf. Sci. 2002, 500, 218–241. [Google Scholar] [CrossRef]
- Ma, X. Natural gas and energy revolution: A case study of Sichuan–Chongqing gas province. Nat. Gas Ind. B 2017, 4, 91–99. [Google Scholar] [CrossRef] [Green Version]
- David, Z.; Ruilan, L.; James, D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef] [Green Version]
- Ismail, A.F.; Khulbe, K.C.; Matsuura, T. Gas Separation Membranes: Polymeric and Inorganic; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Kim, S.; Lee, Y.M. High performance polymer membranes for CO2 separation. Curr. Opin. Chem. Eng. 2013, 2, 238–244. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, 1138–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Liang, C.Z.; Chung, T.S.; Lai, J.Y. A review of polymeric composite membranes for gas separation and energy production. Prog. Polym. Sci. 2019, 97, 101141. [Google Scholar] [CrossRef]
- Baker, R.W.; Lokhandwala, K. Natural gas processing with membranes: An overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Carter, D.; Tezel, F.H.; Kruczek, B.; Kalipcilar, H. Investigation and comparison of mixed matrix membranes composed of polyimide matrimid with ZIF-8, silicalite, and SAPO-34. J. Memb. Sci. 2017, 544, 35–46. [Google Scholar] [CrossRef]
- Mohamed, A.; Yousef, S.; Tonkonogovas, A.; Makarevicius, V.; Stankevičius, A. High performance of PES-GNs MMMs for gas separation and selectivity. Arab. J. Chem. 2022, 15, 103565. [Google Scholar] [CrossRef]
- Tanco, M.A.L.; Medrano, J.A.; Gallucci, F.; Tanaka, D.A.P. Membrane Optimization and Process Condition Investigation for Enhancing the CO2 Separation from Natural Gas; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Yong, W.F.; Zhang, H. Recent advances in polymer blend membranes for gas separation and pervaporation. Prog. Mater. Sci. 2021, 116, 100713. [Google Scholar] [CrossRef]
- Mannan, H.A.; Mukhtar, H.; Murugesan, T. Polyethersulfone (PES) membranes for CO2/CH4 separation: Effect of polymer blending. Appl. Mech. Mater. 2014, 625, 172–175. [Google Scholar] [CrossRef]
- Mohamad, M.B.; Fong, Y.Y.; Shariff, A. Gas Separation of Carbon Dioxide from Methane Using Polysulfone Membrane Incorporated with Zeolite-T. Procedia Eng. 2016, 148, 621–629. [Google Scholar] [CrossRef] [Green Version]
- Ismail, A.F.; Rahim, N.H.; Mustafa, A.; Matsuura, T.; Ng, B.C.; Abdullah, S.; Hashemifard, S.A. Gas separation performance of polyethersulfone/multi-walled carbon nanotubes mixed matrix membranes. Sep. Purif. Technol. 2011, 80, 20–31. [Google Scholar] [CrossRef]
- Sajjan, P.; Nayak, V.; Padaki, M.; Zadorozhnyy, V.Y.; Klyamkin, S.N.; Konik, P.A. Fabrication of Cellulose Acetate Film through Blending Technique with Palladium Acetate for Hydrogen Gas Separation. Energy Fuels 2020, 34, 11699–11707. [Google Scholar] [CrossRef]
- Farnam, M.; Mukhtar, H.; Shariff, A.M. An investigation of blended polymeric membranes and their gas separation performance. RSC Adv. 2016, 6, 102671–102679. [Google Scholar] [CrossRef]
- Dilshad, M.R.; Islam, A.; Hamidullah, U.; Jamshaid, F.; Ahmad, A.; Butt, M.T.Z.; Ijaz, A. Effect of alumina on the performance and characterization of cross-linked PVA/PEG 600 blended membranes for CO2/N2 separation. Sep. Purif. Technol. 2019, 210, 627–635. [Google Scholar] [CrossRef]
