Ultra-Low-Pressure Membrane Filtration for Simultaneous Recovery of Detergent and Water from Laundry Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laundry Wastewater and Analytical Methods
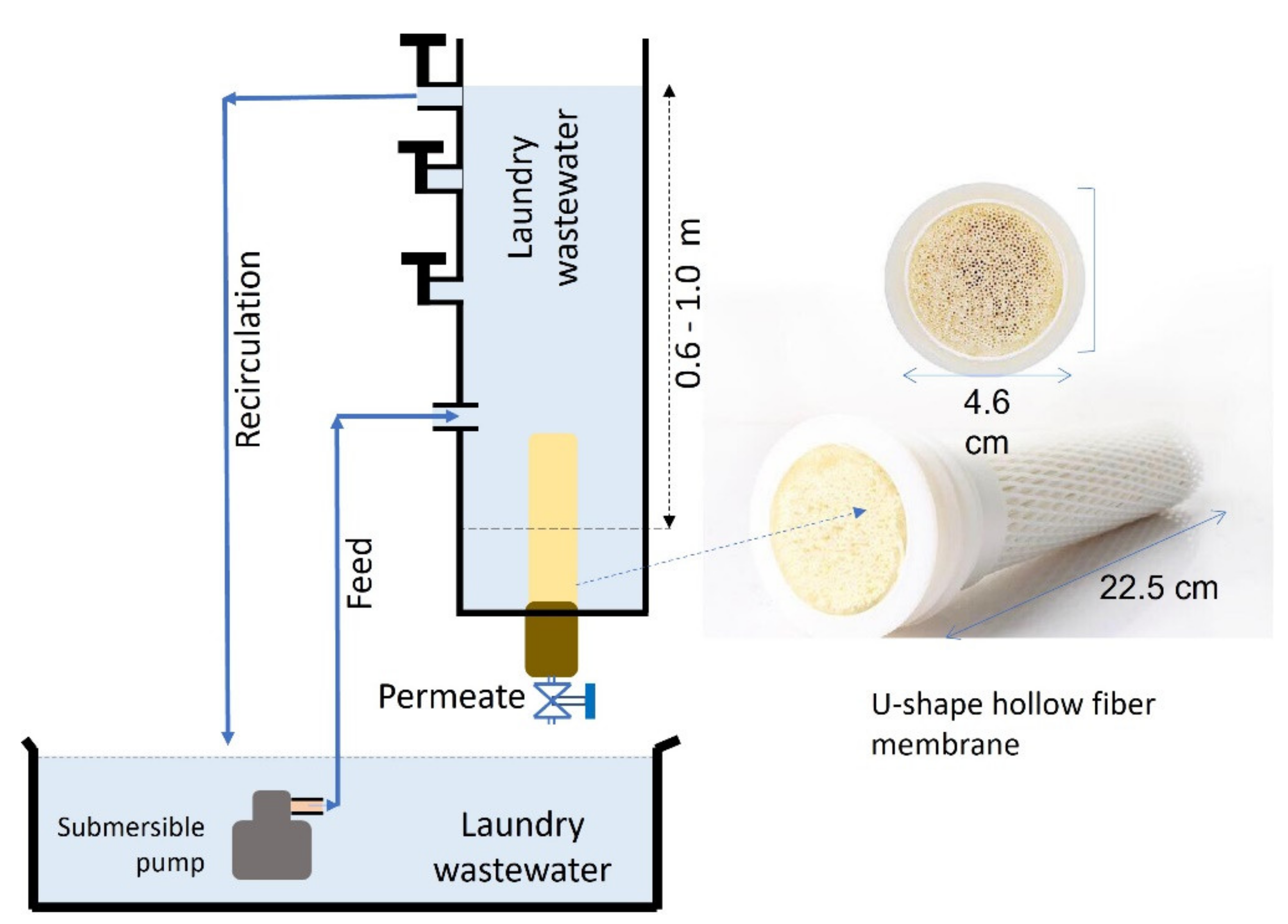
2.2. Gravity-Driven Filtration Set-Up
2.3. Filtration Test
3. Results and Discussion
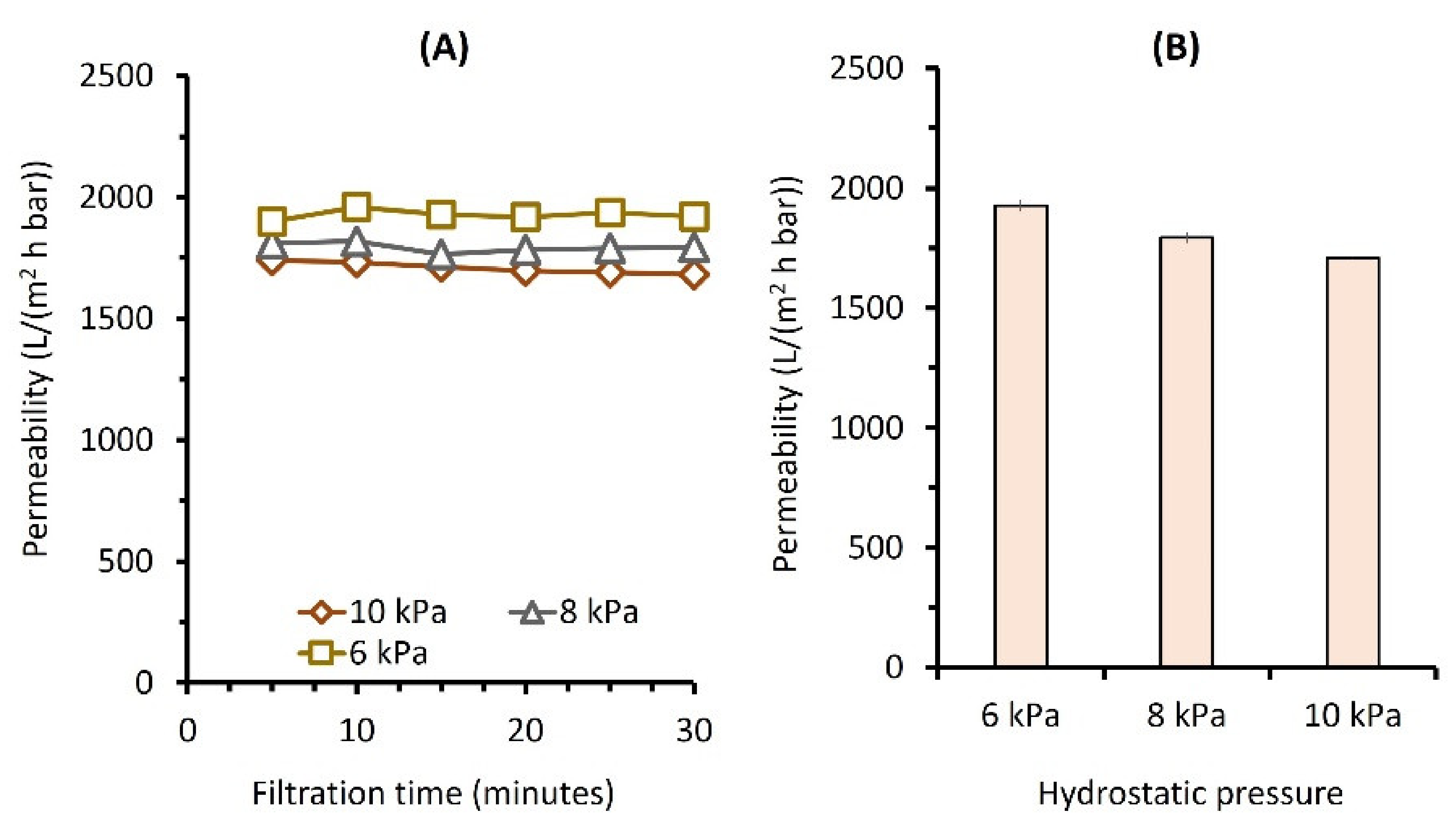
3.1. Effect of Pressure on Clean Water Permeability
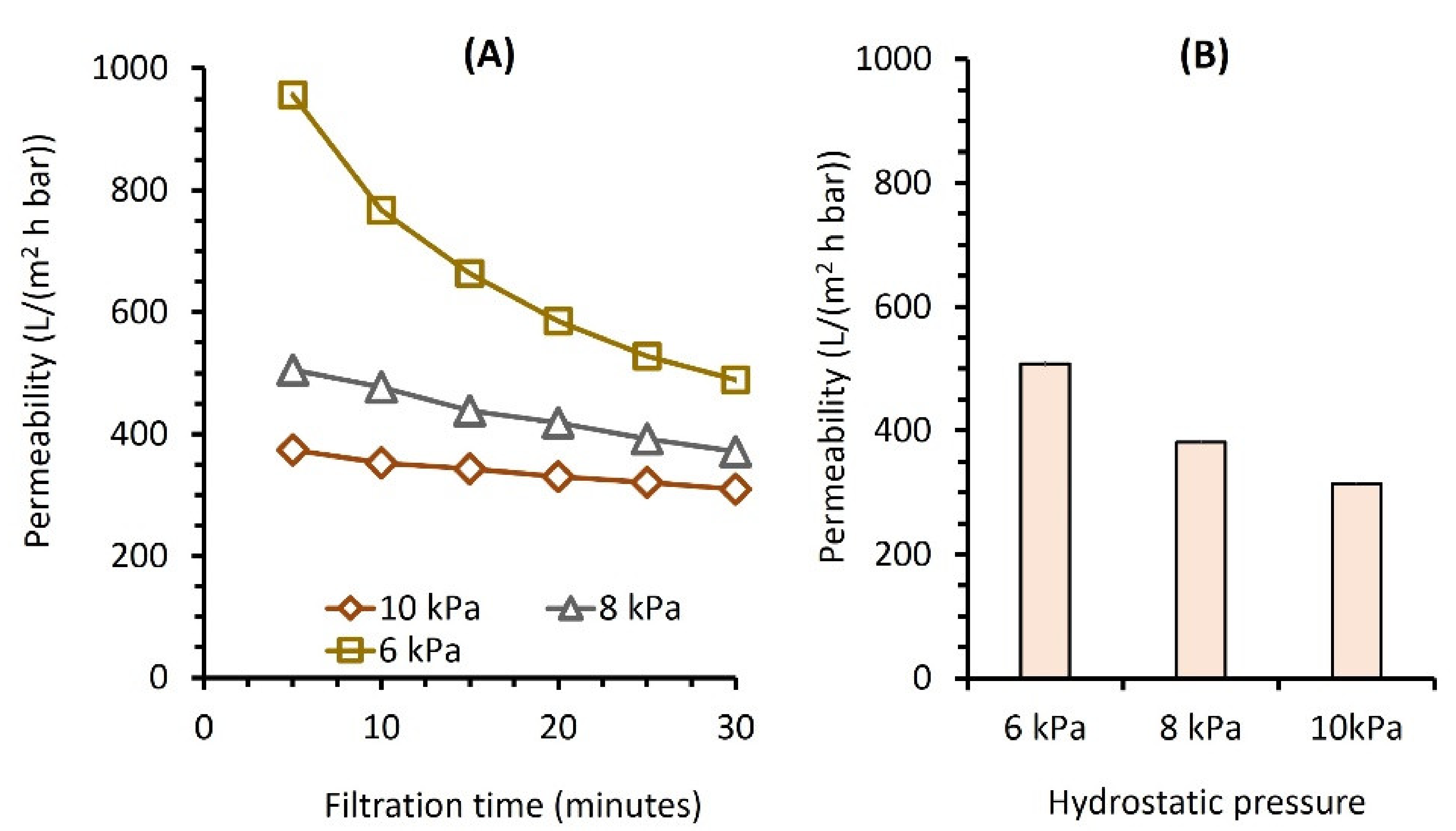
3.2. Detergent Solution and Laundry Wastewater Filtration
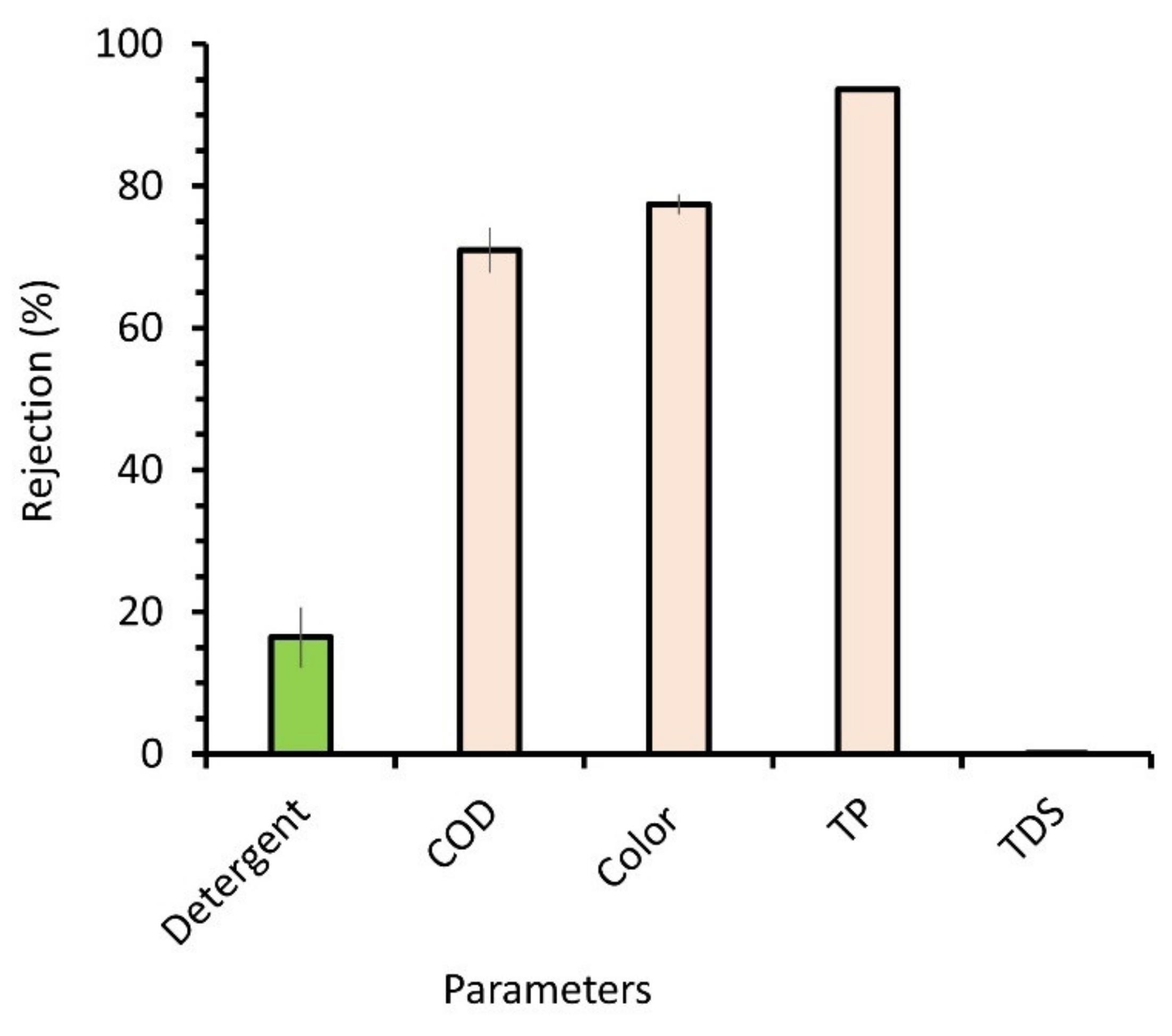
3.3. Rejection Performance and Detergent Recovery Rate
3.4. Practical Implementation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Santiago, D.E.; Rodríguez, M.J.H.; Pulido-Melián, E. Laundry Wastewater Treatment: Review and Life Cycle Assessment. J. Environ. Eng. 2021, 147, 03121001. [Google Scholar] [CrossRef]
- Ho, K.C.; Teow, Y.H.; Sum, J.Y.; Ng, Z.J.; Mohammad, A.W. Water pathways through the ages: Integrated laundry wastewater treatment for pollution prevention. Sci. Total Environ. 2020, 760, 143966. [Google Scholar] [CrossRef] [PubMed]
- Mainardi-Remis, J.M.; Gutiérrez-Cacciabue, D.; Romero, D.S.; Rajal, V.B. Setting boundaries within a bottled water plant aid to better visualize the water use: An approach through the water footprint indicator. J. Water Process Eng. 2021, 43, 102199. [Google Scholar] [CrossRef]
