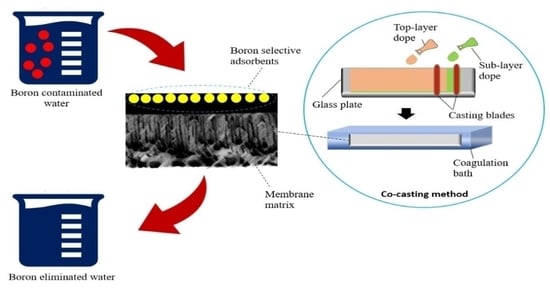
Adsorptive Membrane for Boron Removal: Challenges and Future Prospects
Abstract
1. Introduction
2. Boron Study
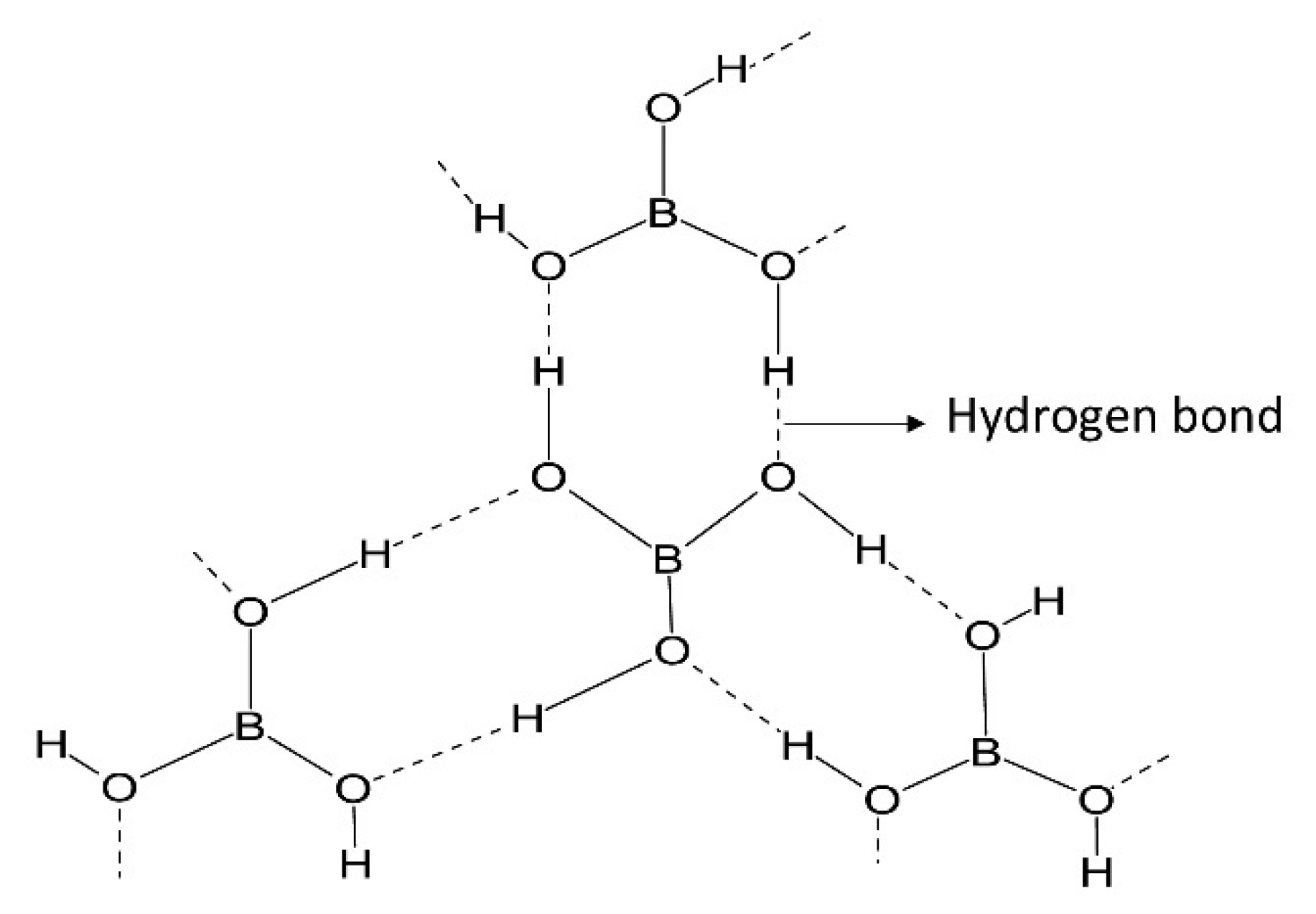
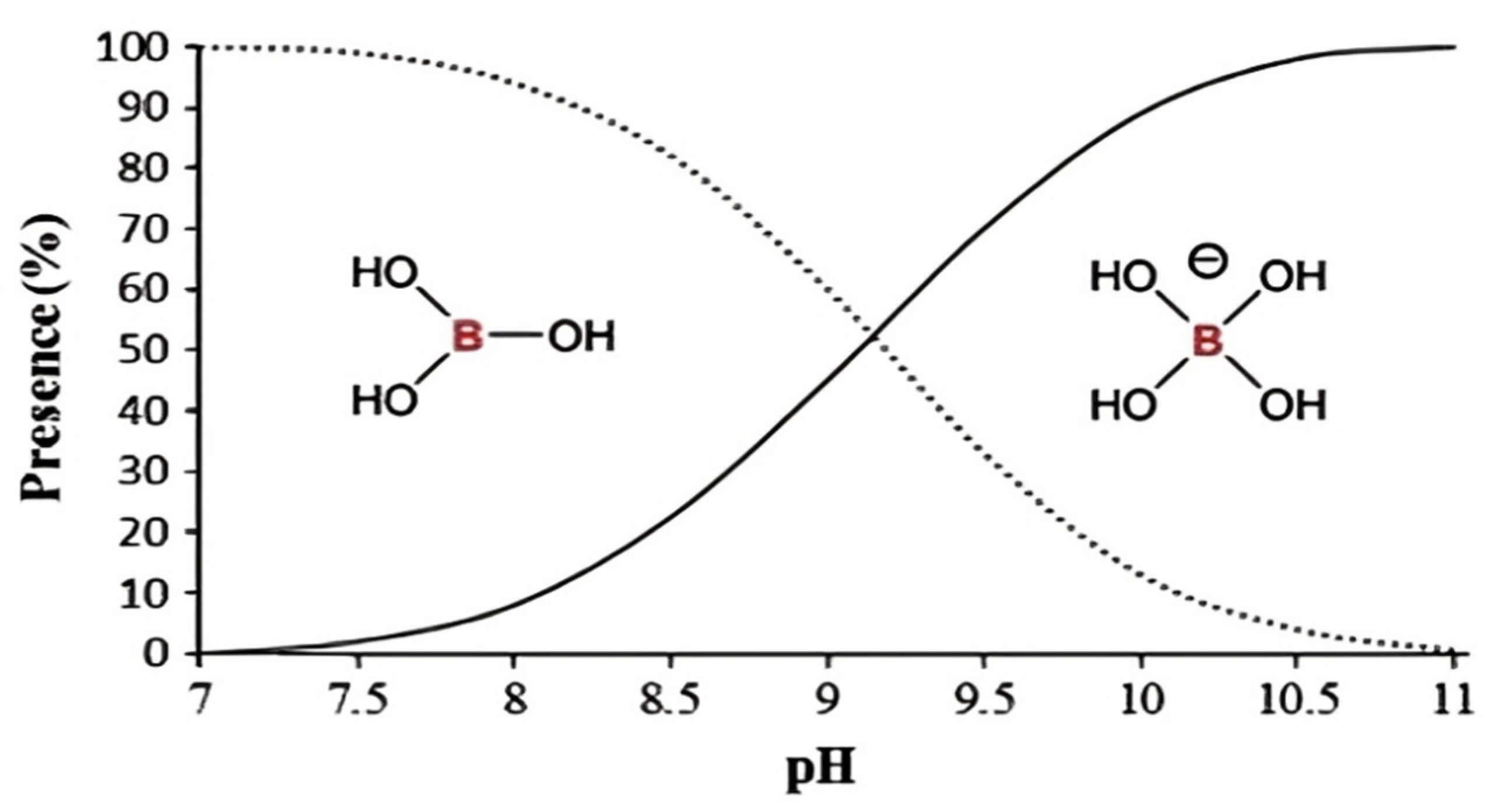
2.1. Chemistry of Boron
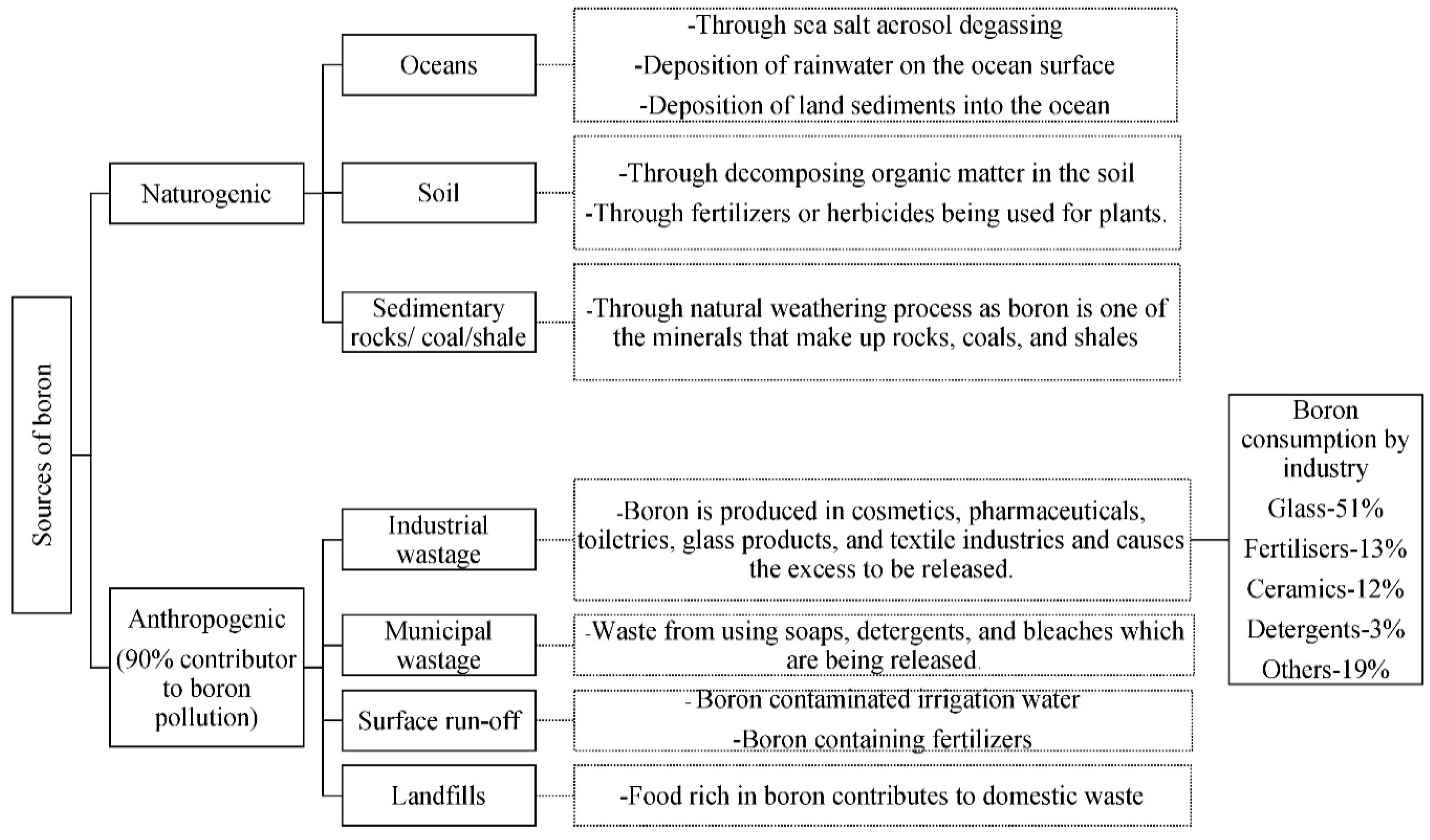
2.2. Sources of Boron
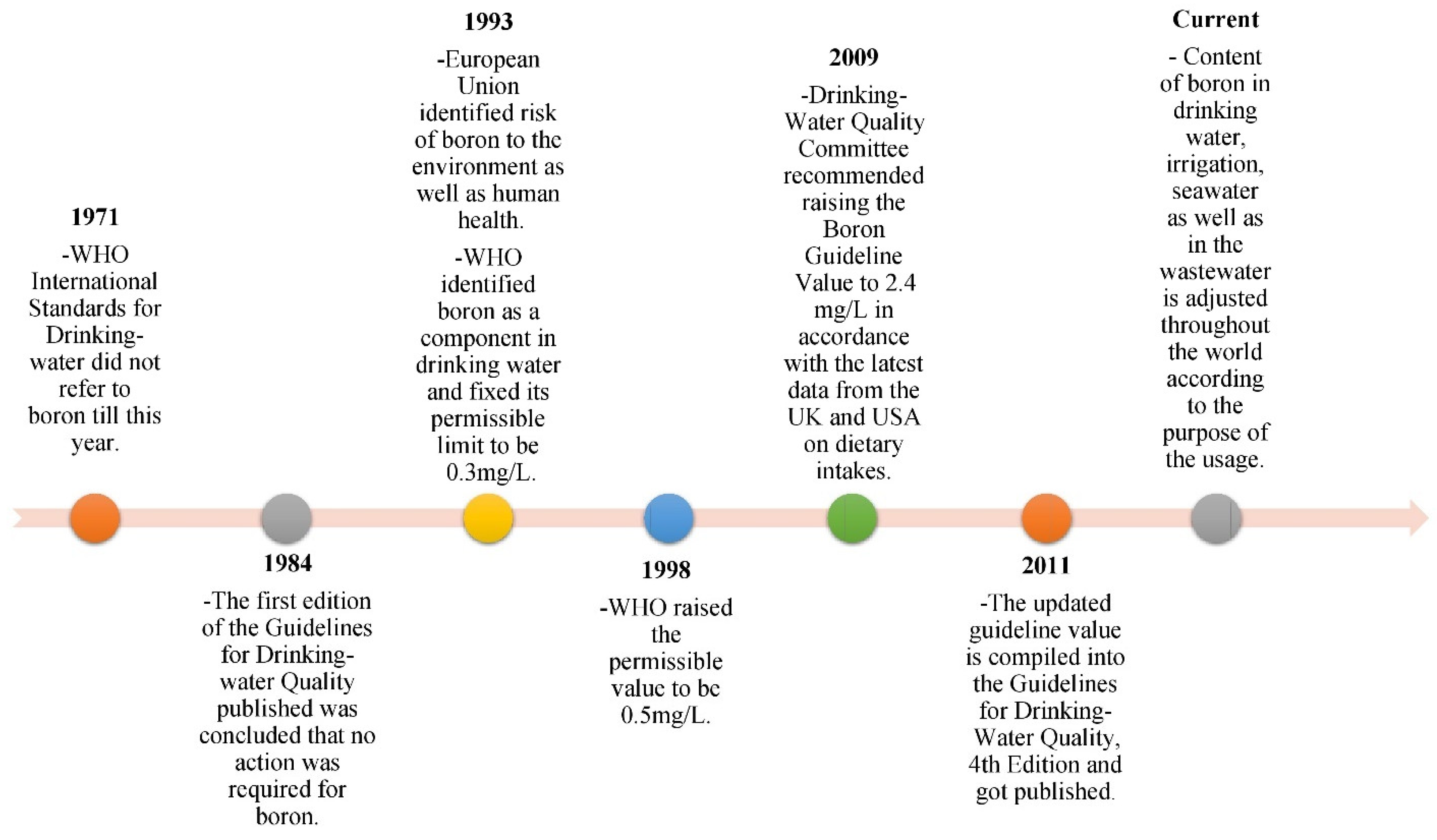
2.3. Toxicity of Boron
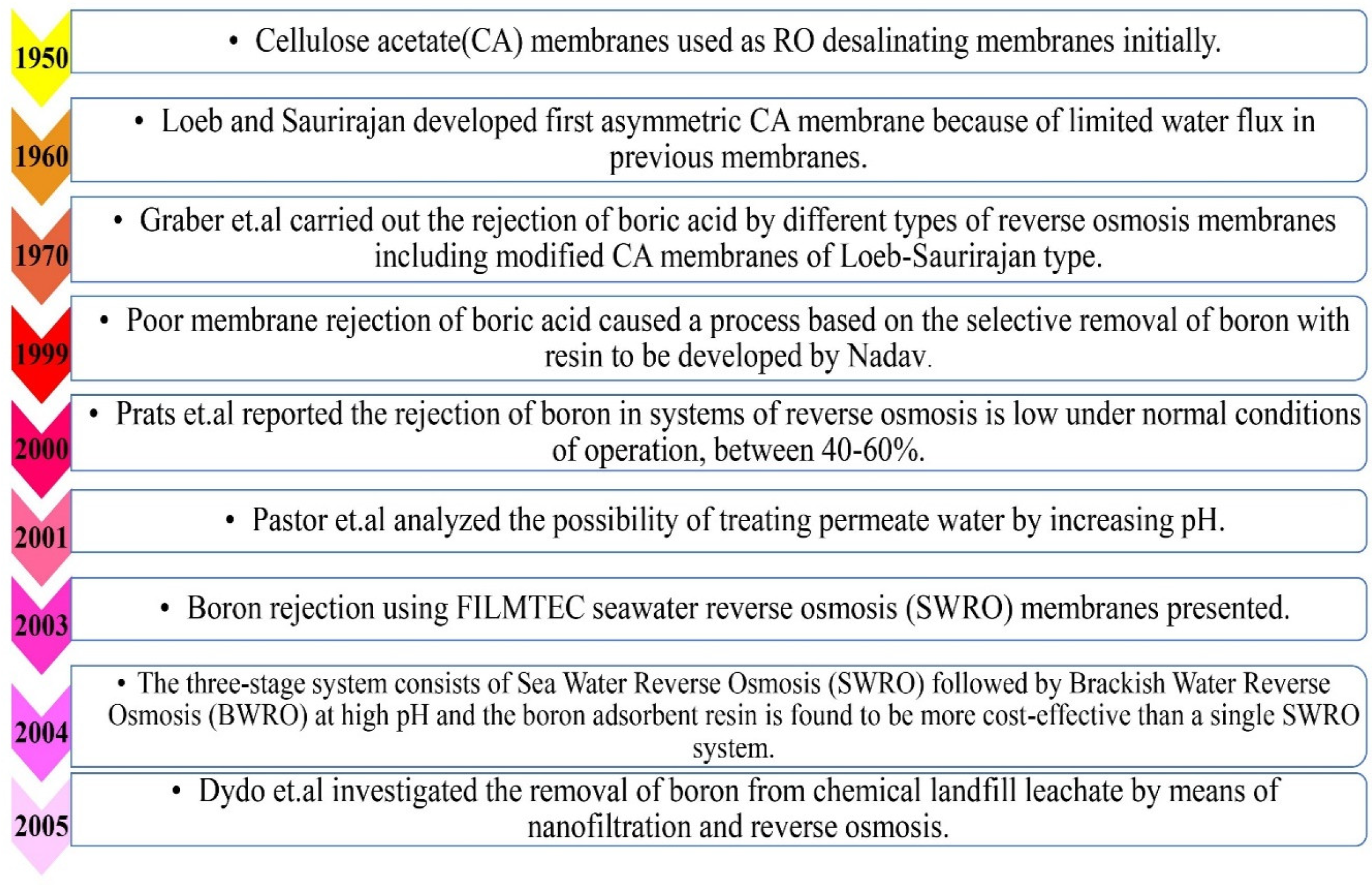
2.4. Limitations of Available Conventional Boron-Removing Applications
| Conventional Boron Technology | Process | Advantages | Disadvantages |
|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3. Adsorptive Membrane Technology
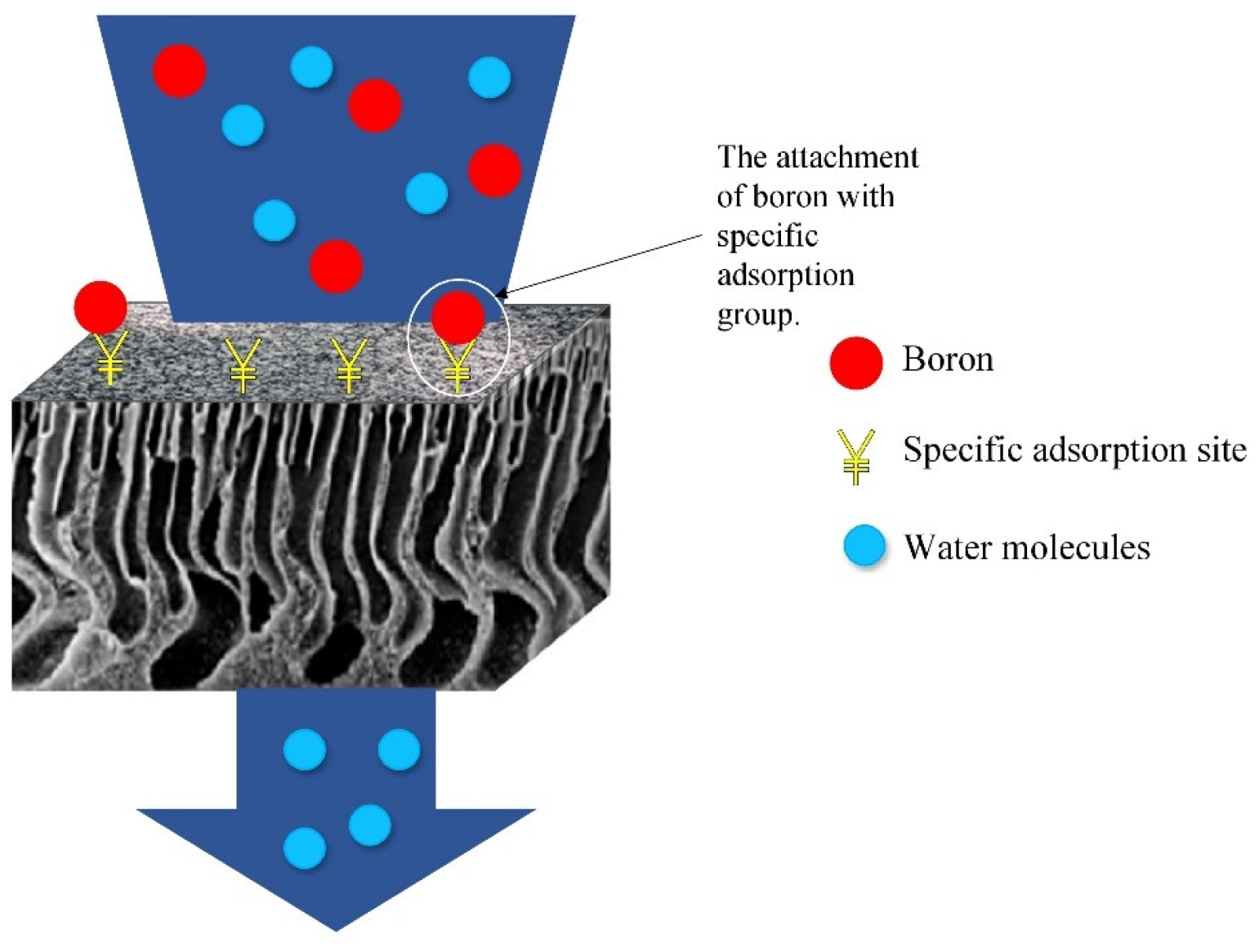
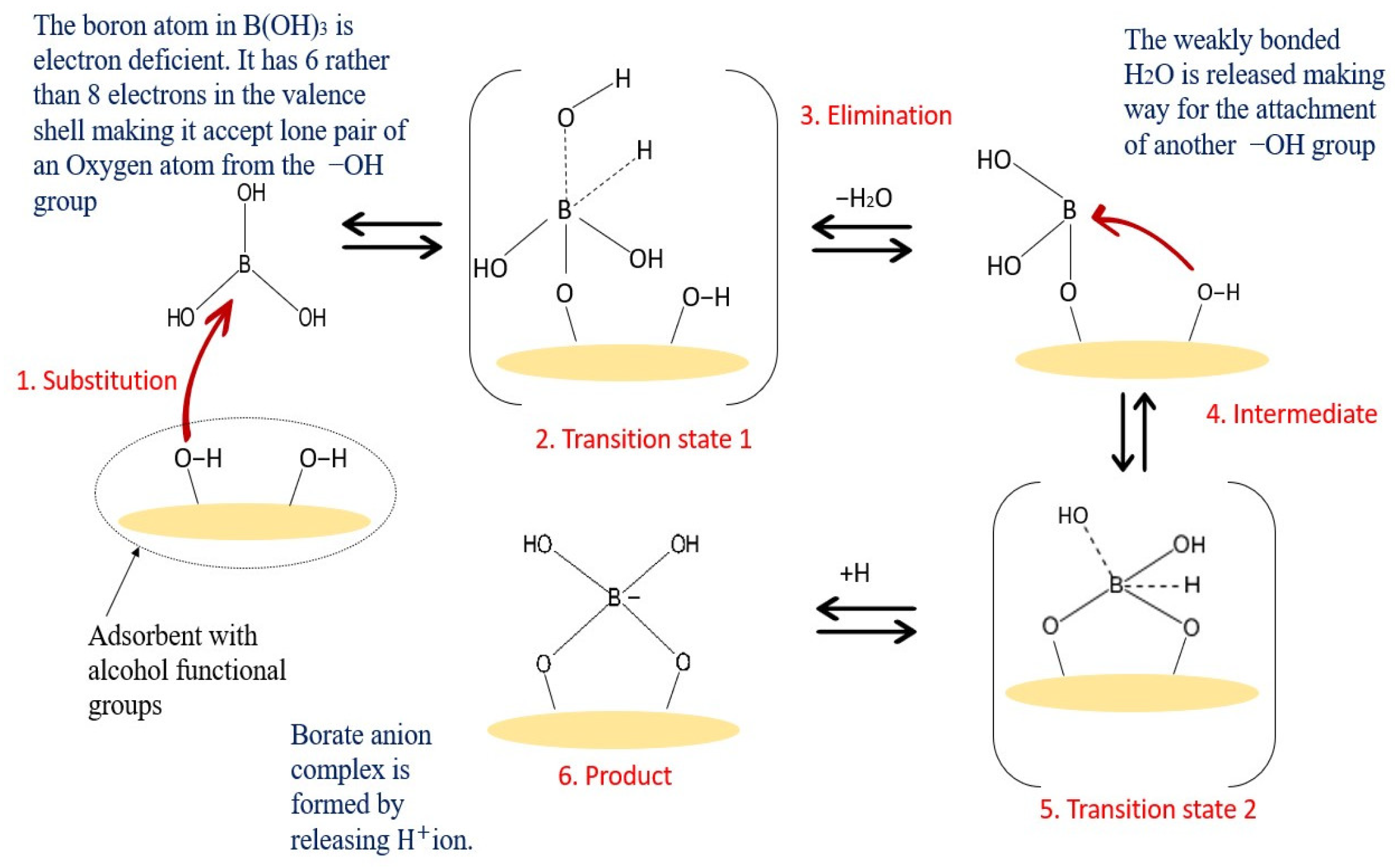
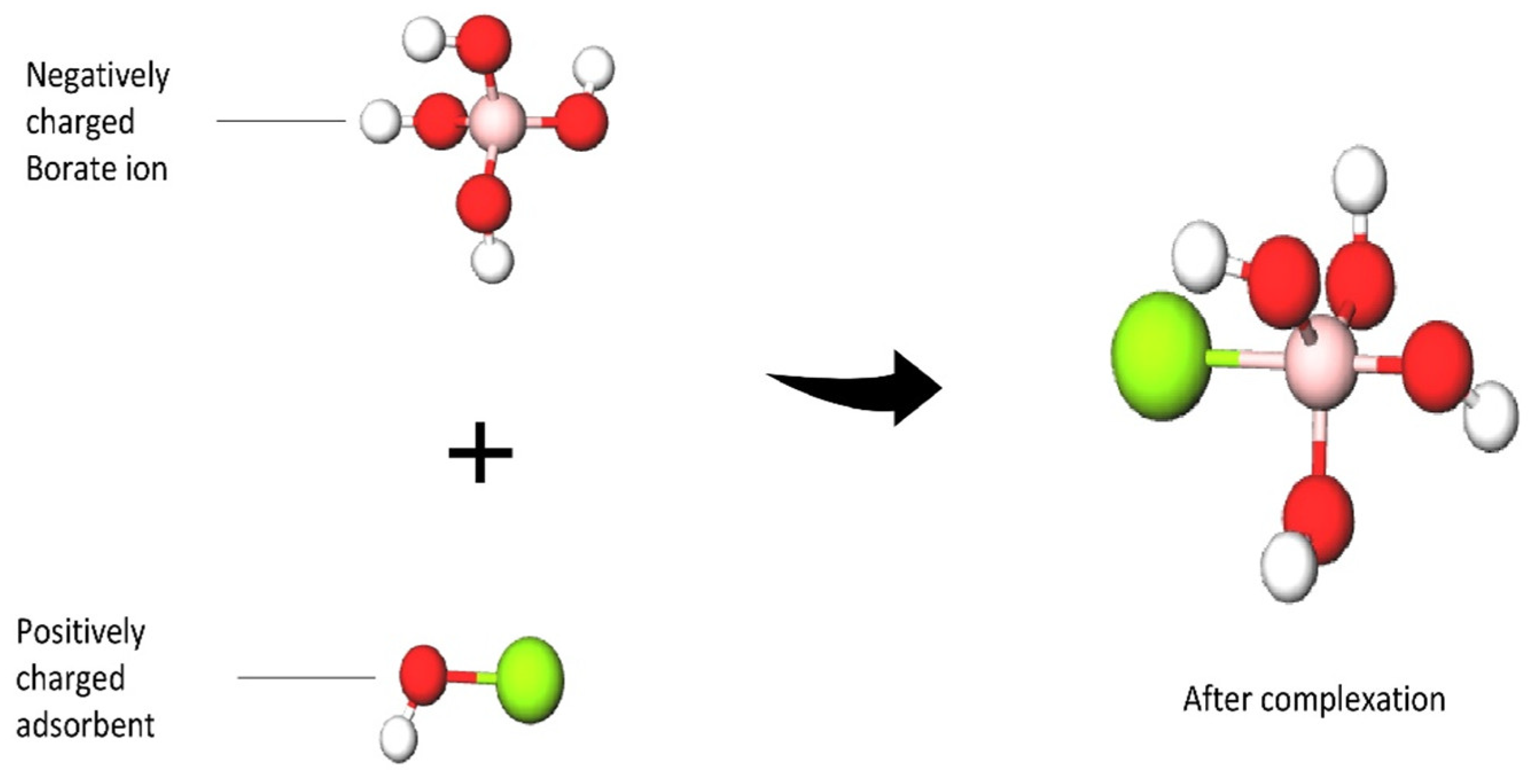
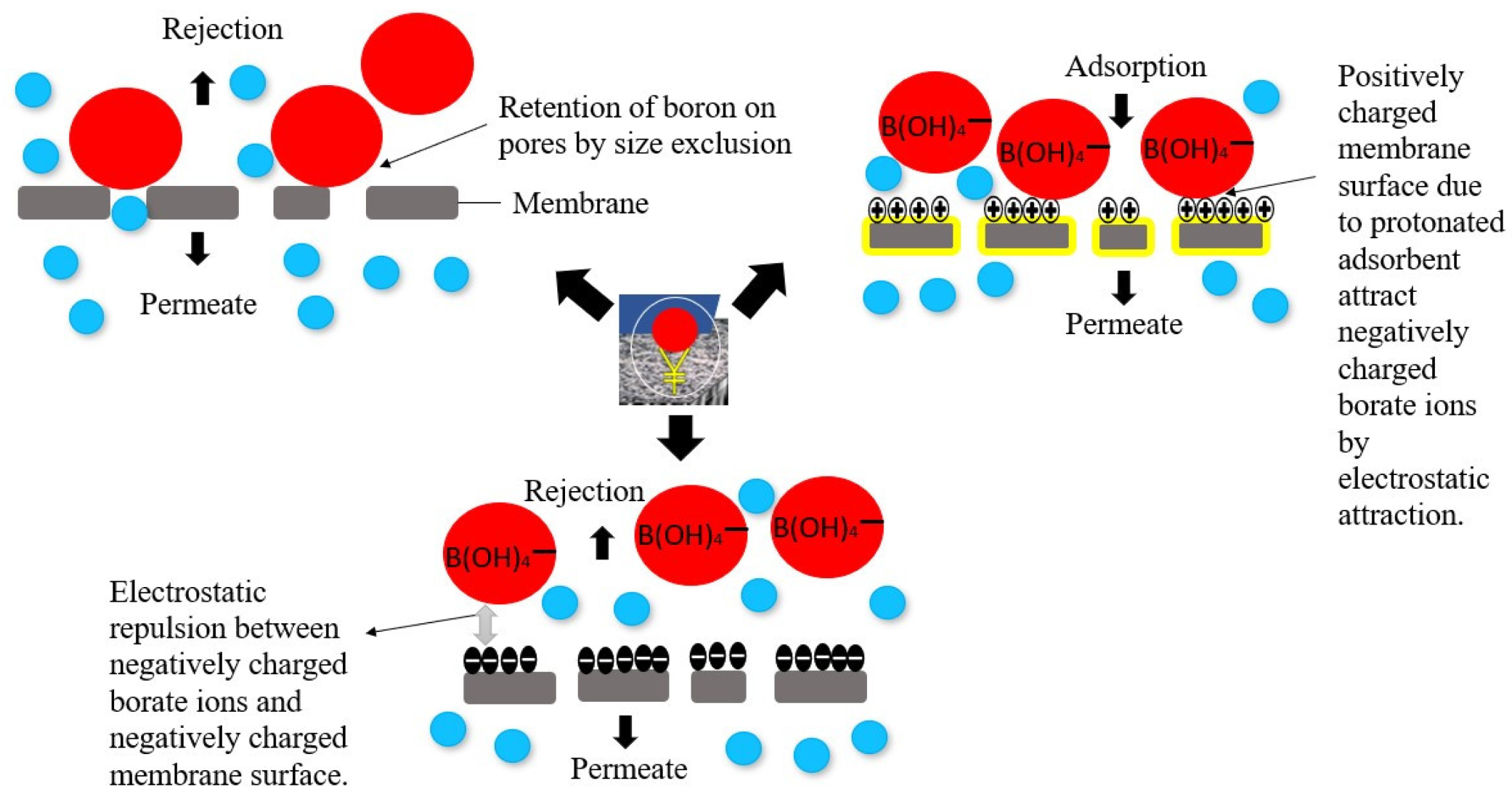
3.1. The Mechanism of the Boron Adsorptive Membrane
3.1.1. Selectivity
3.1.2. Hydrophilicity and Permeability
3.1.3. Mechanical Strength
3.2. Effective Operating Parameters Responsible for Boron Adsorption by an Adsorptive Membrane
3.2.1. Effect of pH
3.2.2. Effect of Temperature
3.2.3. Effect of Contact Time
3.2.4. Effect of Adsorbent Concentration
3.2.5. Effect of Initial Feed Solute Concentration
3.3. Types of Adsorptions Associated with Boron Removal