- Li, T.; Pan, Y.; Peinemann, K.V.; Lai, Z. Carbon dioxide selective mixed matrix composite membrane containing ZIF-7 nano-fillers. J. Memb. Sci. 2013, 425–426, 235–242. [Google Scholar] [CrossRef]
- Mazinani, S.; Ramezani, R.; Darvishmanesh, S.; Molelekwa, G.F.; di Felice, R.; van der Bruggen, B. A ground breaking polymer blend for CO2/N2 separation. J. CO2 Util. 2018, 27, 536–546. [Google Scholar] [CrossRef]
- Esposito, E.; Mazzei, I.; Monteleone, M.; Fuoco, A.; Carta, M.; McKeown, N.B.; Malpass-Evans, R.; Jansen, J.C. Highly permeable Matrimid®/PIM-EA(H2)-TB blend membrane for gas separation. Polymers 2019, 11, 46. [Google Scholar] [CrossRef] [Green Version]
- Rajati, H.; Navarchian, A.H.; Tangestaninejad, S. Preparation and characterization of mixed matrix membranes based on Matrimid/PVDF blend and MIL-101(Cr) as filler for CO2/CH4 separation. Chem. Eng. Sci. 2018, 185, 92–104. [Google Scholar] [CrossRef]
- Khaki, E.; Abyar, H.; Nowrouzi, M.; Younesi, H.; Abdollahi, M.; Enderati, M.G. Comparative life cycle assessment of polymeric membranes: Polyacrylonitrile, polyvinylimidazole and poly (acrylonitrile-co-vinylimidazole) applied for CO2 sequestration. Environ. Technol. Innov. 2021, 22, 101507. [Google Scholar] [CrossRef]
- Hamid, A.S.A.; Ibrahim, A.; Mat, S.; Sopian, K. Experimental Evaluation on Large Scale Solar Dryer for Drying Natural Fiber in Malaysia. Int. J. Renew. Energy Res. 2019, 9, 598–604. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85070940342&partnerID=40&md5=857f3ffce8c2c9e0dc3b3b7c4402d356 (accessed on 8 April 2022).
- Mubashir, M.; Fong, Y.Y.; Leng, C.T.; and Keong, L.K. Enhanced gases separation of cellulose acetate membrane using N-methyl-1-2 pyrrolidone as fabrication solvent. Int. J. Automot. Mech. Eng. 2018, 15, 4978–4986. [Google Scholar] [CrossRef]
- Murali, R.S.; Sridhar, S.; Sankarshana, T.; Ravikumar, Y.V.L. Gas permeation behavior of pebax-1657 nanocomposite membrane incorporated with multiwalled carbon nanotubes. Ind. Eng. Chem. Res. 2010, 49, 6530–6538. [Google Scholar] [CrossRef]
- Lu, G.Q.; Da Costa, J.D.; Duke, M.; Giessler, S.; Socolow, R.; Williams, R.H.; Kreutz, T. Inorganic membranes for hydrogen production and purification: A critical review and perspective. J. Colloid Interface Sci. 2007, 314, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Goh, P.S.; Ismail, A.F.; Sanip, S.M.; Ng, B.C.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Abedini, S.; Parvin, N.; Ashtari, P.; Jazi, F.S. Microstructure, strength and CO2 separation characteristics of α-alumina supported γ-alumina thin film membrane. Adv. Appl. Ceram. 2013, 112, 17–22. [Google Scholar] [CrossRef]
- Ameri, E.; Sadeghi, M.; Zarei, N.; Pournaghshband, A. Enhancement of the gas separation properties of polyurethane membranes by alumina nanoparticles. J. Memb. Sci. 2015, 479, 11–19. [Google Scholar] [CrossRef]
- Yoshimune, M.; Haraya, K. CO2/CH4 mixed gas separation using carbon hollow fiber membranes. Energy Procedia 2013, 37, 1109–1116. [Google Scholar] [CrossRef] [Green Version]
