Chemical and Physical Ionic Liquids in CO2 Capture System Using Membrane Vacuum Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
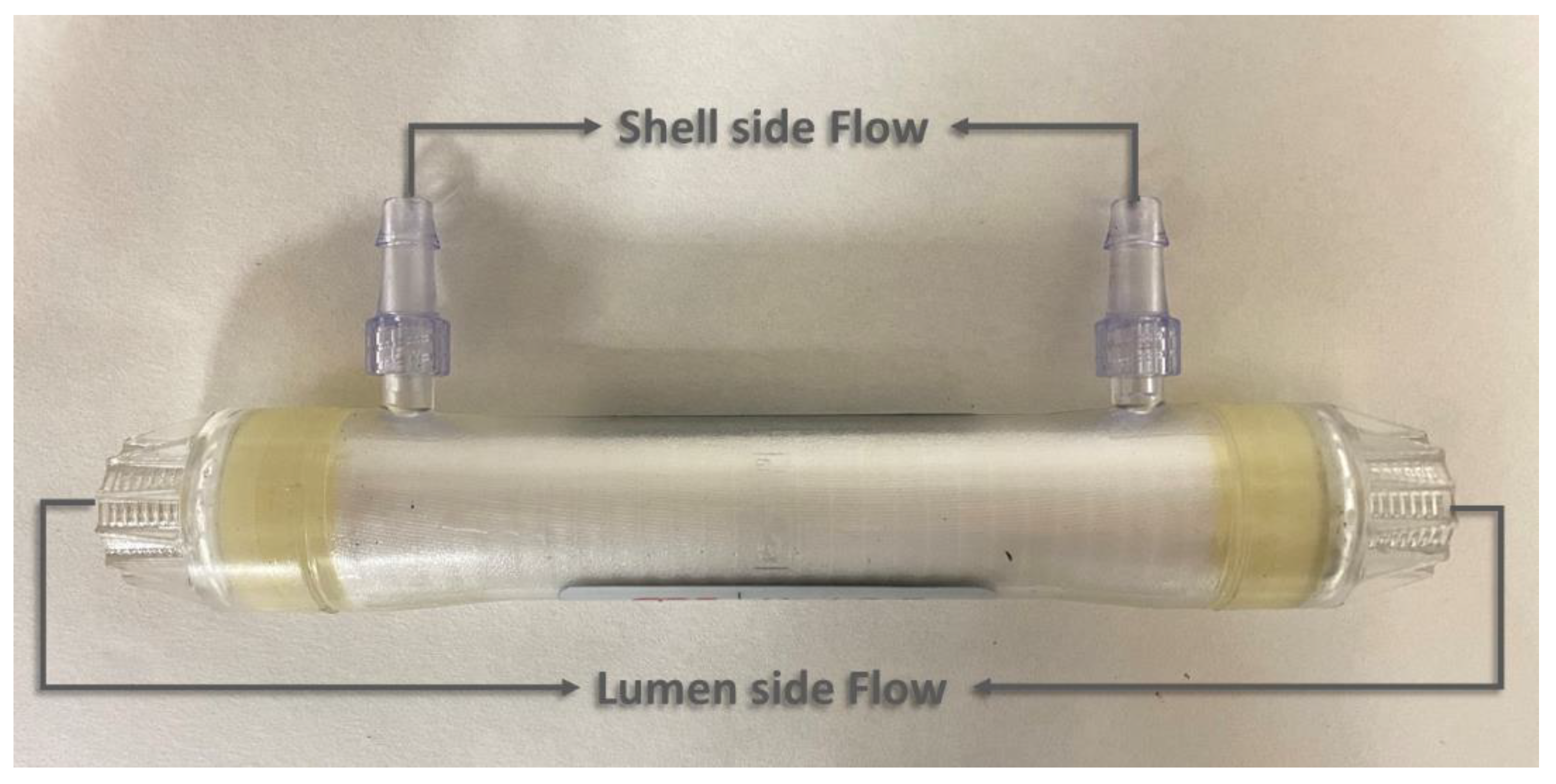
2.2. Membrane Contactor
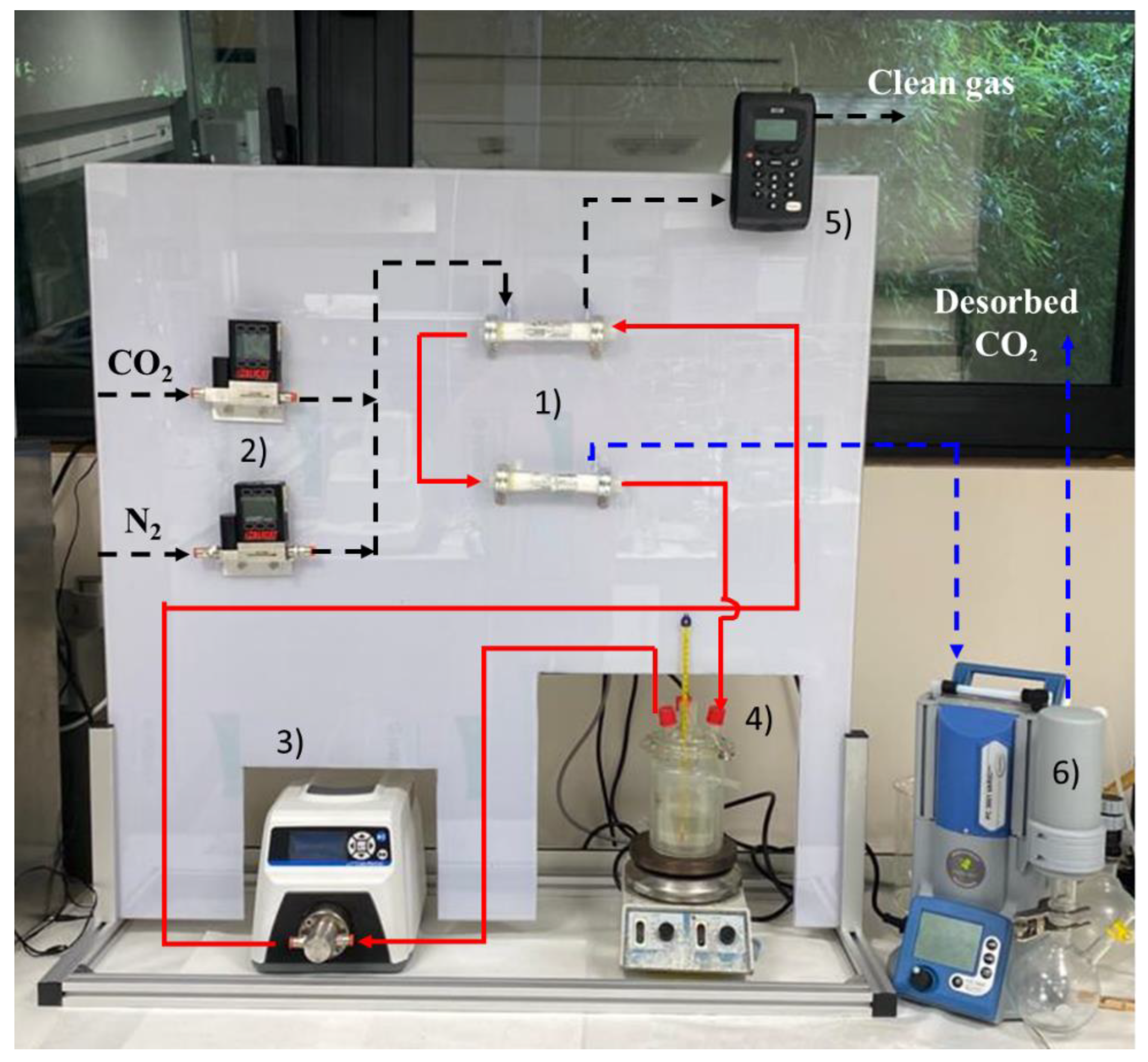
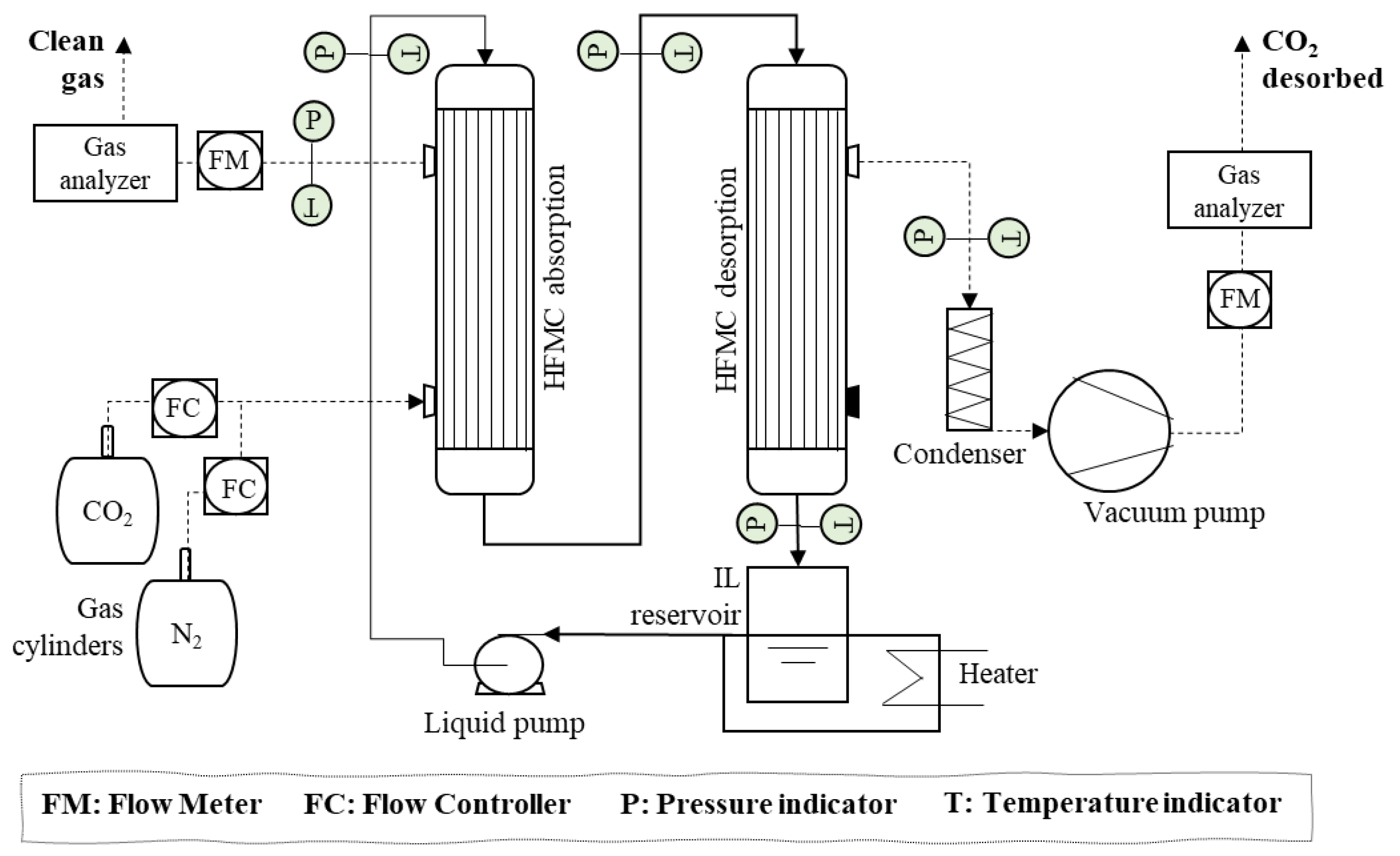
2.3. Experimental Set-Up
- (1)
- Two hollow fiber membrane contactors that can operate interconnected, for both the non-dispersive absorption of the CO2 from the feed gas and the CO2 desorption by applying vacuum.
- (2)
- Two mass flow controllers (Alicat ScientificTM, Duiven, The Netherlands MC-gas mass flow controller Tucson, AZ, USA) to control the flows coming from the pure gas cylinders (CO2 and N2) to set the concentration and flow of the feed gas.