- Uc-Peraza, R.G.; Blas, V.H.D. Acute toxicity and risk assessment of three commercial detergents using the polychaete Capitella sp. C from Chetumal Bay, Quintana Roo, Mexico. Int. Aquat. Res. 2015, 7, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Warne, M.; Schifko, A. Toxicity of Laundry Detergent Components to a Freshwater Cladoceran and Their Contribution to Detergent Toxicity. Ecotoxicol. Environ. Saf. 1999, 44, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Ciabattia, I.; Cesaro, F.; Faralli, L.; Fatarella, E.; Tognotti, F. Demonstration of a treatment system for purification and reuse of laundry wastewater. Desalination 2009, 245, 451–459. [Google Scholar] [CrossRef]
- Manouchehri, M.; Kargari, A. Water recovery from laundry wastewater by the cross flow microfiltration process: A strategy for water recycling in residential buildings. J. Clean. Prod. 2017, 168, 227–238. [Google Scholar] [CrossRef]
- Kundu, S.; Coumar, M.V.; Rajendiran, S.; Rao, A.; Rao, A.S. Phosphates from detergents and eutrophication of surface water ecosystem in India. Curr. Sci. 2015, 108, 1320–1325. [Google Scholar]
- Verdia, P.; Gunaratne, H.Q.N.; Goh, T.Y.; Jacquemin, J.; Blesic, M. A class of efficient short-chain fluorinated catanionic surfactants. Green Chem. 2015, 18, 1234–1239. [Google Scholar] [CrossRef]
- Linclau, E.; Ceulemans, J.; De Sitter, K.; Cauwenberg, P. Water and detergent recovery from rinsing water in an industrial environment. Water Resour. Ind. 2016, 14, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Klimonda, A.; Kowalska, I. Membrane technology for the treatment of industrial wastewater containing cationic surfactants. Water Resour. Ind. 2021, 26, 100157. [Google Scholar] [CrossRef]