3.4. Commercial/Published Work for Boron Removal by Adsorptive Membranes
| Adsorptive Membrane | Membrane Configuration | Source | Initial Boron Concentration (mg/L) | Adsorption Capacity (mg/L/mmol/g) | Boron Removal (%) | Water Flux | Reference |
|---|---|---|---|---|---|---|---|
| N-methyl-D-glucamine-functionalized adsorptive membrane | Hollow fiber | Saline geothermal water | 11.0 mg/L | 0.52 mg/L | 95.3 | - | [120] |
| 3-amino-1,2-propanediol (APD) and tobramycin (TOB)-grafted commercial SW30XLE RO membrane | Flat sheet | Seawater | - | - | 92.2 | 33.4% improved flux | [110] |
| Polysulfones-grafted polyol polymers | Flat sheet | - | 5 mg/L | - | [121] | ||
| 0.20 mmol/g | 70 L/m2 h | |||||
| 0.44 mmol/g | 90 L/m2 h | |||||
| 0.46 mmol/g | 80 L/m2 h | |||||
| Hybrid PVDF-PVP membranes with nano TiO2 as an additive | Hollow fiber | Leachate | 8.2 mg/L | 0.43 mg/L | 94.75 | 223 L/m2 h | [123] |
| Charge aggregate-induced RO membrane-4,4′-(1,2-ethanediyldiimino)bis(benzenesulfonic acid) (EDBSA) with trimesoyl chloride (TMC) on a poly(ether sulfone) (PES) substrate | Flat sheet | Seawater | 5 mg/L | 0.5 mg/L | 90.6 | 8.5 L/m2 h | [124] |
| Thin-film nanocomposite RO membrane with UiO-66 nanoparticles | Flat sheet | Brackish water | 5 mg/L | - | 99.08 | 56.83 L/m2 h | [84] |
| Seawater | 5 mg/L | - | 99.27 | 61.32 L/m2 h | [84] | ||