- (3)
- A digital gear pump (Cole-Parmer Gear Pump SystemTM Vernon Hills, IL, USA, Mount Prospect, Vernon Hills, IL, USA, Benchtop Digital Drive, 0.017 mL·rev−1, 220 VAC, Saint Louis, MO, USA) to drive and maintain constant liquid flow during the continuous absorption–desorption process.
- (4)
- A closed vessel of tempered borosilicate glass (PyrexTM, Paris, France) to contain and keep constant the temperature of the IL by means of a heater-stirrer.
- (5)
- Two gas analyzers (GeotechTM, G110 0-100%, Suffolk, UK) to measure the mass flow rate and CO2 concentration of the gas streams (feed gas, clean gas, and desorbed CO2 output). The analyzer is based on non-dispersive infrared spectroscopy (NDIR). The CO2 concentration in the output gas stream was monitored using the NGA Win-Control software.
- (6)
- A vacuum pump, with condenser included (VacuubrandTM, PC 3001 VARIO PRO, Wertheim, Germany), to set the gas phase of the membrane contactor (used for CO2 desorption) at the desired vacuum pressure.
2.4. Data Analysis
- (1)
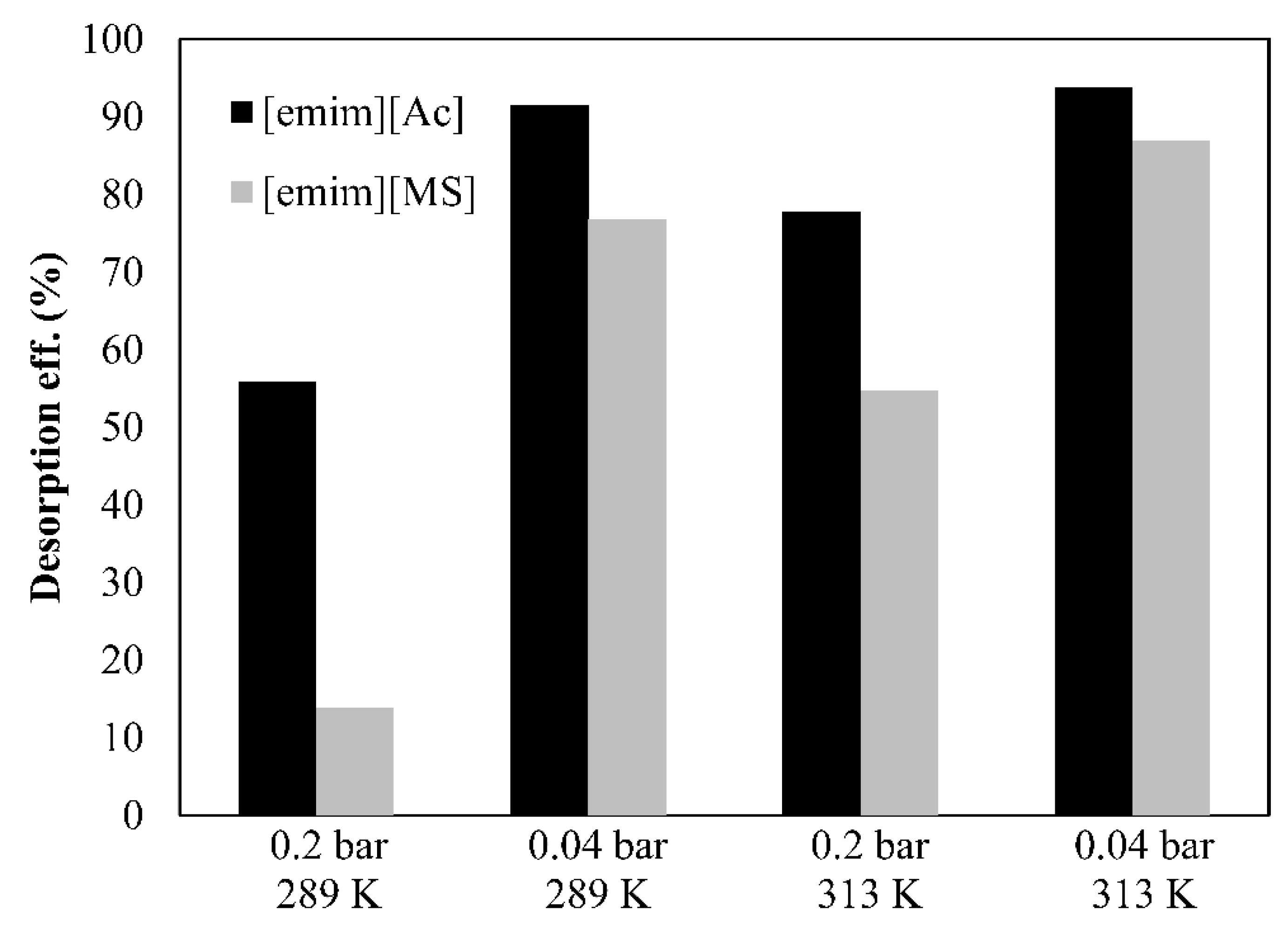
- The CO2 desorption efficiency is calculated by Equation (1), where αrich and αlean are the CO2 loading in the IL (molCO2·mol_IL−1) before and after one pass of IL through the HFMC desorber, respectively.