- Shang, X.; Kim, H.-C.; Huang, J.-H.; Dempsey, B.A. Coagulation strategies to decrease fouling and increase critical flux and contaminant removal in microfiltration of laundry wastewater. Sep. Purif. Technol. 2015, 147, 44–50. [Google Scholar] [CrossRef]
- Seo, G.; Lee, T.; Moon, B.; Lim, J. Ultrafiltration combined with ozone for domestic laundry wastewater reclamation and reuse. Water Supply 2001, 1, 387–392. [Google Scholar] [CrossRef]
- Suárez, L.; Diez, M.A.; García, R.; Riera, F.A. Membrane technology for the recovery of detergent compounds: A review. J. Ind. Eng. Chem. 2012, 18, 1859–1873. [Google Scholar] [CrossRef]
- Choobar, B.G.; Shahmirzadi, M.A.A.; Kargari, A.; Manouchehri, M. Fouling mechanism identification and analysis in microfiltration of laundry wastewater. J. Environ. Chem. Eng. 2019, 7, 103030. [Google Scholar] [CrossRef]
- Cheryan, M. Ultrafiltration and Microfiltration Handbook; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Bilad, M.R.; Nawi, N.I.M.; Subramaniam, D.D.; Shamsuddin, N.; Khan, A.L.; Jaafar, J.; Nandiyanto, A.B.D. Low-pressure submerged membrane filtration for potential reuse of detergent and water from laundry wastewater. J. Water Process Eng. 2020, 36, 101264. [Google Scholar] [CrossRef]
- Barambu, N.U.; Peter, D.; Yusoff, M.H.M.; Bilad, M.R.; Shamsuddin, N.; Marbelia, L.; Nordin, N.A.H.; Jaafar, J. Detergent and Water Recovery from Laundry Wastewater Using Tilted Panel Membrane Filtration System. Membranes 2020, 10, 260. [Google Scholar] [CrossRef]
- Nascimento, C.O.C.; Veit, M.; Palácio, S.M.; Gonçalves, G.C.; Fagundes-Klen, M.R. Combined Application of Coagulation/Flocculation/Sedimentation and Membrane Separation for the Treatment of Laundry Wastewater. Int. J. Chem. Eng. 2019, 2019, 8324710. [Google Scholar] [CrossRef] [Green Version]