| Graphene oxide-modified porous P84 co-polyimide membranes | Flat sheet | - | 100 mg/L | - | 76.6 | - | [129] |
| Polyol-functional polysulfone membranes | Flat sheet | - | 300 mg/L | 1.61 mmol/g | - | 45 L/m2 h | [130] |
| Hyperbranched-polyol-tethered poly(amic acid)electrospun nanofiber membrane | Hollow fiber | - | 5 mg/L | 5.68 mmol/g | - | - | [94] |
| Thin-film composite with a phosphonic acid derivative of TiO2 | Flat sheet | Seawater | 5 mg/L | - | 96 | 38 L/m2 h | [131] |
| Polysulfone membrane with an amphilic graft glycopolymer | Flat sheet | - | 300 mg/L | 0.193 mmol/g | - | 475 L/m2 h | [132] |
| Varied Polytetrafluoroethylene (PTFE) micro powder-added optimized PVDF nanofiber-based membrane distillation | Hollow fiber | Geothermal water | 60.84 mg B/L | 0.5 mg B/L | - | 27.7 kg/m2 h | [133] |
4. Single-Layered Adsorptive Membranes
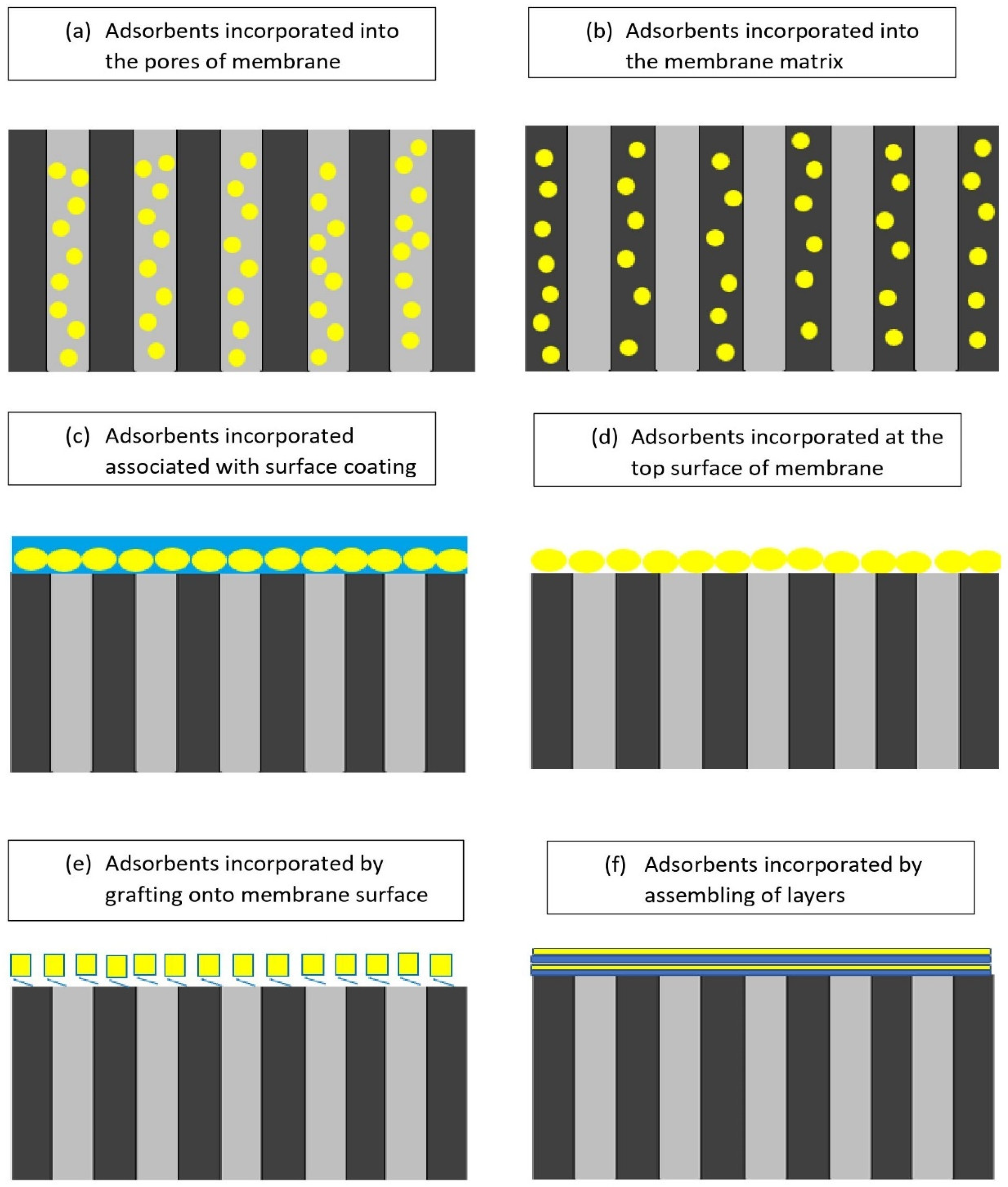
4.1. Methods of Incorporating Adsorbents
4.2. Issues/Problems Found in Single-Layered Adsorptive Membranes
5. Dual-Layered Adsorptive Membranes
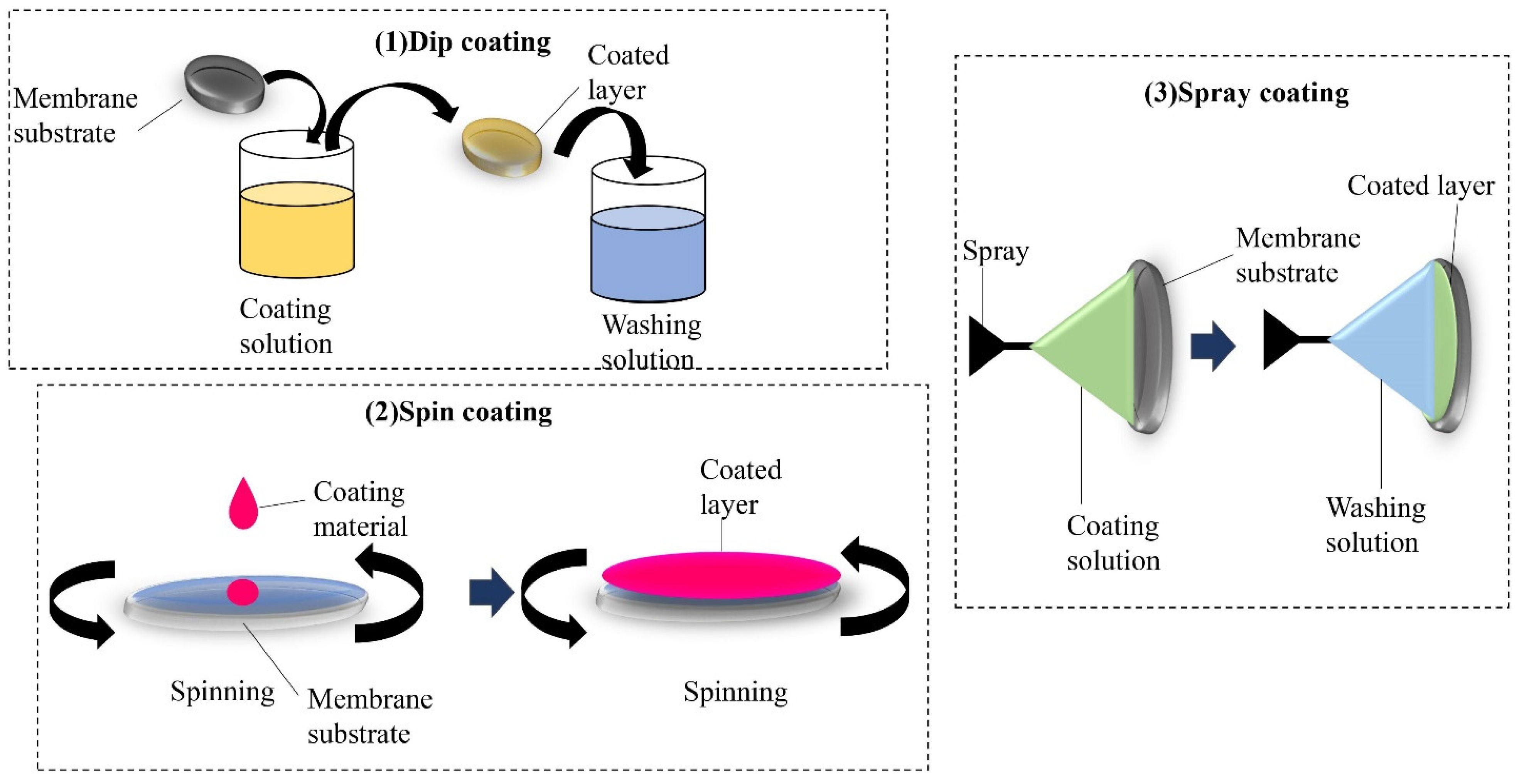
5.1. Various Methods of Fabricating Dual-Layered Membranes
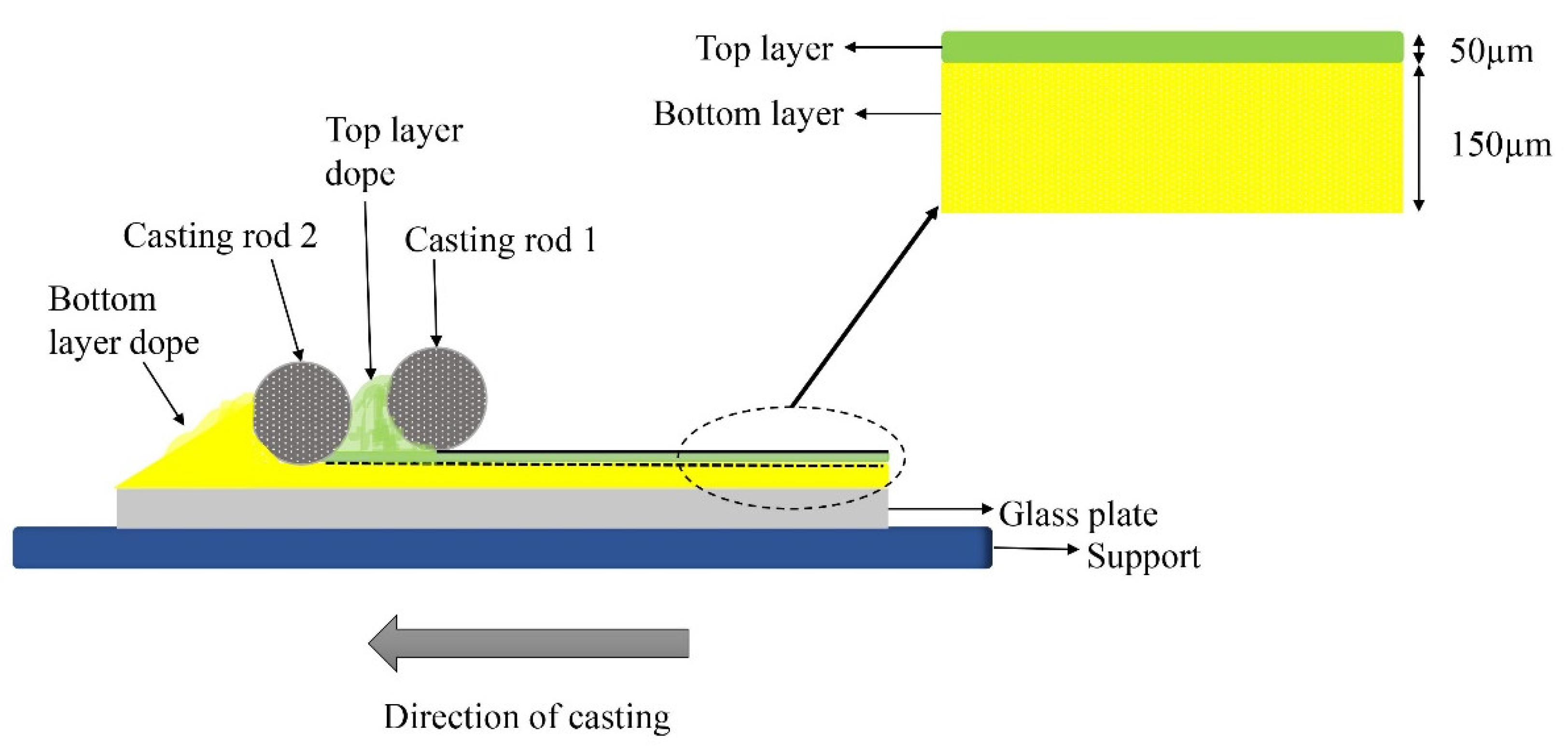
5.2. The Proposed Co-Casting Technique
5.2.1. Parameters Involved in the Process of Co-Casting