- (2)
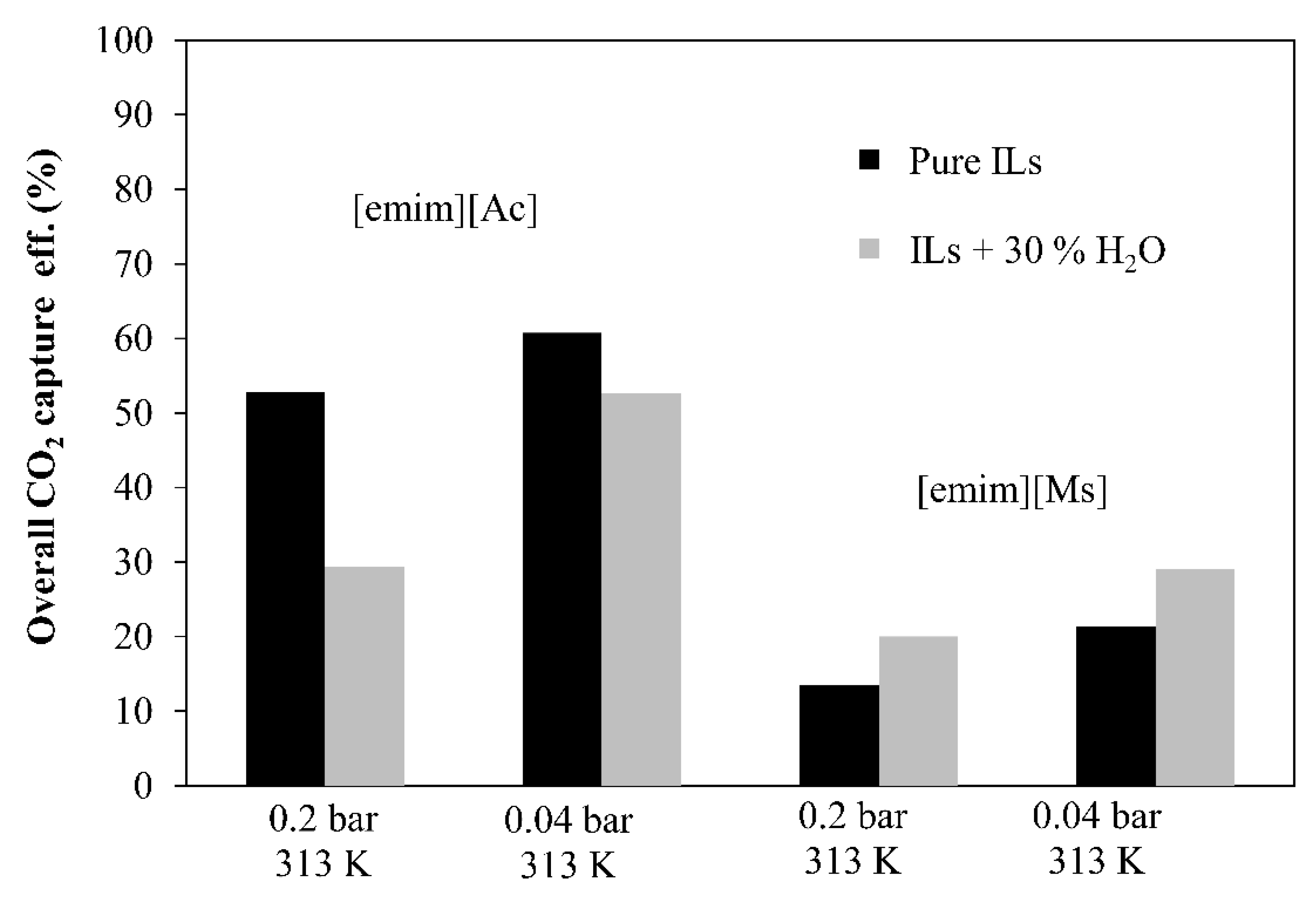
- The overall CO2 capture efficiency, which is defined as the concentration difference in the HFMC absorber between the feed gas and the clean gas, is obtained by Equation (2), where C(CO2,g)in (mol CO2·L−1 gas) is the CO2 concentration in the feed gas and C(CO2,g)out (mol CO2·L−1 gas) is the CO2 concentration at the outlet of the module. The overall CO2 capture efficiency is important in order to study the influence of the MVR technology on the continuous absorption–desorption process.
- (3)
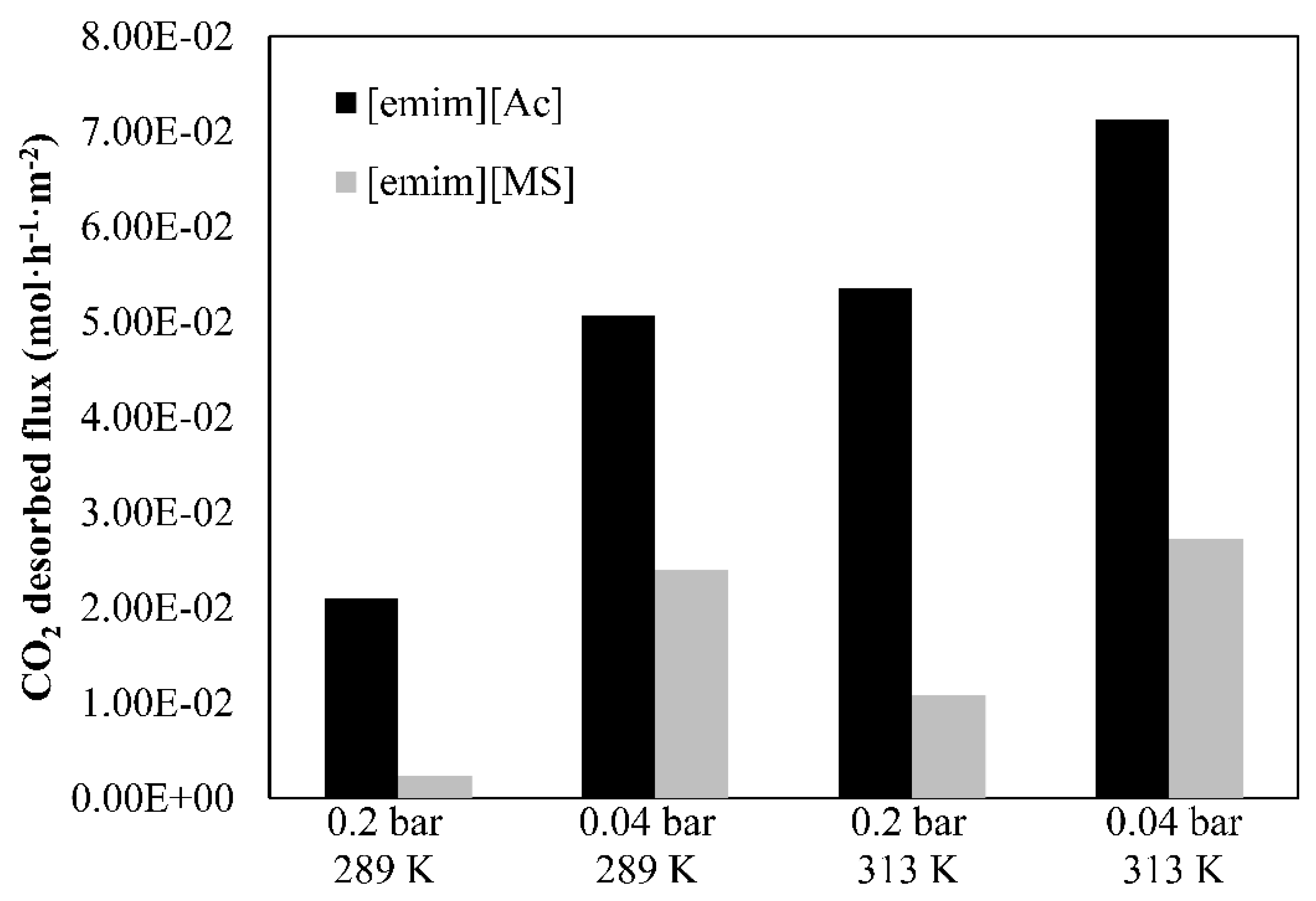
- The CO2 desorbed flux (GV, mol·h−1 m−2) is estimated by Equation (3), where FV is the CO2 flow rate desorbed from the HFMC desorber measured on the vacuum pump output (L·h−1), vm is the molar volume of CO2 in ideal gas conditions (L·molCO2-1), and A is the specific membrane area (m2).