- Huang, A.K.; Veit, M.T.; Juchen, P.T.; Gonçalves, G.D.C.; Palácio, S.M.; Cardoso, C.D.O. Sequential process of coagulation/flocculation/sedimentation- adsorption–microfiltration for laundry effluent treatment. J. Environ. Chem. Eng. 2019, 7, 103226. [Google Scholar] [CrossRef]
- Sumisha, A.; Arthanareeswaran, G.; Thuyavan, Y.L.; Ismail, A.; Chakraborty, S. Treatment of laundry wastewater using polyethersulfone/polyvinylpyrollidone ultrafiltration membranes. Ecotoxicol. Environ. Saf. 2015, 121, 174–179. [Google Scholar] [CrossRef]
- Šostar-Turk, S.; Petrinić, I.; Simonič, M. Conservation; Recycling. Laundry wastewater treatment using coagulation and membrane filtration. Resour. Conserv. Recycl. 2005, 44, 185–196. [Google Scholar] [CrossRef]
- Luogo, B.D.P.; Salim, T.; Zhang, W.; Hartmann, N.B.; Malpei, F.; Candelario, V.M. Reuse of Water in Laundry Applications with Micro- and Ultrafiltration Ceramic Membrane. Membranes 2022, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Pronk, W.; Ding, A.; Morgenroth, E.; Derlon, N.; Desmond, P.; Burkhardt, M.; Wu, B.; Fane, A.G. Gravity-driven membrane filtration for water and wastewater treatment: A review. Water Res. 2018, 149, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Peter-Varbanets, M.; Hammes, F.; Vital, M.; Pronk, W. Stabilization of flux during dead-end ultra-low pressure ultrafiltration. Water Res. 2010, 44, 3607–3616. [Google Scholar] [CrossRef]