Solvent and Non-Solvent Selection
Polymer Concentration and Properties
Additives in the Polymer Concentration
Film Casting Conditions
5.3. The Advantages of Bilayer Membranes by the Co-Casting Technique in Ensuring the Boron Adsorption
6. Challenges and Future Prospects
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bond, N.R.; Burrows, R.M.; Kennard, M.J.; Bunn, S.E. Chapter 6—Water Scarcity as a Driver of Multiple Stressor Effects. In Multiple Stressors in River Ecosystems; Sabater, S., Elosegi, A., Ludwig, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 111–129. [Google Scholar] [CrossRef]
- Rakib, M.A.; Sasaki, J.; Matsuda, H.; Fukunaga, M. Severe salinity contamination in drinking water and associated human health hazards increase migration risk in the southwestern coastal part of Bangladesh. J. Environ. Manag. 2019, 240, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Bixio, D.; Thoeye, C.; De Koning, J.; Joksimovic, D.; Savic, D.; Wintgens, T.; Melin, T. Wastewater reuse in Europe. Desalination 2006, 187, 89–101. [Google Scholar] [CrossRef]
- Kharal, N. Selection of Novel Technology For Wastewater Treatment. 2017. Available online: https://www.researchgate.net/publication/318115987_Selection_of_Novel_Technology_For_Wastewater_Treatment (accessed on 20 July 2022).