- Kruse, N.; Schießer, Y.; Kämnitz, S.; Richter, H.; Voigt, I.; Braun, G.; Repke, J.U. Carbon membrane gas separation of binary CO2 mixtures at high pressure. Sep. Purif. Technol. 2016, 164, 132–137. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.; Yuan, S.; Zhou, J.; Wang, B. Challenges and recent advances in MOF-polymer composite membranes for gas separation. Inorg. Chem. Front. 2016, 3, 896–909. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, H.; Ma, Q.; Mo, K.; Mao, H.; Feldhoff, A.; Cao, X.; Li, Y.; Pan, F.; Jiang, Z. A MOF Glass Membrane for Gas Separation. Angew. Chemie-Int. Ed. 2020, 59, 4365–4369. [Google Scholar] [CrossRef]
- Zarshenas, K.; Raisi, A.; Aroujalian, A. Mixed matrix membrane of nano-zeolite NaX/poly (ether-block-amide) for gas separation applications. J. Memb. Sci. 2016, 510, 70–283. [Google Scholar] [CrossRef]
- Sebastián, V.; Kumakiri, I.; Bredesen, R.; Menéndez, M. Zeolite membrane for CO2 removal: Operating at high pressure. J. Memb. Sci. 2007, 292, 92–97. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T.; Feng, C.Y.; Ismail, A.F. Recent development on the effect of water/moisture on the performance of zeolite membrane and MMMs containing zeolite for gas separation; Review. RSC Adv. 2016, 6, 42943–42961. [Google Scholar] [CrossRef]
- Koresh, J.E.; Soffer, A. The Carbon Molecular Sieve Membranes. General Properties and the Permeability of CH4/H2 Mixture. Sep. Sci. Technol. 1987, 22, 973–982. [Google Scholar] [CrossRef]
- Ismail, A.F.; David, L.I.B. A review on the latest development of carbon membranes for gas separation. J. Memb. Sci. 2001, 193, 1–18. [Google Scholar] [CrossRef]
- Li, L.; Xu, R.; Song, C.; Zhang, B.; Liu, Q.; Wang, T. A review on the progress in nanoparticle/C hybrid CMS membranes for gas separation. Membranes 2018, 8, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, C.; Khulbe, K.C.; Matsuura, T.; Farnood, R.; Ismail, A.F. Recent progress in zeolite/zeotype membranes. J. Membr. Sci. Res. 2015, 1, 49–72. [Google Scholar]
- Vinoba, M.; Bhagiyalakshmi, M.; Alqaheem, Y.; Alomair, A.A.; Pérez, A.; Rana, M.S. Recent progress of fillers in mixed matrix membranes for CO2 separation: A review. Sep. Purif. Technol. 2017, 188, 431–450. [Google Scholar] [CrossRef]
- Iarikov, D.D.; Oyama, S.T. Review of CO2/CH4 Separation Membranes, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2011; Volume 14. [Google Scholar]
- Popov, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. R Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Memb. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Robeson, L.M.; Liu, Q.; Freeman, B.D.; Paul, D.R. Comparison of transport properties of rubbery and glassy polymers and the relevance to the upper bound relationship. J. Memb. Sci. 2015, 476, 421–431. [Google Scholar] [CrossRef]
- Dechnik, J.; Gascon, J.; Doonan, C.J.; Janiak, C.; Sumby, C.J. Mixed-Matrix Membranes. Angew. Chemie-Int. Ed. 2017, 56, 9292–9310. [Google Scholar] [CrossRef] [PubMed]
- Azizi, N.; Mohammadi, T.; Behbahani, R.M. Comparison of permeability performance of PEBAX-1074/TiO2, PEBAX-1074/SiO2 and PEBAX-1074/Al2O3 nanocomposite membranes for CO2/CH4 separation. Chem. Eng. Res. Des. 2017, 117, 177–189. [Google Scholar] [CrossRef]