- Lawson, K.W.; Hall, M.S.; Lloyd, D.R. Compaction of microporous membranes used in membrane distillation. I. Effect on gas permeability. J. Membr. Sci. 1995, 101, 99–108. [Google Scholar] [CrossRef]
- Bert, J.L. Membrane compaction: A theoretical and experimental explanation. J. Polym. Sci. Part B Polym. Lett. 1969, 7, 685–691. [Google Scholar] [CrossRef]
- Stade, S.; Kallioinen, M.; Mikkola, A.; Tuuva, T.; Mänttäri, M. Reversible and irreversible compaction of ultrafiltration membranes. Sep. Purif. Technol. 2013, 118, 127–134. [Google Scholar] [CrossRef]
- Osman, W.N.A.W.; Nawi, N.I.M.; Samsuri, S.; Bilad, M.R.; Khan, A.L.; Hunaepi, H.; Jaafar, J.; Lam, M.K. Ultra low-pressure filtration system for energy efficient microalgae filtration. Heliyon 2021, 7, e07367. [Google Scholar] [CrossRef]
- Ashfaq, M.Y.; Qiblawey, H. Laundry wastewater treatment using ultrafiltration under different operating conditions. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; p. 020002. [Google Scholar]
- Ma’ruf, A.; Al Fathoni, M.; Purnawanto, A.; Astiyani, I. Ultrafiltration of Laundry Wastewater Using Natural Zeolite-PVA Hybrid Membrane. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Chennai, India, 16–17 September 2020; p. 012042. [Google Scholar]
- Balakrishnan, M.; Dua, M.; Bhagat, J. Effect of operating parameters on sugarcane juice ultrafiltration: Results of a field experience. Sep. Purif. Technol. 2000, 19, 209–220. [Google Scholar] [CrossRef]
- Mat Nawi, N.I.; Sait, N.R.; Bilad, M.R.; Shamsuddin, N.; Jaafar, J.; Nordin, N.A.H.; Narkkun, T.; Faungnawakij, K.; Mohshim, D.F. Polyvinylidene Fluoride Membrane Via Vapour Induced Phase Separation for Oil/Water Emulsion Filtration. Polymers 2021, 13, 427. [Google Scholar] [CrossRef]