- Zuo, K.; Wang, K.; DuChanois, R.M.; Fang, Q.; Deemer, E.M.; Huang, X.; Xin, R.; Said, I.A.; He, Z.; Feng, Y.; et al. Selective membranes in water and wastewater treatment: Role of advanced materials. Mater. Today 2021, 50, 516–532. [Google Scholar] [CrossRef]
- Jevons, K.; Awe, M. Economic benefits of membrane technology vs. evaporator. Desalination 2010, 250, 961–963. [Google Scholar] [CrossRef]
- Bera, S.P.; Godhaniya, M.; Kothari, C. Emerging and advanced membrane technology for wastewater treatment: A review. J. Basic Microbiol. 2022, 62, 245–259. [Google Scholar] [CrossRef]
- Rahimpour, M. Membrane reactors for biodiesel production and processing. In Membrane Reactors for Energy Applications and Basic Chemical Production; Elsevier: Amsterdam, The Netherlands, 2015; pp. 289–312. [Google Scholar]
- Obotey Ezugbe, E.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Siddique, T.; Dutta, N.; Roy Choudhury, N. Mixed-Matrix Membrane Fabrication for Water Treatment. Membranes 2021, 11, 557. [Google Scholar] [CrossRef]
- Jomekian, A.; Behbahani, R.M.; Mohammadi, T.; Kargari, A. CO2/CH4 separation by high performance co-casted ZIF-8/Pebax 1657/PES mixed matrix membrane. J. Nat. Gas Sci. Eng. 2016, 31, 562–574. [Google Scholar] [CrossRef]
- Loizou, E.; Kanari, P.; Kyriacou, G.; Aletrari, M. Boron determination in a multi element national water monitoring program: The absence of legal limits. J. Für Verbrauch. Und Lebensm. 2010, 5, 459–463. [Google Scholar] [CrossRef][Green Version]
- Koseoglu, H.; Kabay, N.; Yüksel, M.; Sarp, S.; Arar, Ö.; Kitis, M. Boron Removal in Seawater Desalination by Reverse Osmosis Membranes—The Impacts of Operating Conditions. In Environmental Earth Sciences; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Reddy, K.V.; Ghaffour, N. Overview of the cost of desalinated water and costing methodologies. Desalination 2007, 205, 340–353. [Google Scholar] [CrossRef]
- Matsuura, T. Progress in membrane science and technology for seawater desalination—A review. Desalination 2001, 134, 47–54. [Google Scholar] [CrossRef]
- Graber, F.M.; Lonsdale, H.K.; Milstead, C.E.; Cross, B.P. Boron rejection by cellulose acetate reverse osmosis membranes. Desalination 1970, 7, 249–258. [Google Scholar] [CrossRef]
- Nadav, N. Boron removal from seawater reverse osmosis permeate utilizing selective ion exchange resin. Desalination 1999, 124, 131–135. [Google Scholar] [CrossRef]
- Prats, D.; Chillon-Arias, M.F.; Rodriguez-Pastor, M. Analysis of the influence of pH and pressure on the elimination of boron in reverse osmosis. Desalination 2000, 128, 269–273. [Google Scholar] [CrossRef]
- Rodríguez Pastor, M.; Ferrándiz Ruiz, A.; Chillón, M.F.; Prats Rico, D. Influence of pH in the elimination of boron by means of reverse osmosis. Desalination 2001, 140, 145–152. [Google Scholar] [CrossRef]
- Redondo, J.; Busch, M.; Witte, J.-P. Boron removal from seawater using FILMTEC ™ high rejection SWRO membranes. Desalination 2003, 156, 229–238. [Google Scholar] [CrossRef]
- Taniguchi, M.; Fusaoka, Y.; Nishikawa, T.; Kurihara, M. Boron removal in RO seawater desalination. Desalination 2004, 167, 419–426. [Google Scholar] [CrossRef]
- Dydo, P.; Turek, M.; Ciba, J.; Trojanowska, J.; Kluczka, J. Boron removal from landfill leachate by means of nanofiltration and reverse osmosis. Desalination 2005, 185, 131–137. [Google Scholar] [CrossRef]
- Kuru, R.; Mutlu, E.; Cempel, E.L.A.; ÇElİK, S.; Yarat, A. Evaluation of Dietary Boron in terms of Health: A Retrospective Study. Clin. Exp. Health Sci. 2018, 8, 296–300. [Google Scholar] [CrossRef]
- Baboo, P. Boron removal in drinking water. GSJ 2021, 9, 1581–1590. [Google Scholar] [CrossRef]
- Wolska, J.; Bryjak, M. Methods for boron removal from aqueous solution—A review. Desalination 2013, 310, 18–24. [Google Scholar] [CrossRef]
- Kabay, N.; Bryjak, M. Boron Removal From Seawater Using Reverse Osmosis Integrated Processes. In Boron Separation Processes; Elsevier: Amsterdam, The Netherlands, 2015; pp. 219–235. [Google Scholar] [CrossRef]
- McCarthy, T.S.; Ellery, W.N. The effect of vegetation on soil and ground water chemistry and hydrology of islands in the seasonal swamps of the Okavango Fan, Botswana. J. Hydrol. 1994, 154, 169–193. [Google Scholar] [CrossRef]
- Gaillardet, J.; Lemarchand, D.; Göpel, C.; Manhès, G. Evaporation and Sublimation of Boric Acid: Application for Boron Purification from Organic Rich Solutions. Geostand. Newsl. 2001, 25, 67–75. [Google Scholar] [CrossRef]
- Brdar-Jokanović, M. Boron Toxicity and Deficiency in Agricultural Plants. Int. J. Mol. Sci. 2020, 21, 1424. [Google Scholar] [CrossRef]
- Parks, J.L.; Edwards, M. Boron in the Environment. Crit. Rev. Environ. Sci. Technol. 2005, 35, 81–114. [Google Scholar] [CrossRef]
- Bakirdere, S.; Örenay, S.; Korkmaz, M. Effect of Boron on Human Health. Open Miner. Process. J. 2010, 3, 54–59. [Google Scholar] [CrossRef]
- Nasef, M.M.; Nallappan, M.; Ujang, Z. Polymer-based chelating adsorbents for the selective removal of boron from water and wastewater: A review. React. Funct. Polym. 2014, 85, 54–68. [Google Scholar] [CrossRef]
- Hilal, N.; Kim, G.J.; Somerfield, C. Boron removal from saline water: A comprehensive review. Desalination 2011, 273, 23–35. [Google Scholar] [CrossRef]
- Chruszcz-Lipska, K.; Winid, B.; Madalska, G.A.; Macuda, J.; Łukańko, Ł. High Content of Boron in Curative Water: From the Spa to Industrial Recovery of Borates? (Poland as a Case Study). Minerals 2020, 11, 8. [Google Scholar] [CrossRef]
- Argust, P. Distribution of boron in the environment. Biol. Trace Elem. Res. 1998, 66, 131–143. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Vengosh, A. Global boron cycle in the Anthropocene. Glob. Biogeochem. Cycles 2016, 30, 219–230. [Google Scholar] [CrossRef]
- Landi, M.; Margaritopoulou, T.; Papadakis, I.E.; Araniti, F. Boron toxicity in higher plants: An update. Planta 2019, 250, 1011–1032. [Google Scholar] [CrossRef]
- Heindel, J.J.; Price, C.J.; Field, E.A.; Marr, M.C.; Myers, C.B.; Morrissey, R.E.; Schwetz, B.A. Developmental toxicity of boric acid in mice and rats. Fundam. Appl. Toxicol. 1992, 18, 266–277. [Google Scholar] [CrossRef]
- Watson, A.T.D.; Sutherland, V.L.; Cunny, H.; Miller-Pinsler, L.; Furr, J.; Hebert, C.; Collins, B.; Waidyanatha, S.; Smith, L.; Vinke, T.; et al. Postnatal Effects of Gestational and Lactational Gavage Exposure to Boric Acid in the Developing Sprague Dawley Rat. Toxicol. Sci. 2020, 176, 65–73. [Google Scholar] [CrossRef]
- Białek, M.; Czauderna, M.; Krajewska, K.A.; Przybylski, W. Selected physiological effects of boron compounds for animals and humans. A review. J. Anim. Feed Sci. 2019, 28, 307–320. [Google Scholar] [CrossRef]
- Donoiu, I.; Militaru, C.; Obleagă, O.; Hunter, J.M.; Neamţu, J.; Biţă, A.; Scorei, I.R.; Rogoveanu, O.C. Effects of boron-containing compounds on cardiovascular disease risk factors—A review. J. Trace Elem. Med. Biol. 2018, 50, 47–56. [Google Scholar] [CrossRef]
- Rahmawati, K. Boron Removal in Seawater Reverse Osmosis System. 2011. Available online: https://core.ac.uk/download/pdf/132719375.pdf (accessed on 20 July 2022).
- Reid, R. Can we really increase yields by making crop plants tolerant to boron toxicity? Plant Sci. 2010, 178, 9–11. [Google Scholar] [CrossRef]
- Princi, M.P.; Lupini, A.; Araniti, F.; Longo, C.; Mauceri, A.; Sunseri, F.; Abenavoli, M.R. Chapter 5—Boron Toxicity and Tolerance in Plants: Recent Advances and Future Perspectives. In Plant Metal Interaction; Ahmad, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 115–147. [Google Scholar] [CrossRef]
- Liu, M.; Guo, Q.; Luo, L.; He, T. Environmental impacts of geothermal waters with extremely high boron concentrations: Insight from a case study in Tibet, China. J. Volcanol. Geotherm. Res. 2020, 397, 106887. [Google Scholar] [CrossRef]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef]
- Emiroglu, O.; Cicek, A.; Arslan, N.; Aksan, S.; Ruzgar, M. Boron concentration in water, sediment and different organisms around large borate deposits of Turkey. Bull. Environ. Contam. Toxicol. 2010, 84, 427–431. [Google Scholar] [CrossRef]
- Schoderboeck, L.; Muhlegger, S.; Losert, A.; Gausterer, C.; Hornek, R. Effects assessment: Boron compounds in the aquatic environment. Chemosphere 2011, 82, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Tsuchiya, K.; Toba, Y.; Eguchi, M.; Tokoro, C. Rapid boron removal from wastewater using low-crystalline magnesium oxide. J. Environ. Chem. Eng. 2020, 8, 104171. [Google Scholar] [CrossRef]
- Parsaei, M.; Goodarzi, M.; Nasef, M. Mohamed Mahmoud Nasef. Adsorption Study for Removal of Boron Using Ion Exchange Resin in Bach System. Proccedings of the 2011 2nd International Conference on Environmental Science and Technology, Singapore, 26–28 February 2011; Volume 1, pp. 398–402. [Google Scholar]
- Biçak, N.; Şenkal, B.F. Sorbitol-modified poly(N-glycidyl styrene sulfonamide) for removal of boron. J. Appl. Polym. Sci. 1998, 68, 2113–2119. [Google Scholar] [CrossRef]
- Hoshina, H.; Seko, N.; Ueki, Y.; Tamada, M. Synthesis of Graft Adsorbent with N-Methyl-D-glucamine for Boron Adsorption. J. Ion Exch. Soc. Jpn. 2007, 18, 236–239. [Google Scholar] [CrossRef]
- Bai, C.; Guo, M.; Liu, Z.; Wu, Z.; Li, Q. A novel method for removal of boron from aqueous solution using sodium dodecyl benzene sulfonate and D-mannitol as the collector. Desalination 2018, 431, 47–55. [Google Scholar] [CrossRef]
- Isa, M.H.; Ezechi, E.H.; Ahmed, Z.; Magram, S.F.; Kutty, S.R.M. Boron removal by electrocoagulation and recovery. Water Res. 2014, 51, 113–123. [Google Scholar] [CrossRef]
- Linares-Hernández, I.; Barrera-Díaz, C.; Bilyeu, B.; Juárez-GarcíaRojas, P.; Campos-Medina, E. A combined electrocoagulation–electrooxidation treatment for industrial wastewater. J. Hazard. Mater. 2010, 175, 688–694. [Google Scholar] [CrossRef]
- Dinçer, A.R. Use of activated sludge in biological treatment of boron containing wastewater by fed-batch operation. Process Biochem. 2004, 39, 723–730. [Google Scholar] [CrossRef]
- Shpiner, R.; Liu, G.; Stuckey, D.C. Treatment of oilfield produced water by waste stabilization ponds: Biodegradation of petroleum-derived materials. Bioresour. Technol. 2009, 100, 6229–6235. [Google Scholar] [CrossRef]
- Tu, K.L.; Nghiem, L.D.; Chivas, A.R. Boron removal by reverse osmosis membranes in seawater desalination applications. Sep. Purif. Technol. 2010, 75, 87–101. [Google Scholar] [CrossRef]
- Edzwald, J.K.; Haarhoff, J. Seawater pretreatment for reverse osmosis: Chemistry, contaminants, and coagulation. Water Res. 2011, 45, 5428–5440. [Google Scholar] [CrossRef]
- Tagliabue, M.; Reverberi, A.P.; Bagatin, R. Boron removal from water: Needs, challenges and perspectives. J. Clean. Prod. 2014, 77, 56–64. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C. Distillation vs. membrane filtration: Overview of process evolutions in seawater desalination. Desalination 2002, 143, 207–218. [Google Scholar] [CrossRef]
- Ghaffour, N.; Missimer, T.M.; Amy, G.L. Technical review and evaluation of the economics of water desalination: Current and future challenges for better water supply sustainability. Desalination 2013, 309, 197–207. [Google Scholar] [CrossRef]