3. Results and Discussion
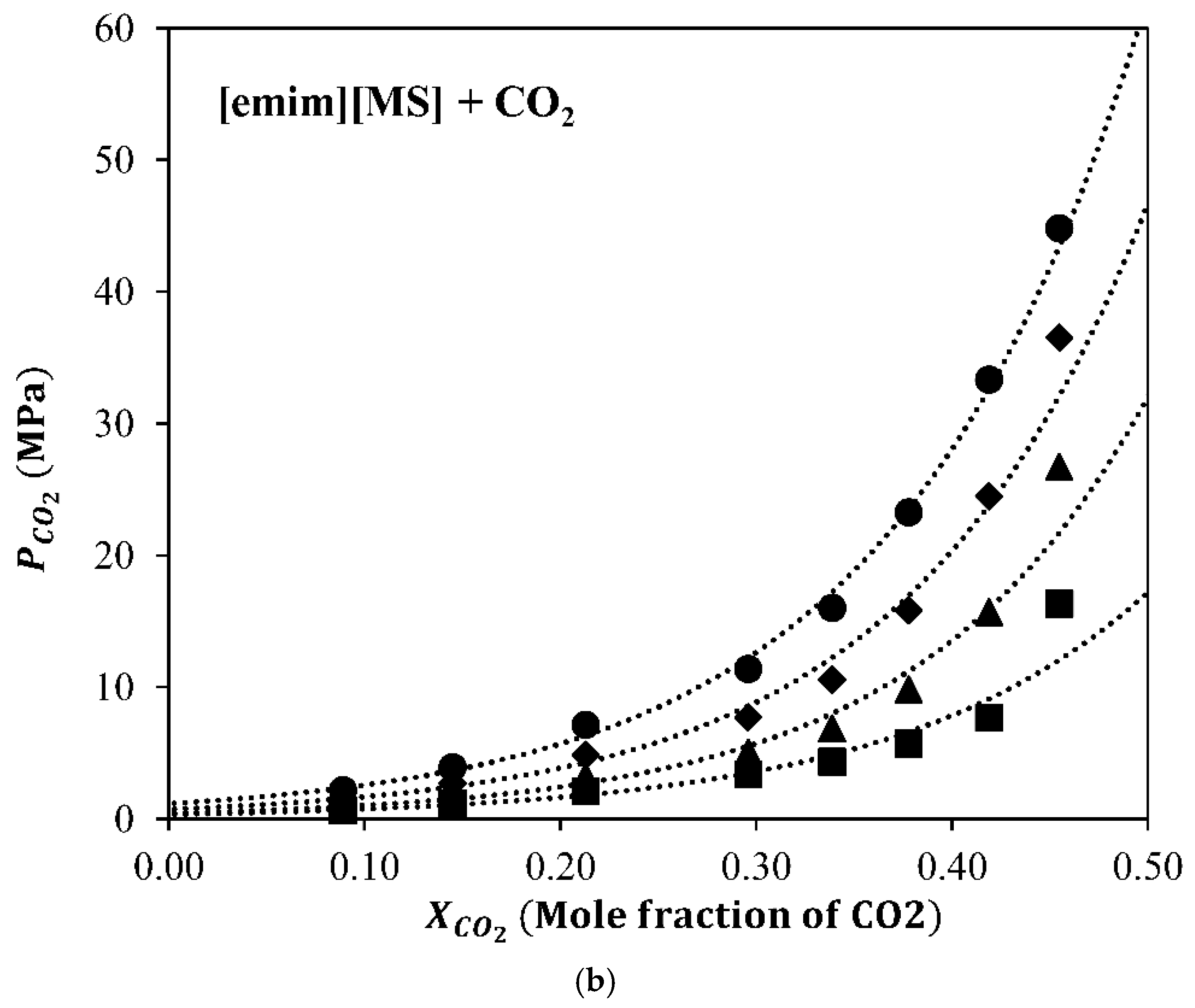
3.1. Absorption Properties
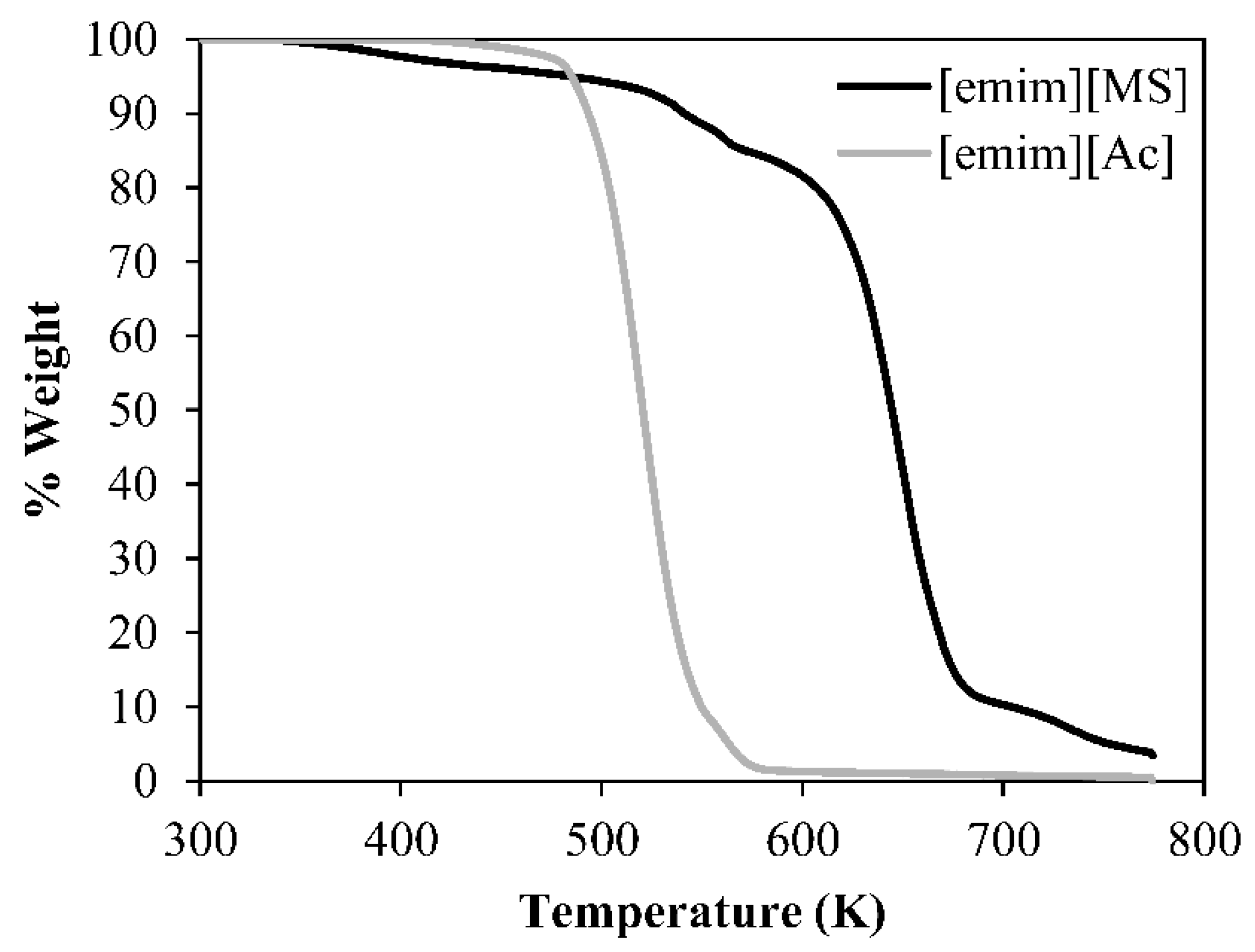
3.2. ILs Characterization
3.3. Parametric Study of Desorption Process
3.4. ILs Comparison in the Overall CO2 Capture
3.5. Influence of IL Water Content in the Process Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsiropoulos, I.; Nijs, W.; Tarvydas, D.; Ruiz Castello, P. Towards Net-Zero Emissions in the EU Energy System by 2050, EUR 29981 EN; Publications Office of the European Union: Luxembourg, 2020; ISBN 978-92-76-13097-0. [Google Scholar]
- Rúa, J.; Bui, M.; Nord, L.O.; Mac Dowell, N. Does CCS Reduce Power Generation Flexibility? A Dynamic Study of Combined Cycles with Post-Combustion CO2 Capture. Int. J. Greenh. Gas Control 2020, 95, 102984. [Google Scholar] [CrossRef]
- Díaz-Sainz, G.; Alvarez-Guerra, M.; Solla-Gullón, J.; García-Cruz, L.; Montiel, V.; Irabien, A. Catalyst Coated Membrane Electrodes for the Gas Phase CO2 Electroreduction to Formate. Catal. Today 2020, 346, 58–64. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, M.K.; Park, J.H. Decompression Stripping of Carbon Dioxide from Rich Monoethanolamine through Porous Hydrophobic Modified Ceramic Hollow Fiber Membrane Contactor. Sep. Purif. Technol. 2020, 236, 116304. [Google Scholar] [CrossRef]
- Lau, H.S.; Lau, S.K.; Soh, L.S.; Hong, S.U.; Gok, X.Y.; Yi, S.; Yong, W.F. State-of-the-Art Organicand Inorganic-Based Hollow Fiber Membranes in Liquid and Gas Applications: Looking Back and Beyond. Membranes 2022, 12, 539. [Google Scholar] [CrossRef]
- Intan Listiyana, N.; Rahmawati, Y.; Nurkhamidah, S.; Rofiq Syahnur, H.; Zaelana, Y. CO2 Desorption from Activated DEA Using Membrane Contactor with Vacuum Regeneration Technology. In Proceedings of the 24th Regional Symposium on Chemical Engineering (RSCE 2017), Semarang, Indonesia, 15–16 November 2007; Volume 156. [Google Scholar] [CrossRef]
- He, Q.; Xi, J.; Wang, W.; Meng, L.; Yan, S. CO2 Absorption Using Biogas Slurry: Recovery of Absorption Performance through CO2 Vacuum Regeneration. Int. J. Greenh. Gas Control 2017, 58, 103–113. [Google Scholar] [CrossRef]
- Vadillo, J.M.; Gomez-Coma, L.; Garea, A.; Irabien, A. Hollow Fiber Membrane Contactors in CO2 Desorption: A Review. Energy Fuels 2021, 35, 111–136. [Google Scholar] [CrossRef]
- Albarracin Zaidiza, D.; Belaissaoui, B.; Rode, S.; Favre, E. Intensification Potential of Hollow Fiber Membrane Contactors for CO2 Chemical Absorption and Stripping Using Monoethanolamine Solutions. Sep. Purif. Technol. 2017, 188, 38–51. [Google Scholar] [CrossRef]
- Vadillo, J.M.; Gómez-coma, L.; Garea, A.; Irabien, A. CO2 Desorption Performance from Imidazolium Ionic Liquids by Membrane Vacuum Regeneration Technology. Membranes 2020, 10, 234. [Google Scholar] [CrossRef]