- Kim, H.-C.; Shang, X.; Huang, J.-H.; Dempsey, B.A. Treating laundry waste water: Cationic polymers for removal of contaminants and decreased fouling in microfiltration. J. Membr. Sci. 2014, 456, 167–174. [Google Scholar] [CrossRef]
- Alresheedi, M.T.; Basu, O.D. Interplay of water temperature and fouling during ceramic ultrafiltration for drinking water production. J. Environ. Chem. Eng. 2020, 8, 104354. [Google Scholar] [CrossRef]
- Gilbert, P.; De Jong, A. The Use of Phosphate in Detergents and Possible Replacements for Phosphate. Ciba Found Symp. 1977, 57, 253–268. [Google Scholar]
- Watiniasih, N.; Purnama, I.; Padmanabha, G.; Merdana, I.; Antara, I. Managing laundry wastewater. IOP Conf. Ser. Earth Environ. Sci. 2019, 248, 012084. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.C.-T.; Lee, D.-J.; Huang, C. Membrane Fouling Mitigation: Membrane Cleaning. Sep. Sci. Technol. 2010, 45, 858–872. [Google Scholar] [CrossRef]
- Giagnorio, M.; Amelio, A.; Gruttner, H.; Tiraferri, A. Environmental impacts of detergents and benefits of their recovery in the laundering industry. J. Clean. Prod. 2017, 154, 593–601. [Google Scholar] [CrossRef]


| System/Source of Wastewater | Purpose of Study | Pressure (kPa) | Permeability (Lm−2h−1bar−1) | Removal (%) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| COD | Color | TDSs | NTU | TP | |||||
| UF/residential laundry | Detergent and water recovery | Vacuum of <10 | 150–297 | 52 | - | - | 97.9 | 65.3 | [17] |