- Bhagyaraj, S.; Al-Ghouti, M.A.; Kasak, P.; Krupa, I. An updated review on boron removal from water through adsorption processes. Emergent Mater. 2021, 4, 1167–1186. [Google Scholar] [CrossRef]
- Ruiz-Aguirre, A.; Andrés-Mañas, J.A.; Zaragoza, G. Evaluation of Permeate Quality in Pilot Scale Membrane Distillation Systems. Membranes 2019, 9, 69. [Google Scholar] [CrossRef]
- Lou, X.-Y.; Xu, Z.; Bai, A.-P.; Resina-Gallego, M.; Ji, Z.-G. Separation and Recycling of Concentrated Heavy Metal Wastewater by Tube Membrane Distillation Integrated with Crystallization. Membranes 2020, 10, 19. [Google Scholar] [CrossRef]
- Eryildiz, B.; Yuksekdag, A.; Korkut, S.; Zeytuncu, B.; Pasaoglu, M.E.; Koyuncu, I. Effect of operating parameters on removal of boron from wastewater containing high boron concentration by vacuum assisted air gap membrane distillation. J. Water Process Eng. 2020, 38, 101579. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Bin Darwish, N.; Hakami, M.W.; Abdullah, A.; Alsadun, A.; Abu Homod, H. Boron Removal by Membrane Distillation: A Comparison Study. Membranes 2020, 10, 263. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, J.-Q. Technologies for Boron Removal. Ind. Eng. Chem. Res. 2007, 47, 16–24. [Google Scholar] [CrossRef]
- Kayser, H. Ueber die Verdichtung von Gasen an Oberflächen in ihrer Abhängigkeit von Druck und Temperatur. Ann. Der Phys. 1881, 248, 526–537. [Google Scholar] [CrossRef]
- Robertson, M.A.F.; Yeager, H.L. A Fluorescent Probe Investigation of the Origin of Superselectivity in Perfluorinated Ionomer Membranes. Macromolecules 1996, 29, 5166–5171. [Google Scholar] [CrossRef]
- Hao, S.; Jia, Z.; Wen, J.; Li, S.; Peng, W.; Huang, R.; Xu, X. Progress in adsorptive membranes for separation—A review. Sep. Purif. Technol. 2021, 255, 117772. [Google Scholar] [CrossRef]
- Nasir, A.M.; Goh, P.S.; Abdullah, M.S.; Ng, B.C.; Ismail, A.F. Adsorptive nanocomposite membranes for heavy metal remediation: Recent progresses and challenges. Chemosphere 2019, 232, 96–112. [Google Scholar] [CrossRef]
- Aguero, R.; Bringas, E.; San Román, M.; Ortiz, I.; Ibanez, R. Membrane processes for whey proteins separation and purification. A review. Curr. Org. Chem. 2017, 21, 1740–1752. [Google Scholar] [CrossRef]
- Rohani, R.; Yusoff, I.I.; Zaman, N.K.; Ali, A.M.; Rusli, N.A.B.; Tajau, R.; Basiron, S.A. Ammonia removal from raw water by using adsorptive membrane filtration process. Sep. Purif. Technol. 2021, 270, 118757. [Google Scholar] [CrossRef]
- Adam, M.R.; Matsuura, T.; Othman, M.H.D.; Puteh, M.H.; Pauzan, M.A.B.; Ismail, A.F.; Mustafa, A.; Rahman, M.A.; Jaafar, J.; Abdullah, M.S. Feasibility study of the hybrid adsorptive hollow fibre ceramic membrane (HFCM) derived from natural zeolite for the removal of ammonia in wastewater. Process Saf. Environ. Prot. 2019, 122, 378–385. [Google Scholar] [CrossRef]
- Alosaimi, A.M. Polysulfone Membranes Based Hybrid Nanocomposites for the Adsorptive Removal of Hg(II) Ions. Polymers 2021, 13, 2792. [Google Scholar] [CrossRef]
- Zeng, J.; Qi, P.; Wang, Y.; Liu, Y.; Sui, K. Electrostatic assembly construction of polysaccharide functionalized hybrid membrane for enhanced antimony removal. J. Hazard. Mater. 2021, 410, 124633. [Google Scholar] [CrossRef]
- Yurekli, Y. Removal of heavy metals in wastewater by using zeolite nano-particles impregnated polysulfone membranes. J. Hazard. Mater. 2016, 309, 53–64. [Google Scholar] [CrossRef]
- Keshtkar, A.R.; Irani, M.; Moosavian, M.A. Removal of uranium (VI) from aqueous solutions by adsorption using a novel electrospun PVA/TEOS/APTES hybrid nanofiber membrane: Comparison with casting PVA/TEOS/APTES hybrid membrane. J. Radioanal. Nucl. Chem. 2013, 295, 563–571. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, L.; Yang, Z.; Zhang, R.; Liu, Y.-N.; He, M.; Su, Y.; Jiang, Z. Loose nanofiltration membrane for dye/salt separation through interfacial polymerization with in-situ generated TiO2 nanoparticles. Appl. Surf. Sci. 2017, 410, 494–504. [Google Scholar] [CrossRef]
- Irani, M.; Keshtkar, A.R.; Mousavian, M.A. Removal of Cd(II) and Ni(II) from aqueous solution by PVA/TEOS/TMPTMS hybrid membrane. Chem. Eng. J. 2011, 175, 251–259. [Google Scholar] [CrossRef]
- Power, P.P.; Woods, W.G. The chemistry of boron and its speciation in plants. Plant Soil 1997, 193, 1–13. [Google Scholar] [CrossRef]
- Qalyoubi, L.; Al-Othman, A.; Al-Asheh, S. Recent progress and challenges on adsorptive membranes for the removal of pollutants from wastewater. Part I: Fundamentals and classification of membranes. Case Stud. Chem. Environ. Eng. 2021, 3, 100086. [Google Scholar] [CrossRef]
- Liu, L.; Xie, X.; Qi, S.; Li, R.; Zhang, X.; Song, X.; Gao, C. Thin film nanocomposite reverse osmosis membrane incorporated with UiO-66 nanoparticles for enhanced boron removal. J. Membr. Sci. 2019, 580, 101–109. [Google Scholar] [CrossRef]
- Li, X.; Liu, R.; Wu, S.; Liu, J.; Cai, S.; Chen, D. Efficient removal of boron acid by N-methyl-D-glucamine functionalized silica–polyallylamine composites and its adsorption mechanism. J. Colloid Interface Sci. 2011, 361, 232–237. [Google Scholar] [CrossRef]
- Ng, Z.-C.; Chong, C.-Y.; Lau, W.-J.; Karaman, M.; Ismail, A.F. Boron removal and antifouling properties of thin-film nanocomposite membrane incorporating PECVD-modified titanate nanotubes. J. Chem. Technol. Biotechnol. 2019, 94, 2772–2782. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, Y.; Liu, K. Extremely effective boron removal from water by stable metal organic framework ZIF-67. Ind. Eng. Chem. Res. 2019, 58, 4199–4207. [Google Scholar] [CrossRef]
- Gao, C.; Liao, J.; Lu, J.; Ma, J.; Kianfar, E. The effect of nanoparticles on gas permeability with polyimide membranes and network hybrid membranes: A review. Rev. Inorg. Chem. 2021, 41, 1–20. [Google Scholar] [CrossRef]
- Wolf, C.; Angellier-Coussy, H.; Gontard, N.; Doghieri, F.; Guillard, V. How the shape of fillers affects the barrier properties of polymer/non-porous particles nanocomposites: A review. J. Membr. Sci. 2018, 556, 393–418. [Google Scholar] [CrossRef]
- Nasir, A.; Masood, F.; Yasin, T.; Hameed, A. Progress in polymeric nanocomposite membranes for wastewater treatment: Preparation, properties and applications. J. Ind. Eng. Chem. 2019, 79, 29–40. [Google Scholar] [CrossRef]
- Mohammad, F.; Al-Lohedan, H.A.; Al-Haque, H.N. Chitosan-mediated fabrication of metal nanocomposites for enhanced biomedical applications. Adv. Mater. Lett. 2016, 8, 89–100. [Google Scholar] [CrossRef]
- Elele, E.; Shen, Y.; Tang, J.; Lei, Q.; Khusid, B.; Tkacik, G.; Carbrello, C. Mechanical properties of polymeric microfiltration membranes. J. Membr. Sci. 2019, 591, 117351. [Google Scholar] [CrossRef]
- Li, D.; Song, S.; Zuo, D.; Wu, W. Effect of pore defects on mechanical properties of graphene reinforced aluminum nanocomposites. Metals 2020, 10, 468. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Zhang, Y.; Meng, J. Hyperbranched-polyol-tethered poly (amic acid) electrospun nanofiber membrane with ultrahigh adsorption capacity for boron removal. Appl. Surf. Sci. 2017, 402, 21–30. [Google Scholar] [CrossRef]
- Kluczka, J.; Pudło, W.; Krukiewicz, K. Boron adsorption removal by commercial and modified activated carbons. Chem. Eng. Res. Des. 2019, 147, 30–42. [Google Scholar] [CrossRef]
- Oo, M.H.; Song, L. Effect of pH and ionic strength on boron removal by RO membranes. Desalination 2009, 246, 605–612. [Google Scholar] [CrossRef]
- Jiang, L.-l.; Yu, H.-T.; Pei, L.-f.; Hou, X.-g. The Effect of Temperatures on the Synergistic Effect between a Magnetic Field and Functionalized Graphene Oxide-Carbon Nanotube Composite for Pb2+ and Phenol Adsorption. J. Nanomater. 2018, 2018, 9167938. [Google Scholar] [CrossRef]
- Yılmaz, A.E.; Boncukcuoglu, R.; Yılmaz, M.T.; Kocakerim, M.M. Adsorption of boron from boron-containing wastewaters by ion exchange in a continuous reactor. J. Hazard. Mater. 2005, 117, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.-Y.; Yi, W.-T. Preparation, characterization, and boron adsorption behavior of gluconate-intercalated hydrotalcite. Environ. Prog. Sustain. Energy 2010, 29, 450–456. [Google Scholar] [CrossRef]
- Öztürk, N.; Kavak, D. Adsorption of boron from aqueous solutions using fly ash: Batch and column studies. J. Hazard. Mater. 2005, 127, 81–88. [Google Scholar] [CrossRef]
- Ghiasi, S.; Mohammadi, T.; Tofighy, M.A. Hybrid nanofiltration thin film hollow fiber membranes with adsorptive supports containing bentonite and LDH nanoclays for boron removal. J. Membr. Sci. 2022, 655, 120576. [Google Scholar] [CrossRef]
- Banerjee, S.; Chattopadhyaya, M.C. Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arab. J. Chem. 2017, 10, S1629–S1638. [Google Scholar] [CrossRef]
- Che Man, H.; Chin, W.; Zadeh, M.; Rashid, M. Adsorption potential of unmodified rice husk for boron removal. Bioresources 2012, 7, 1167–1186. [Google Scholar] [CrossRef]
- Kavak, D. Removal of boron from aqueous solutions by batch adsorption on calcined alunite using experimental design. J. Hazard. Mater. 2009, 163, 308–314. [Google Scholar] [CrossRef]
- Deng, F.; Luo, X.-B.; Ding, L.; Luo, S.-L. 5—Application of Nanomaterials and Nanotechnology in the Reutilization of Metal Ion From Wastewater. In Nanomaterials for the Removal of Pollutants and Resource Reutilization; Luo, X., Deng, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 149–178. [Google Scholar] [CrossRef]
- Kalebaila, K.; Maseka, K.; Mbulo, M. Selected Adsorbents for Removal of Contaminants from Wastewater: Towards Engineering Clay Minerals. Open J. Appl. Sci. 2018, 8, 355–369. [Google Scholar] [CrossRef]
- Yang-Zhou, C.H.; Cao, J.X.; Dong, S.S.; Chen, S.H.; Michael, R.N. Phosphorus Co-Existing in Water: A New Mechanism to Boost Boron Removal by Calcined Oyster Shell Powder. Molecules 2021, 27, 54. [Google Scholar] [CrossRef]
- Ruiz-García, A.; León, F.A.; Ramos-Martín, A. Different boron rejection behavior in two RO membranes installed in the same full-scale SWRO desalination plant. Desalination 2019, 449, 131–138. [Google Scholar] [CrossRef]
- Bao, X.; Long, W.; Liu, H.; She, Q. Boron and salt ion transport in electrically assisted reverse osmosis. J. Membr. Sci. 2021, 637, 119639. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Wang, Y.; Gao, B.; Wang, Z. Surface Modification of Reverse Osmosis Membranes for Enhanced Boron Removal and Fouling Resistance. ACS EST Water 2021, 1, 2284–2292. [Google Scholar] [CrossRef]
- Lyu, J.; Liu, H.; Zeng, Z.; Zhang, J.; Xiao, Z.; Bai, P.; Guo, X. Metal–Organic Framework UiO-66 as an Efficient Adsorbent for Boron Removal from Aqueous Solution. Ind. Eng. Chem. Res. 2017, 56, 2565–2572. [Google Scholar] [CrossRef]
- Rupiasih, N.N.; Purnomo, R.R.; Sumadiyasa, M. Preparation and Application of Chitosan Membranes to Filter Silver from X-ray Film Processing Wastes. J. Phys. Conf. Ser. 2016, 710, 012009. [Google Scholar] [CrossRef]
- Sahebjamee, N.; Soltanieh, M.; Mousavi, S.M.; Heydarinasab, A. Preparation and characterization of porous chitosan–based membrane with enhanced copper ion adsorption performance. React. Funct. Polym. 2020, 154, 104681. [Google Scholar] [CrossRef]