- Kosaraju, P.; Kovvali, A.S.; Korikov, A.; Sirkar, K.K. Hollow Fiber Membrane Contactor Based CO2 Absorption-Stripping Using Novel Solvents and Membranes. Ind. Eng. Chem. Res. 2005, 44, 1250–1258. [Google Scholar] [CrossRef]
- Fang, M.; Wang, Z.; Yan, S.; Cen, Q.; Luo, Z. CO2 Desorption from Rich Alkanolamine Solution by Using Membrane Vacuum Regeneration Technology. Int. J. Greenh. Gas Control 2012, 9, 507–521. [Google Scholar] [CrossRef]
- Yan, S.; Fang, M.; Wang, Z.; Luo, Z. Regeneration Performance of CO2-Rich Solvents by Using Membrane Vacuum Regeneration Technology: Relationships between Absorbent Structure and Regeneration Efficiency. Appl. Energy 2012, 98, 357–367. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, M.; Pan, Y.; Yan, S.; Luo, Z. Amine-Based Absorbents Selection for CO2 Membrane Vacuum Regeneration Technology by Combined Absorption-Desorption Analysis. Chem. Eng. Sci. 2013, 93, 238–249. [Google Scholar] [CrossRef]
- Nii, S.; Iwata, Y.; Takahashi, K.; Takeuchi, H. Regeneration of CO2-Loaded Carbonate Solution by Reducing Pressure. J. Chem. Eng. Jpn. 1995, 28, 148–153. [Google Scholar] [CrossRef]
- Rosli, A.; Shoparwe, N.F.; Ahmad, A.L.; Low, S.C.; Lim, J.K. Dynamic Modelling and Experimental Validation of CO2 Removal Using Hydrophobic Membrane Contactor with Different Types of Absorbent. Sep. Purif. Technol. 2019, 219, 230–240. [Google Scholar] [CrossRef]
- Yan, X.; Anguille, S.; Bendahan, M.; Moulin, P. Ionic Liquids Combined with Membrane Separation Processes: A Review. Sep. Purif. Technol. 2019, 222, 230–253. [Google Scholar] [CrossRef]
- Carvalho, P.J.; Kurnia, K.A.; Coutinho, J.A.P. Dispelling Some Myths about the CO2 Solubility in Ionic Liquids. Phys. Chem. Chem. Phys. 2016, 18, 14757–14771. [Google Scholar] [CrossRef]
- Sohaib, Q.; Vadillo, J.M.; Gómez-Coma, L.; Albo, J.; Druon-Bocquet, S.; Irabien, A.; Sanchez-Marcano, J. Post-Combustion CO2 Capture by Coupling [Emim] Cation Based Ionic Liquids with a Membrane Contactor; Pseudo-Steady-State Approach. Int. J. Greenh. Gas Control 2020, 99, 103076. [Google Scholar] [CrossRef]
- Mulukutla, T.; Obuskovic, G.; Sirkar, K.K. Novel Scrubbing System for Post-Combustion CO2 Capture and Recovery: Experimental Studies. J. Memb. Sci. 2014, 471, 16–26. [Google Scholar] [CrossRef]
- Bazhenov, S.; Malakhov, A.; Bakhtin, D.; Khotimskiy, V.; Bondarenko, G.; Volkov, V.; Ramdin, M.; Vlugt, T.J.H.; Volkov, A. CO2 Stripping from Ionic Liquid at Elevated Pressures in Gas-Liquid Membrane Contactor. Int. J. Greenh. Gas Control 2018, 71, 293–302. [Google Scholar] [CrossRef]
- Lu, J.G.; Lu, Z.Y.; Chen, Y.; Wang, J.T.; Gao, L.; Gao, X.; Tang, Y.Q.; Liu, D.G. CO2 Absorption into Aqueous Blends of Ionic Liquid and Amine in a Membrane Contactor. Sep. Purif. Technol. 2015, 150, 278–285. [Google Scholar] [CrossRef]