- Aoki, T. Macromolecular design of permselective membranes. Prog. Polym. Sci. 1999, 24, 951–993. [Google Scholar] [CrossRef]
- Strathmann, H. Membrane separation processes: Current relevance and future opportunities. AIChE J. 2001, 47, 1077–1087. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Yave, W.; Golemme, G. Some approaches for high performance polymer based membranes for gas separation: Block copolymers, carbon molecular sieves and mixed matrix membranes. RSC Adv. 2012, 2, 10745–10773. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Peinemann, K.V. Hybrid membrane materials with different metal-organic frameworks (MOFs) for gas separation. Desalination 2006, 200, 424–426. [Google Scholar] [CrossRef]
- Jomekian, A.; Behbahani, R.M.; Mohammadi, T.; Kargari, A. CO2/CH4 separation by high performance co-casted ZIF-8/Pebax 1657/PES mixed matrix membrane. J. Nat. Gas Sci. Eng. 2016, 31, 562–574. [Google Scholar] [CrossRef]
- Mohamed, M.J.B.G.; Mannan, H.A.; Nasir, R.; Mohshim, D.F.; Mukhtar, H.; Abdulrahman, A.; Ahmed, A. Composite mixed matrix membranes incorporating microporous carbon molecular sieve as filler in polyethersulfone for CO2/CH4 separation. J. Appl. Polym. Sci. 2020, 137, 1–9. [Google Scholar] [CrossRef]
- Dong, G.; Hou, J.; Wang, J.; Zhang, Y.; Chen, V.; Liu, J. Enhanced CO2/ N2 separation by porous reduced graphene oxide/Pebax mixed matrix membranes. J. Membr. Sci. 2016, 520, 860–868. [Google Scholar] [CrossRef]
- Endo, M.; Hayashi, T.; Kim, Y.A.; Muramatsu, H. Development and application of carbon nanotubes. Jpn. J. Appl. Phys. 2006, 45, 4883–4892. [Google Scholar] [CrossRef] [Green Version]
- Bethune, D.S.; Klang, C.H.; de Vries, M.S.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature 1993, 363, 605–607. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R. Physics of carbon nanotubes. Carbon N. Y. 1995, 33, 883–891. [Google Scholar] [CrossRef]
- Baddour, C.E.; Briens, C. Carbon nanotube synthesis: A review. Int. J. Chem. React. Eng. 2005, 3. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Franceschi, W.; Menezes, B.R.C.; Biagioni, A.F.; Coutinho, A.R.; Cividanes, L.S. Applications of Carbon Nanotubes; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Skoulidas, A.I.; Ackerman, D.M.; Johnson, J.K.; Sholl, D.S. Rapid Transport of Gases in Carbon Nanotubes. Phys. Rev. Lett. 2002, 89, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Rehman, S.A.U.; Luan, H.Y.; Farid, M.U.; Huang, H. Challenges and opportunities in functional carbon nanotubes for membrane-based water treatment and desalination. Sci. Total Environ. 2019, 646, 1126–1139. [Google Scholar] [CrossRef]