- Haider, S.; Ali, F.A.A.; Haider, A.; Al-Masry, W.A.; Al-Zeghayer, Y. Novel route for amine grafting to chitosan electrospun nanofibers membrane for the removal of copper and lead ions from aqueous medium. Carbohydr. Polym. 2018, 199, 406–414. [Google Scholar] [CrossRef]
- Salehi, E.; Daraei, P.; Shamsabadi, A.A. A review on chitosan-based adsorptive membranes. Carbohydr. Polym. 2016, 152, 419–432. [Google Scholar] [CrossRef]
- Chen, J.; Lian, H.; Sun, X.; Liu, B. Development of a chitosan molecularly imprinted electrochemical sensor for trichlorphon determination. Int. J. Environ. Anal. Chem. 2012, 92, 1046–1058. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Gazi, M.; Yilmaz, E. Single and binary adsorption of azo and anthraquinone dyes by chitosan-based hydrogel: Selectivity factor and Box-Behnken process design. Chem. Eng. Res. Des. 2015, 104, 264–279. [Google Scholar] [CrossRef]
- Huang, Z.-Q.; Cheng, Z.-F. Recent advances in adsorptive membranes for removal of harmful cations. J. Appl. Polym. Sci. 2020, 137, 48579. [Google Scholar] [CrossRef]
- Raval, H.; Sundarkumar, V. Low-energy reverse osmosis membrane with high boron rejection by surface modification with a polysaccharide. Can. J. Chem. Eng. 2019, 97, 1575–1580. [Google Scholar] [CrossRef]
- Çermikli, E.; Şen, F.; Altıok, E.; Wolska, J.; Cyganowski, P.; Kabay, N.; Bryjak, M.; Arda, M.; Yüksel, M. Performances of novel chelating ion exchange resins for boron and arsenic removal from saline geothermal water using adsorption-membrane filtration hybrid process. Desalination 2020, 491, 114504. [Google Scholar] [CrossRef]
- Du, X.; Meng, J.; Xu, R.; Shi, Q.; Zhang, Y. Polyol-grafted polysulfone membranes for boron removal: Effects of the ligand structure. J. Membr. Sci. 2015, 476, 205–215. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, M.; Zhang, Z.; Shi, X.; Wang, Y. Boron removal by water molecules inside covalent organic framework (COF) multilayers. Desalination 2022, 526, 115548. [Google Scholar] [CrossRef]
- Man, H.C.; Abba, M.U.; Abdulsalam, M.; Azis, R.a.S.; Idris, A.I.; Hamzah, M.H. Utilization of Nano-TiO2 as an Influential Additive for Complementing Separation Performance of a Hybrid PVDF-PVP Hollow Fiber: Boron Removal from Leachate. Polymers 2020, 12, 2511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, H.; Fan, X.; Lv, F.; Chen, S.; Quan, X. Fabrication of TiO2 nanofiber membranes by a simple dip-coating technique for water treatment. Surf. Coat. Technol. 2016, 298, 45–52. [Google Scholar] [CrossRef]
- You, S.; Lu, J.; Tang, C.Y.; Wang, X. Rejection of heavy metals in acidic wastewater by a novel thin-film inorganic forward osmosis membrane. Chem. Eng. J. 2017, 320, 532–538. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef]
- Zelmanov, G.; Semiat, R. Boron removal from water and its recovery using iron (Fe+3) oxide/hydroxide-based nanoparticles (NanoFe) and NanoFe-impregnated granular activated carbon as adsorbent. Desalination 2014, 333, 107–117. [Google Scholar] [CrossRef]
- Khakpor, R.; Yavari, R.; Aroon, M.A.; Alipor, H. Investigation of comparative behavior of boron ion rejection from aqueous solution using mixed matrix membranes in nanofiltration process. J. Nucl. Sci. Technol. 2021, 42, 18–27. [Google Scholar] [CrossRef]
- Sun, M.; Li, M.; Zhang, X.; Wu, C.; Wu, Y. Graphene oxide modified porous P84 co-polyimide membranes for boron recovery by bipolar membrane electrodialysis process. Sep. Purif. Technol. 2020, 232, 115963. [Google Scholar] [CrossRef]
- Jin, J.; Du, X.; Yu, J.; Qin, S.; He, M.; Zhang, K.; Yang, J. Synthesis of Negatively Charged Polyol-Functional PSF Membranes with Good Hydrophilic and Efficient Boron Removal Properties. Polymers 2019, 11, 780. [Google Scholar] [CrossRef]
- Kumar, R.; Ahmed, M.; Bhadrachari, G.; Al- Muqahwi, S.; Thomas, J.P. Thin-film nanocomposite membrane comprised of a novel phosphonic acid derivative of titanium dioxide for efficient boron removal. J. Environ. Chem. Eng. 2021, 9, 105722. [Google Scholar] [CrossRef]
- Shi, Q.; Meng, J.-Q.; Xu, R.-S.; Du, X.-L.; Zhang, Y.-F. Synthesis of hydrophilic polysulfone membranes having antifouling and boron adsorption properties via blending with an amphiphilic graft glycopolymer. J. Membr. Sci. 2013, 444, 50–59. [Google Scholar] [CrossRef]
- Ozbey-Unal, B.; Gezmis-Yavuz, E.; Eryildiz, B.; Koseoglu-Imer, D.Y.; Keskinler, B.; Koyuncu, I. Boron removal from geothermal water by nanofiber-based membrane distillation membranes with significantly improved surface hydrophobicity. J. Environ. Chem. Eng. 2020, 8, 104113. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Manoharan, B.; Sivakumar, N. Chapter 1—Introduction: Why Perovskite and Perovskite Solar Cells? In Perovskite Photovoltaics; Thomas, S., Thankappan, A., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–24. [Google Scholar] [CrossRef]
- Smith, R.; Inomata, H.; Peters, C. Chapter 4—Historical Background and Applications. In Supercritical Fluid Science and Technology; Smith, R., Inomata, H., Peters, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 4, pp. 175–273. [Google Scholar]
- Sherazi, T. Spray Coating. In Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–2. [Google Scholar] [CrossRef]
- Yilmaz, Y. Principles of Membrane Surface Modification for Water Applications. In Wastewater Treatment; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Kato, K.; Uchida, E.; Kang, E.-T.; Uyama, Y.; Ikada, Y. Polymer surface with graft chains. Prog. Polym. Sci. 2003, 28, 209–259. [Google Scholar] [CrossRef]
- Abu Seman, M.N.; Khayet, M.; Bin Ali, Z.I.; Hilal, N. Reduction of nanofiltration membrane fouling by UV-initiated graft polymerization technique. J. Membr. Sci. 2010, 355, 133–141. [Google Scholar] [CrossRef]
- Puro, L.; Mänttäri, M.; Pihlajamäki, A.; Nyström, M. Characterization of Modified Nanofiltration Membranes by Octanoic Acid Permeation and FTIR Analysis. Chem. Eng. Res. Des. 2006, 84, 87–96. [Google Scholar] [CrossRef]
- Khraisheh, M.; Elhenawy, S.; AlMomani, F.; Al-Ghouti, M.; Hassan, M.K.; Hameed, B.H. Recent Progress on Nanomaterial-Based Membranes for Water Treatment. Membranes 2021, 11, 995. [Google Scholar] [CrossRef]
- Liu, H.; Qing, B.; Ye, X.; Li, Q.; Lee, K.; Wu, Z. Boron adsorption by composite magnetic particles. Chem. Eng. J. 2009, 151, 235–240. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Q.; Lyu, J.; Bai, P.; Guo, X. Boron removal and reclamation by magnetic magnetite (Fe3O4) nanoparticle: An adsorption and isotopic separation study. Sep. Purif. Technol. 2019, 231, 115930. [Google Scholar] [CrossRef]
- Ismail, A.F.; Padaki, M.; Hilal, N.; Matsuura, T.; Lau, W.J. Thin film composite membrane—Recent development and future potential. Desalination 2015, 356, 140–148. [Google Scholar] [CrossRef]
- Dlamini, D.S.; Mamba, B.B.; Li, J. The role of nanoparticles in the performance of nano-enabled composite membranes—A critical scientific perspective. Sci. Total Environ. 2019, 656, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Donato, L.; Drioli, E. Imprinted membranes for sustainable separation processes. Front. Chem. Sci. Eng. 2021, 15, 775–792. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2018, 8, 19. [Google Scholar] [CrossRef]
- Erdem, Ö.; Saylan, Y.; Andaç, M.; Denizli, A. Molecularly Imprinted Polymers for Removal of Metal Ions: An Alternative Treatment Method. Biomimetics 2018, 3, 38. [Google Scholar] [CrossRef]
- Yanar, N.; Son, M.; Park, H.; Choi, H. Toward greener membranes with 3D printing technology. Environ. Eng. Res. 2021, 26, 200020–200027. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Yu, L.-Y.; Shen, H.-M.; Xu, Z.-L. PVDF–TiO2 composite hollow fiber ultrafiltration membranes prepared by TiO2 sol–gel method and blending method. J. Appl. Polym. Sci. 2009, 113, 1763–1772. [Google Scholar] [CrossRef]
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Park, M.J.; Phuntsho, S.; He, T.; Nisola, G.M.; Tijing, L.D.; Li, X.-M.; Chen, G.; Chung, W.-J.; Shon, H.K. Graphene oxide incorporated polysulfone substrate for the fabrication of flat-sheet thin-film composite forward osmosis membranes. J. Membr. Sci. 2015, 493, 496–507. [Google Scholar] [CrossRef]
- Wu, D.; Huang, Y.; Yu, S.; Lawless, D.; Feng, X. Thin film composite nanofiltration membranes assembled layer-by-layer via interfacial polymerization from polyethylenimine and trimesoyl chloride. J. Membr. Sci. 2014, 472, 141–153. [Google Scholar] [CrossRef]
- Yu, S.; Yao, G.; Dong, B.; Zhu, H.; Peng, X.; Liu, J.; Liu, M.; Gao, C. Improving fouling resistance of thin-film composite polyamide reverse osmosis membrane by coating natural hydrophilic polymer sericin. Sep. Purif. Technol. 2013, 118, 285–293. [Google Scholar] [CrossRef]
- Liu, X.; Ng, H.Y. Fabrication of layered silica–polysulfone mixed matrix substrate membrane for enhancing performance of thin-film composite forward osmosis membrane. J. Membr. Sci. 2015, 481, 148–163. [Google Scholar] [CrossRef]
- Dlamini, D.S.; Tesha, J.M.; Vilakati, G.D.; Mamba, B.B.; Mishra, A.K.; Thwala, J.M.; Li, J. A critical review of selected membrane- and powder-based adsorbents for water treatment: Sustainability and effectiveness. J. Clean. Prod. 2020, 277, 123497. [Google Scholar] [CrossRef]
- Di Vincenzo, M.; Barboiu, M.; Tiraferri, A.; Legrand, Y.M. Polyol-functionalized thin-film composite membranes with improved transport properties and boron removal in reverse osmosis. J. Membr. Sci. 2017, 540, 71–77. [Google Scholar] [CrossRef]
- Ali, Z.; Al Sunbul, Y.; Pacheco, F.; Ogieglo, W.; Wang, Y.; Genduso, G.; Pinnau, I. Defect-free highly selective polyamide thin-film composite membranes for desalination and boron removal. J. Membr. Sci. 2019, 578, 85–94. [Google Scholar] [CrossRef]
- Hashemifard, S.A.; Ismail, A.F.; Matsuura, T. Co-casting technique for fabricating dual-layer flat sheet membranes for gas separation. J. Membr. Sci. 2011, 375, 258–267. [Google Scholar] [CrossRef]
- Fu, F.-J.; Sun, S.-P.; Zhang, S.; Chung, T.-S. Pressure retarded osmosis dual-layer hollow fiber membranes developed by co-casting method and ammonium persulfate (APS) treatment. J. Membr. Sci. 2014, 469, 488–498. [Google Scholar] [CrossRef]
- Li, X.-M.; Ji, Y.; Yin, Y.; Zhang, Y.-Y.; Wang, Y.; He, T. Origin of delamination/adhesion in polyetherimide/polysulfone co-cast membranes. J. Membr. Sci. 2010, 352, 173–179. [Google Scholar] [CrossRef]
- Xia, Q.-C.; Liu, M.-L.; Cao, X.-L.; Wang, Y.; Xing, W.; Sun, S.-P. Structure design and applications of dual-layer polymeric membranes. J. Membr. Sci. 2018, 562, 85–111. [Google Scholar] [CrossRef]
- Yeow, M.L.; Liu, Y.T.; Li, K. Morphological study of poly (vinylidene fluoride) asymmetric membranes: Effects of the solvent, additive, and dope temperature. J. Appl. Polym. Sci. 2004, 92, 1782–1789. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Z.-L.; Yu, L.-Y. Effects of mixed solvents and PVDF types on performances of PVDF microporous membranes. J. Appl. Polym. Sci. 2010, 115, 2277–2287. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and Characterization of Membranes Formed by Nonsolvent Induced Phase Separation: A Review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Preparation, characterization and performance studies of polysulfone membranes using PVP as an additive. J. Membr. Sci. 2008, 315, 36–47. [Google Scholar] [CrossRef]