- Simons, T.J.; Hield, P.; Pas, S.J. A Novel Experimental Apparatus for the Study of Low Temperature Regeneration CO2 Capture Solvents Using Hollow Fibre Membrane Contactors. Int. J. Greenh. Gas Control 2018, 78, 228–235. [Google Scholar] [CrossRef]
- Hospital-Benito, D.; Lemus, J.; Moya, C.; Santiago, R.; Palomar, J. Process Analysis Overview of Ionic Liquids on CO2 Chemical Capture. Chem. Eng. J. 2020, 390, 124509. [Google Scholar] [CrossRef]
- Zhao, S.; Feron, P.H.M.; Deng, L.; Favre, E.; Chabanon, E.; Yan, S.; Hou, J.; Chen, V.; Qi, H. Status and Progress of Membrane Contactors in Post-Combustion Carbon Capture: A State-of-the-Art Review of New Developments. J. Memb. Sci. 2016, 511, 180–206. [Google Scholar] [CrossRef]
- Ramdin, M.; De Loos, T.W.; Vlugt, T.J.H. State-of-the-Art of CO2 Capture with Ionic Liquids. Ind. Eng. Chem. Res. 2012, 51, 8149–8177. [Google Scholar] [CrossRef]
- Shiflett, M.B.; Elliott, B.A.; Lustig, S.R.; Sabesan, S.; Kelkar, M.S.; Yokozeki, A. Phase Behavior of CO2 in Room-Temperature Ionic Liquid 1-Ethyl-3-Ethylimidazolium Acetate. ChemPhysChem 2012, 13, 1806–1817. [Google Scholar] [CrossRef] [PubMed]
- Yim, J.H.; Ha, S.J.; Lim, J.S. Measurement and Correlation of CO2 Solubility in 1-Ethyl-3-Methylimidazolium ([EMIM]) Cation-Based Ionic Liquids: [EMIM][Ac], [EMIM][Cl], and [EMIM][MeSO4]. J. Chem. Eng. Data 2018, 63, 508–518. [Google Scholar] [CrossRef]
- Zhou, L.; Shang, X.; Fan, J.; Wang, J. Solubility and Selectivity of CO2 in Ether-Functionalized Imidazolium Ionic Liquids. J. Chem. Thermodyn. 2016, 103, 292–298. [Google Scholar] [CrossRef]
- Freire, M.G.; Teles, A.R.R.; Rocha, M.A.A.; Schröder, B.; Neves, C.M.S.S.; Carvalho, P.J.; Evtuguin, D.V.; Santos, L.M.N.B.F.; Coutinho, J.A.P. Thermophysical characterization of ionic liquids able to dissolve biomass. J. Chem. Eng. Data 2011, 56, 4813–4822. [Google Scholar] [CrossRef]
- Fröba, A.P.; Kremer, H.; Leipertz, A. Density, refractive index, interfacial tension, and viscosity of ionic liquids [EMIM][EtSO4], [EMIM][NTf2], [EMIM][N(CN)2], and [OMA][NTf2] in dependence on temperature at atmospheric pressure. J. Phys. Chem. B 2008, 112, 12420–12430. [Google Scholar] [CrossRef]
- Vadillo, J.M.; Hospital-benito, D.; Moya, C.; Gomez-, L.; Palomar, J.; Garea, A.; Irabien, A. Modelling and Simulation of Hollow Fiber Membrane Vacuum Regeneration for CO2 Desorption Processes Using Ionic Liquids. Sep. Purif. Technol. 2021, 277, 119465. [Google Scholar] [CrossRef]
- Qazi, S.; Manuel Vadillo, J.; Gómez-Coma, L.; Albo, J.; Druon-Bocquet, S.; Irabien, A.; Sanchez-Marcano, J. CO2 Capture with Room Temperature Ionic Liquids; Coupled Absorption/Desorption and Single Module Absorption in Membrane Contactor. Chem. Eng. Sci. 2020, 223, 115719. [Google Scholar] [CrossRef]