- Ma, J.; Ping, D.; Dong, X. Fabrication and water treatment application of carbon nanotubes (CNTs)-based composite membranes: A review. Membranes 2017, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Iranagh, F.R.; Asghari, M.; Parnian, M.J. Dispersion engineering of MWCNT to control structural and gas transport properties of PU mixed matrix membranes. J. Environ. Chem. Eng. 2020, 8, 104493. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Ribeiro, B.; Botelho, E.; Costa, M.; Bandeira, C. Carbon nanotube buckypaper reinforced polymer composites: A review. Polímeros 2017, 27, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Ghaemi, N.; Madaeni, S.S.; Daraei, P.; Rajabi, H.; Shojaeimehr, T.; Rahimpour, F.; Shirvani, B. PES mixed matrix nanofiltration membrane embedded with polymer wrapped MWCNT: Fabrication and performance optimization in dye removal by RSM. J. Hazard. Mater. 2015, 298, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Amirkhani, F.; Mosadegh, M.; Asghari, M.; Parnian, M.J. The beneficial impacts of functional groups of CNT on structure and gas separation properties of PEBA mixed matrix membranes. Polym. Test. 2020, 82, 106285. [Google Scholar] [CrossRef]
- Singh, S.; Varghese, A.M.; Reddy, K.S.K.; Romanos, G.E.; Karanikolos, G.N. Polysulfone Mixed-Matrix Membranes Comprising Poly(ethylene glycol)-Grafted Carbon Nanotubes: Mechanical Properties and CO2 Separation Performance. Ind. Eng. Chem. Res. 2021, 60, 11289–11308. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Asghari, M.; Mahmoodi, N.M. Chitosan-wrapped multiwalled carbon nanotube as filler within PEBA thin film nanocomposite (TFN) membrane to improve dye removal. Carbohydr. Polym. 2020, 237, 116128. [Google Scholar] [CrossRef] [PubMed]
- Borgohain, R.; Jain, N.; Prasad, B.; Mandal, B.; Su, B. Carboxymethyl chitosan/carbon nanotubes mixed matrix membranes for CO2 separation. React. Funct. Polym. 2019, 143, 104331. [Google Scholar] [CrossRef]
- Fernandes, R.M.F.; Dai, J.; Regev, O.; Marques, E.F.; Furó, I. Block Copolymers as Dispersants for Single-Walled Carbon Nanotubes: Modes of Surface Attachment and Role of Block Polydispersity. Langmuir 2018, 34, 13672–13679. [Google Scholar] [CrossRef]
- Hussain, A.; Farrukh, S.; Hussain, A.; Ayoub, M. Carbon Capture from Natural Gas Using Multi-Walled CNTs Based Mixed Matrix Membranes; Taylor & Francis: Oxford, UK, 2019; Volume 40. [Google Scholar]
- Modi, A.; Verma, S.K.; Bellare, J. Carboxylated Carbon Nanotubes/Polyethersulfone Hollow Fiber Mixed Matrix Membranes: Development and Characterization for Enhanced Gas Separation Performance. MRS Adv. 2018, 3, 3135–3141. [Google Scholar] [CrossRef]
- Yousef, S.; Tuckute, S.; Tonkonogovas, A.; Stankevičius, A.; Mohamed, A. Ultra-permeable CNTs/PES membranes with a very low CNTs content and high H2/N2 and CH4/N2 selectivity for clean energy extraction applications. J. Mater. Res. Technol. 2021, 15, 5114–5127. [Google Scholar] [CrossRef]
- Yu, B.; Cong, H.; Li, Z.; Tang, J.; Zhao, X.S. Pebax-1657 nanocomposite membranes incorporated with nanoparticles/colloids/carbon nanotubes for CO2/N2 and CO2/H2 separation. J. Appl. Polym. Sci. 2013, 130, 2867–2876. [Google Scholar] [CrossRef]
- Dai, Z.; Deng, J.; Peng, K.J.; Liu, Y.L.; Deng, L. Pebax/PEG grafted CNT hybrid membranes for enhanced CO2/N2 separation. Ind. Eng. Chem. Res. 2019, 58, 12226–12234. [Google Scholar] [CrossRef]
- Lee, R.J.; Jawad, Z.A.; Chua, H.B.; Ahmad, A.L. Blend cellulose acetate butyrate/functionalised multi-walled carbon nanotubes mixed matrix membrane for enhanced CO2/N2 separation with kinetic sorption study. J. Environ. Chem. Eng. 2020, 8, 104212. [Google Scholar] [CrossRef]