| UF/residential laundry | Detergent and water recovery | 10 | 100–200 | 57 | - | - | 77.0 | 30.0 | [18] |
| MF/residential laundry | Water recycle | 50 | 18.72 | 5.5 | - | 2.5 | 98.4 | - | [7] |
| Physico-chemical pre-treatment, sand filtration, ozonation, GAC filtration and UF/domestic laundry | Water reuse | - | 84.0 | 87.0 | - | - | 99.0 | - | [6] |
| Combined coagulation, flocculation, sedimentation, and MF or UF/industrial laundry | Wastewater treatment | 140 | 12.5–92.2 | 68.8 | 98.4 | 55.0 | 99.1 | - | [19] |
| Combined coagulation, flocculation, sedimentation, adsorption, MF/industrial laundry | Wastewater treatment | 140 | 43.2 | 80.0 | 99.9 | - | 99.4 | - | [20] |
| UF/laundry center | Wastewater treatment | 100 600 | 25.0 11.04 | 88.0 | - | 82.0 | 98.0 | - | [21] |
| Coagulation + MF/industrial laundry | Wastewater treatment | 70 | 160–450 | 65.0 | - | - | 100.0 | - | [12] |
| UF/hospital laundry | Effluent treatment | 300–500 | 30–50 | 53.6 | - | - | - | 95.4 | [22] |
| RO/hospital laundry | Effluent treatment | 300–500 | 7.4–12.3 | 98.9 | - | - | - | 98.6 | |
| Ozone + UF/domestic laundry | Water recycle | 40 | 25.0 | 95.0 | - | - | - | - | [13] |
| MF ceramic membrane/domestic laundry | Water recycle | 300 | 30.0 | 80.0 | - | - | 95.0 | - | [23] |
| UF ceramic membrane/domestic laundry | Water recycle | 300 | 16.7 | 83.8 | - | - | 99.5 | - | |
| UF/domestic laundry | Detergent and water recovery | 6 | 500.4 | 71.0 | 78.0 | - | - | 93.6 | This study |
| Parameters | Unit | Value | Analytical Method |
|---|---|---|---|