- Jung, B.; Yoon, J.K.; Kim, B.; Rhee, H.-W. Effect of molecular weight of polymeric additives on formation, permeation properties and hypochlorite treatment of asymmetric polyacrylonitrile membranes. J. Membr. Sci. 2004, 243, 45–57. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Jeong, B.-H.; Huang, X.; Hoek, E.M.V. Impacts of reaction and curing conditions on polyamide composite reverse osmosis membrane properties. J. Membr. Sci. 2008, 311, 34–45. [Google Scholar] [CrossRef]
- Tsai, H.-A.; Ruaan, R.-C.; Wang, D.-M.; Lai, J.-Y. Effect of temperature and span series surfactant on the structure of polysulfone membranes. J. Appl. Polym. Sci. 2002, 86, 166–173. [Google Scholar] [CrossRef]
- Li, D.; Wang, H. Recent developments in reverse osmosis desalination membranes. J. Mater. Chem. 2010, 20, 4551–4566. [Google Scholar] [CrossRef]
- Kim, B.-C.; Phan, H.; Moon, S.-H. Boron removal from seawater by combined system of seawater reverse osmosis membranes and ion exchange process: A pilot-scale study. Desalination Water Treat. 2012, 15, 178–182. [Google Scholar] [CrossRef]
- Kassim Shaari, N.Z.; Abd Rahman, N.; Sulaiman, N.A.; Mohd Tajuddin, R. Thin Film Composite Membranes: Mechanical and Antifouling Properties. MATEC Web Conf. 2017, 103, 06005. [Google Scholar] [CrossRef]
- Ezechi, E.H.; Isa, M.H.; Kutty, S.R.B.M. Boron in Produced VVater: Challenges and Improvements: A Comprehensive Review. J. Appl. Sci. 2012, 12, 402–415. [Google Scholar] [CrossRef]
- Kumar, R.; Ismail, A.F. Fouling control on microfiltration/ultrafiltration membranes: Effects of morphology, hydrophilicity, and charge. J. Appl. Polym. Sci. 2015, 132, 42042. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. Chapter 5—Flat-Sheet Membrane for Power Generation and Desalination Based on Salinity Gradient. In Membrane-Based Salinity Gradient Processes for Water Treatment and Power Generation; Sarp, S., Hilal, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 155–174. [Google Scholar] [CrossRef]
- Goh, P.S.; Wong, K.C.; Wong, T.W.; Fauzi Ismail, A. Surface-tailoring chlorine resistant materials and strategies for polyamide thin film composite reverse osmosis membranes. Front. Chem. Sci. Eng. 2022, 16, 564–591. [Google Scholar] [CrossRef]
- Abia, L.; Armesto, X.L.; Canle, M.L.; García, M.V.; Santaballa, J.A. Oxidation of aliphatic amines by aqueous chlorine. Tetrahedron 1998, 54, 521–530. [Google Scholar] [CrossRef]
- Dam, N.; Ogilby, P.R. On the mechanism of polyamide degradation in chlorinated water. Helv. Chim. Acta 2001, 84, 2540–2549. [Google Scholar] [CrossRef]
- Gabelich, C.J.; Frankin, J.C.; Gerringer, F.W.; Ishida, K.P.; Suffet, I.H. Enhanced oxidation of polyamide membranes using monochloramine and ferrous iron. J. Membr. Sci. 2005, 258, 64–70. [Google Scholar] [CrossRef]
- Kwon, Y.-N.; Leckie, J.O. Hypochlorite degradation of crosslinked polyamide membranes: I. Changes in chemical/morphological properties. J. Membr. Sci. 2006, 283, 21–26. [Google Scholar] [CrossRef]
- Kwon, Y.-N.; Leckie, J.O. Hypochlorite degradation of crosslinked polyamide membranes: II. Changes in hydrogen bonding behavior and performance. J. Membr. Sci. 2006, 282, 456–464. [Google Scholar] [CrossRef]
- Al-Abri, M.; Al-Ghafri, B.; Bora, T.; Dobretsov, S.; Dutta, J.; Castelletto, S.; Rosa, L.; Boretti, A. Chlorination disadvantages and alternative routes for biofouling control in reverse osmosis desalination. Npj Clean Water 2019, 2, 2. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Gazi, M. High boron removal by functionalized magnesium ferrite nanopowders. Environ. Chem. Lett. 2016, 14, 373–379. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.; Xu, H.; Liu, H.; Wang, M.; He, Y.; Pan, B. Nanomaterials-enabled water and wastewater treatment. NanoImpact 2016, 3–4, 22–39. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Chong, W.C.; Choo, Y.L.; Koo, C.H.; Pang, Y.L.; Lai, S.O. Adsorptive membranes for heavy metal removal–A mini review. AIP Conf. Proc. 2019, 2157, 020005. [Google Scholar]
- Shahrin, S.; Lau, W.-J.; Kartohardjono, S.; Jamshidi Gohari, R.; Goh, P.-S.; Jaafar, J.; Ismail, A.F. Chapter 1—Development of adsorptive ultrafiltration membranes for heavy metal removal. In Advanced Nanomaterials for Membrane Synthesis and its Applications; Lau, W.-J., Ismail, A.F., Isloor, A., Al-Ahmed, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–22. [Google Scholar] [CrossRef]
- Khan, A.A.; Maitlo, H.A.; Khan, I.A.; Lim, D.; Zhang, M.; Kim, K.-H.; Lee, J.; Kim, J.-O. Metal oxide and carbon nanomaterial based membranes for reverse osmosis and membrane distillation: A comparative review. Environ. Res. 2021, 202, 111716. [Google Scholar] [CrossRef]
- Sabir, A.; Shafiq, M.; Islam, A.; Jabeen, F.; Shafeeq, A.; Ahmad, A.; Zahid Butt, M.T.; Jacob, K.I.; Jamil, T. Conjugation of silica nanoparticles with cellulose acetate/polyethylene glycol 300 membrane for reverse osmosis using MgSO4 solution. Carbohydr. Polym. 2016, 136, 551–559. [Google Scholar] [CrossRef]
- Teow, Y.H.; Mohammad, A.W. New generation nanomaterials for water desalination: A review. Desalination 2019, 451, 2–17. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Functional materials in desalination: A review. Desalination 2019, 468, 114077. [Google Scholar] [CrossRef]
- Al Aani, S.; Wright, C.J.; Atieh, M.A.; Hilal, N. Engineering nanocomposite membranes: Addressing current challenges and future opportunities. Desalination 2017, 401, 1–15. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S.J. Nanoparticles in reverse osmosis membranes for desalination: A state of the art review. Desalination 2020, 475, 114171. [Google Scholar] [CrossRef]
- Strathmann, H. Membrane separation processes: Current relevance and future opportunities. AIChE J. 2001, 47, 1077–1087. [Google Scholar] [CrossRef]
| Boron Concentration (1 ppm = 1 mg/L) | Tolerance of Crops |
|---|---|
| <0.5 ppm | Satisfactory for all crops. |
| 0.5–1.0 ppm | Can show injury for sensitive crops. |
| 1.0–2.0 ppm | Satisfactory among semi-tolerant crops, can cause retarded growth in sensitive crops. |
| >2.0 ppm | Only satisfactory for certain tolerant crops. |
| Method | Membrane | Removal | Remark | Reference |
|---|---|---|---|---|
| Natural zeolite-based hollow fiber ceramic membrane | Ammonia |
| [75] |
| Polysulfone (PSf)/Organoclay/Organic nanofiller (G, GO, CNTs or CNTOxi) hybrid membranes | Mercury |
| [76] |
| PSf support matrix membrane loaded with a chitosan functionalized iron nanocomposite membrane fabricated using the phase inversion method and then coated with an alginate active layer | Antimony |
| [77] |
| Zeolite nanoparticles-impregnated polysulfone membranes | Lead and nickel cations |
| [78] |
| Polyvinylalcohol/tetraethylorthosilicate/aminopropyltriethoxysilane (PVA/TEOS/APTES) nanofiber membrane | Uranium (IV) |
| [79] |
| Loose nanofiltration membrane with TiO2 nanoparticles on the membrane surface | Salt and dye |
| [80] |
| Functionalized poly(vinyl alcohol)/tetraethyl orthosilicate (PVA/TEOS) hybrid membranes with 3-mercaptopropyltrimethoxysilane (TMPTMS) groups | Cadmium and nickel ions |
| [81] |
| Adsorptive Membrane | Single-Layered | Double-Layered |
|---|---|---|
| Structure | 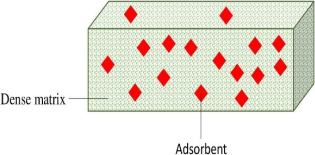 | 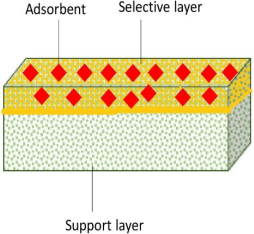 |
| Advantages |
|
|
| Common issues |
|
|
| Method | Feature | Material | Application | Output | References |
|---|---|---|---|---|---|
| 1. Interfacial polymerization | Interfacial polymerization is a type of step-growth polymerization in which polymerization occurs at the interface between two immiscible phases (generally, two liquids), resulting in a polymer that is constrained to the interface. | Active layer: Polyamide (PA) Sub-layer: Polysulfone/graphene oxide | Forward osmosis (FO), salt rejection | Enhanced water permeability, higher selectivity, improved performance as a TFC-FO membrane | [153] |
| 2. Layer-by-layer | Deposition of thin films and coatings with a precisely controlled composition and thickness (can be used for multilayer films too) | Polyethylenimine (PEI) and trimesoyl chloride (TMC) on a microporous polyethersulfone (PES) substrate. | Nanofiltration (NF) | Increased permeability, stable and higher salt rejection, more compact structure | [154] |
| 3. Coating and cross-linking | Process of chemically joining two or more molecules by covalent bonding to be spread on the surface of the membrane. | Polyamide reverse osmosis membrane modified through coating a surface layer of natural polymer sericin | Reverse osmosis (RO) | Increased antifouling ability, decreased pure water permeability, increased salt rejection, capability of decreasing the foulant attachment on the membrane surface | [155] |
| 4. Co-casting technique | Simultaneous casting of two dope solutions on a casting plate by controlling several parameters | Silica-impregnated porous bottom layer nano-particle-devoid top surface-interface PA-active layer | Forward osmosis (FO) | Defect-free structure and increased water flux without compromising on the reverse salt flux | [156] |
| Membrane Preparation Method | Adsorbent/Active Layer | Polymer | Boron Rejection | Application | Findings | Reference |
|---|---|---|---|---|---|---|
| 1. Interfacial polymerization | Commercially available NMDG group, (±)-3-amino-1,2 propanediol or serinol | Polyamide-sub layer | 90% | Ultrafiltration | 40% reduction in salt passage; max boron rejection at pH = 5.2 | [158] |
| 2. Interfacial polymerization | Trimesoyl chloride | Polysulfone-sub layer | 99% | Ultrafiltration | Max rejection at pH = 10 | [159] |
| 3. Interfacial polymerization | M-phenylenediamine cross-linked 1,3,5-benzenetricarbonyl trichloride followed by a polyamide layer with the UiO66 nanoparticle | Polysulfone-sub layer | 91.2% | Reverse osmosis | Improved water flux and salt rejection | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehanathan, S.; Jaafar, J.; Nasir, A.M.; Rahman, R.A.; Ismail, A.F.; Illias, R.M.; Othman, M.H.D.; A Rahman, M.; Bilad, M.R.; Naseer, M.N. Adsorptive Membrane for Boron Removal: Challenges and Future Prospects. Membranes 2022, 12, 798. https://doi.org/10.3390/membranes12080798
Mehanathan S, Jaafar J, Nasir AM, Rahman RA, Ismail AF, Illias RM, Othman MHD, A Rahman M, Bilad MR, Naseer MN. Adsorptive Membrane for Boron Removal: Challenges and Future Prospects. Membranes. 2022; 12(8):798. https://doi.org/10.3390/membranes12080798
Chicago/Turabian StyleMehanathan, Shaymala, Juhana Jaafar, Atikah Mohd Nasir, Roshanida A. Rahman, Ahmad Fauzi Ismail, Rosli Md Illias, Mohd Hafiz Dzarfan Othman, Mukhlis A Rahman, Muhammad Roil Bilad, and Muhammad Nihal Naseer. 2022. "Adsorptive Membrane for Boron Removal: Challenges and Future Prospects" Membranes 12, no. 8: 798. https://doi.org/10.3390/membranes12080798
APA StyleMehanathan, S., Jaafar, J., Nasir, A. M., Rahman, R. A., Ismail, A. F., Illias, R. M., Othman, M. H. D., A Rahman, M., Bilad, M. R., & Naseer, M. N. (2022). Adsorptive Membrane for Boron Removal: Challenges and Future Prospects. Membranes, 12(8), 798. https://doi.org/10.3390/membranes12080798