- Lee, S.T.; Pang, J.N.; Jawad, Z.A. Functionalised multi-walled carbon nanotubes/cellulose acetate butyrate mixed matrix membrane for CO2/N2 separation. J. Phys. Sci. 2019, 30, 99–135. [Google Scholar] [CrossRef]
- Moghadassi, A.R.; Rajabi, Z.; Hosseini, S.M.; Mohammadi, M. Preparation and Characterization of Polycarbonate-Blend-Raw/Functionalized Multi-Walled Carbon Nano Tubes Mixed Matrix Membrane for CO2 Separation. Sep. Sci. Technol. 2013, 48, 1261–1271. [Google Scholar] [CrossRef]
- Moghadassi, A.R.; Rajabi, Z.; Hosseini, S.M.; Mohammadi, M. Fabrication and modification of cellulose acetate based mixed matrix membrane: Gas separation and physical properties. J. Ind. Eng. Chem. 2014, 20, 1050–1060. [Google Scholar] [CrossRef]
- Dilshad, M.R.; Islam, A.; Haider, B.; Sabir, A.; Ijaz, A.; Khan, R.U.; Durrani, A.K. Novel PVA/PEG nano-composite membranes tethered with surface engineered multi-walled carbon nanotubes for carbon dioxide separation. Microporous Mesoporous Mater. 2020, 308, 110545. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, J.; Wang, Y.; Qiu, Y.; Li, H.; Hua, K.; Li, X.; Ji, J.; Deng, M. High CO2 Separation Performance of Pebax®/CNTs/GTA Mixed Matrix Membranes; Elsevier: Amsterdam, The Netherlands, 2017; Volume 521. [Google Scholar]
- Dong, G.; Li, H.; Chen, V. Challenges and opportunities for mixed-matrix membranes for gas separation. J. Mater. Chem. A 2013, 1, 4610–4630. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary Perspective: Polymers and Mixed Matrix Membranes for Gas and Vapor Separation: A Review and Prospective Opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Z.; Zhao, D. Mixed Matrix Membranes for Natural Gas Upgrading: Current Status and Opportunities. Ind. Eng. Chem. Res. 2018, 57, 4139–4169. [Google Scholar] [CrossRef]



| Technology | Advantages | Limitation | Refs. |
|---|---|---|---|
| Absorption | - Does not have a pretreatment process - Has recovery rates of up to 95% - Has product purity up to 99% volume. | - Requires high costs - Need to regenerate solvent, and the process has a high energy demand - It requires a large floor area and is not suitable for offshore application | [4] |
| Adsorption | - No solvent - Has better stability for feed with high impurity concentrations - Recovers CO2 concentration higher than 90 vol% | - Low solid-to-gas capacity - Low solvent regeneration rate - Requires a large floor area | [5,6] |
| Cryogenic | - Achieves more than 99% of CO2 capture at -150 °C operating temperature - Produces liquified CO2 for more accessible storage | - High operating cost - Need to operate at high pressure to prevent CO2 sublimation - Requires a large floor area | [7,8] |
| Membranes | - Simplicity - Requires minimum supervision - Small floor area requirement - Bulk removal | - Moderate purity compared to other technologies - Possible recompression of permeate - Possible plugging of impurities in the gas stream. | [7] |