| pH | - | 7.9 | SNI 06-6989.11 |
| Detergent | (mg/L) | 2.56 | SNI 06-6989.51 |
| COD 1 | (mg/L) | 1060 | SNI 6989.2 |
| Color | (Pt.Co) | 229.8 | SNI 06-6989.80 |
| TP 2 | (mg/L) | 1.271 | SNI 06-6989.31 |
| TDSs 3 | (mg/L) | 692.6 | APHA 2540 C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khery, Y.; Daniar, S.E.; Mat Nawi, N.I.; Bilad, M.R.; Wibisono, Y.; Nufida, B.A.; Ahmadi, A.; Jaafar, J.; Huda, N.; Kobun, R. Ultra-Low-Pressure Membrane Filtration for Simultaneous Recovery of Detergent and Water from Laundry Wastewater. Membranes 2022, 12, 591. https://doi.org/10.3390/membranes12060591
Khery Y, Daniar SE, Mat Nawi NI, Bilad MR, Wibisono Y, Nufida BA, Ahmadi A, Jaafar J, Huda N, Kobun R. Ultra-Low-Pressure Membrane Filtration for Simultaneous Recovery of Detergent and Water from Laundry Wastewater. Membranes. 2022; 12(6):591. https://doi.org/10.3390/membranes12060591
Chicago/Turabian StyleKhery, Yusran, Sonia Ely Daniar, Normi Izati Mat Nawi, Muhammad Roil Bilad, Yusuf Wibisono, Baiq Asma Nufida, Ahmadi Ahmadi, Juhana Jaafar, Nurul Huda, and Rovina Kobun. 2022. "Ultra-Low-Pressure Membrane Filtration for Simultaneous Recovery of Detergent and Water from Laundry Wastewater" Membranes 12, no. 6: 591. https://doi.org/10.3390/membranes12060591
APA StyleKhery, Y., Daniar, S. E., Mat Nawi, N. I., Bilad, M. R., Wibisono, Y., Nufida, B. A., Ahmadi, A., Jaafar, J., Huda, N., & Kobun, R. (2022). Ultra-Low-Pressure Membrane Filtration for Simultaneous Recovery of Detergent and Water from Laundry Wastewater. Membranes, 12(6), 591. https://doi.org/10.3390/membranes12060591