- Lu, J.G.; Lu, C.T.; Chen, Y.; Gao, L.; Zhao, X.; Zhang, H.; Xu, Z.W. CO2 Capture by Membrane Absorption Coupling Process: Application of Ionic Liquids. Appl. Energy 2014, 115, 573–581. [Google Scholar] [CrossRef]
- Gomez-Coma, L.; Garea, A.; Irabien, A. Carbon Dioxide Capture by [Emim][Ac] Ionic Liquid in a Polysulfone Hollow Fiber Membrane Contactor. Int. J. Greenh. Gas Control 2016, 52, 401–409. [Google Scholar] [CrossRef]


| Abbreviation | Molecular Formula | Chemical Structure |
|---|---|---|
| 1-ethyl-3-methylimidazolium acetate [emim][Ac] | C7H14N2O4S | 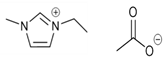 |
| 1-ethyl-3-methylimidazolium methyl sulfate [emim][MS] | C7H14N2O4S | 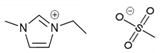 |
| Parameter | Value |
|---|---|
| Membrane Material | Polypropylene |
| Module configuration | Parallel |
| Module i.d., dcont (m) | 25 × 10−3 |
| Fiber outside diameter, do (m) | 3 × 10−4 |
| Fiber inside diameter, di (m) | 22 × 10−5 |
| Fiber length, L (m) | 0.115 |
| Number of fibers, n | 2300 |
| Effective inner membrane area, A (m2) | 0.180 |
| Membrane thickness, δ (m) | 4 × 10−5 |
| Membrane pore diameter, dp (m) | 4 × 10−8 |
| Porosity, ς (%) | 40 |
| Packing factor, φ | 0.390 |
| Tortuosity, τ | 2.500 |
| Parameter/Property | Value | Unit |
|---|---|---|
| Volume, V | 250 | mL |
| Temperature, T | 289–310 | K |
| Feed Gas flow rate, Fg | 60 | mL·min−1 |
| Liquid flow rate, Fl | 60 | mL·min−1 |
| Feed gas pressure, Pg,in | 1.03 | bar |
| Liquid pressure, Pl,in | 1.31 | bar |
| Vacuum pressure, Pv | 0.04–0.50 | bar |
| Property | [emim][Ac] | [emim][MS] |
|---|---|---|
| Viscosity, pure ILs, cP | 138 | 48 |
| Viscosity, ILs + 30% H2O, cP | 12.1 | 5.8 |
| Measured contact angle (°) | 114.5 | 110.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vadillo, J.M.; Díaz-Sainz, G.; Gómez-Coma, L.; Garea, A.; Irabien, A. Chemical and Physical Ionic Liquids in CO2 Capture System Using Membrane Vacuum Regeneration. Membranes 2022, 12, 785. https://doi.org/10.3390/membranes12080785
Vadillo JM, Díaz-Sainz G, Gómez-Coma L, Garea A, Irabien A. Chemical and Physical Ionic Liquids in CO2 Capture System Using Membrane Vacuum Regeneration. Membranes. 2022; 12(8):785. https://doi.org/10.3390/membranes12080785
Chicago/Turabian StyleVadillo, José Manuel, Guillermo Díaz-Sainz, Lucía Gómez-Coma, Aurora Garea, and Angel Irabien. 2022. "Chemical and Physical Ionic Liquids in CO2 Capture System Using Membrane Vacuum Regeneration" Membranes 12, no. 8: 785. https://doi.org/10.3390/membranes12080785
APA StyleVadillo, J. M., Díaz-Sainz, G., Gómez-Coma, L., Garea, A., & Irabien, A. (2022). Chemical and Physical Ionic Liquids in CO2 Capture System Using Membrane Vacuum Regeneration. Membranes, 12(8), 785. https://doi.org/10.3390/membranes12080785