| Membranes | Permeability (Barrer) | Selectivity | Refs. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 | O2 | N2 | CH4 | H2 | CO2/CH4 | CO2/N2 | O2/N2 | H2/N2 | H2/CO2 | ||
| Glassy Polymer Membranes | |||||||||||
| PSF | 39 | 139 | 3.6 | [43] | |||||||
| PES | 10 | 12 | 4 | 10 | 2.5 | 0.8 | 0.8 | 1 | [29] | ||
| Matrimid 5218 | 9.54 | 0.7 | 0.32 | 30.3 | 94.6 | 43.2 | 3.2 | [28] | |||
| Cellulose Acetate | 15.56 | 1.77 | 1.45 | 10.7 | 8.8 | [44] | |||||
| Rubbery Polymer Membranes | |||||||||||
| Pebax | 55.85 | 4.69 | 1.39 | 32.11 | 40.18 | 3.37 | 23.1 | 0.57 | [45] | ||
| PIM-EA(H2)-TB | 1391 | 53.1 | 62.6 | 22.22 | 26.20 | [40] | |||||
| PVDF | 2.11 | 0.08 | 26.37 | [41] | |||||||
| Polymer Blend Membranes | |||||||||||
| PVA/PEG | 52.9 | 2.03 | 26 | [37] | |||||||
| Matrimid/PIM-EA(H2)-TB | 198 | 6.83 | 9.1 | 21.66 | 28.99 | [40] | |||||
| Matrimid/PVDF | 9.42 | 0.08 | 42.81 | [41] | |||||||
| Membranes | Pressure (Bar) | Loading Ratio (wt.%) | Permeability | Selectivity | Refs. | ||||
|---|---|---|---|---|---|---|---|---|---|
| CO2 | N2 | CH4 | H2 | CO2/CH4 | CO2/N2 | ||||
| PES/MWCNT | 2 | 1 | 3.2 | 0.15 | 22 | [2] | |||
| 2 | 3 | 3.5 | 0.17 | 21 | |||||
| 2 | 5 | 4.5 | 0.21 | 21 | |||||
| 2 | 10 | 3.5 | 0.19 | 18.5 | |||||
| Matrimid/MWCNT | 2 | 2 | 13 | 0.84 | 0.81 | 16 | 15.5 | [41] | |
| 2 | 5 | 15 | 1 | 1 | 15 | 15 | |||
| 2 | 8 | 18 | 1.29 | 1.38 | 13 | 14 | |||
| 2 | 10 | 11 | 0.85 | 0.92 | 12 | 13 | |||
| PEBAX/MWCNT with TDI | 1 | 2 | 3.54 | 0.03 | 2.51 | 83.2 | [45] | ||
| 1 | 5 | 17.47 | 0.21 | 7.18 | 84.5 | ||||
| PEBAX/MWCNT–NH2 with GTA | 20 | 1 | 1408 | 213 | [102] | ||||
| PEBAX-MWCNT crosslinked | 10 | 2 | |||||||
| 10 | 5 | ||||||||
| PEBAX/CNT–COOH | 10 | 0.75 | 132.30 | 1.55 | 5.47 | 24.18 | 85.32 | [87] | |
| PEBAX/CNT–NCO | 10 | 0.3 | 148.86 | 1.42 | 5.14 | 28.95 | 104.92 | ||
| PEBAX/CNT–NH2 | 10 | 0.5 | 139.52 | 1.46 | 5.31 | 26.28 | 95.62 | ||
| PC-PEG/ MWCNT–COOH | 2 | 1 | 8.35 | 0.18 | 0.28 | 25.73 | 28.19 | [99] | |
| PC-PEG/MWCNT–COOH | 2 | 2 | 12.53 | 0.26 | 0.37 | 26.59 | 27.45 | ||
| 2 | 5 | 15.47 | 0.31 | 0.46 | 27.38 | 25.42 | |||
| 2 | 10 | 20.32 | 0.39 | 0.57 | 27.28 | 25.37 | |||
| PVA-PEG/MWCNT | 1 | 0.5 | 115.57 | 0.57 | 1.41 | 82.26 | 202.75 | [101] | |
| 5 | 0.5 | 107.78 | 0.55 | 1.38 | 77.88 | 195.96 | |||
| 10 | 0.5 | 104.5 | 0.54 | 1.35 | 77.35 | 193.52 | |||
| 15 | 0.5 | 101.12 | 0.52 | 1.32 | 76.49 | 194.46 | |||
| 20 | 0.5 | 99.62 | 0.51 | 1.33 | 76.45 | 195.33 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazid, A.F.; Mukhtar, H.; Nasir, R.; Mohshim, D.F. Incorporating Carbon Nanotubes in Nanocomposite Mixed-Matrix Membranes for Gas Separation: A Review. Membranes 2022, 12, 589. https://doi.org/10.3390/membranes12060589
Yazid AF, Mukhtar H, Nasir R, Mohshim DF. Incorporating Carbon Nanotubes in Nanocomposite Mixed-Matrix Membranes for Gas Separation: A Review. Membranes. 2022; 12(6):589. https://doi.org/10.3390/membranes12060589
Chicago/Turabian StyleYazid, Aimi Farzana, Hilmi Mukhtar, Rizwan Nasir, and Dzeti Farhah Mohshim. 2022. "Incorporating Carbon Nanotubes in Nanocomposite Mixed-Matrix Membranes for Gas Separation: A Review" Membranes 12, no. 6: 589. https://doi.org/10.3390/membranes12060589







