Membrane Fouling and Electrochemical Regeneration at a PbO2-Reactive Electrochemical Membrane: Study on Experiment and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reactant and Material
2.2. Fabrication of PbO2-REM
2.3. Characterization of PbO2-REM
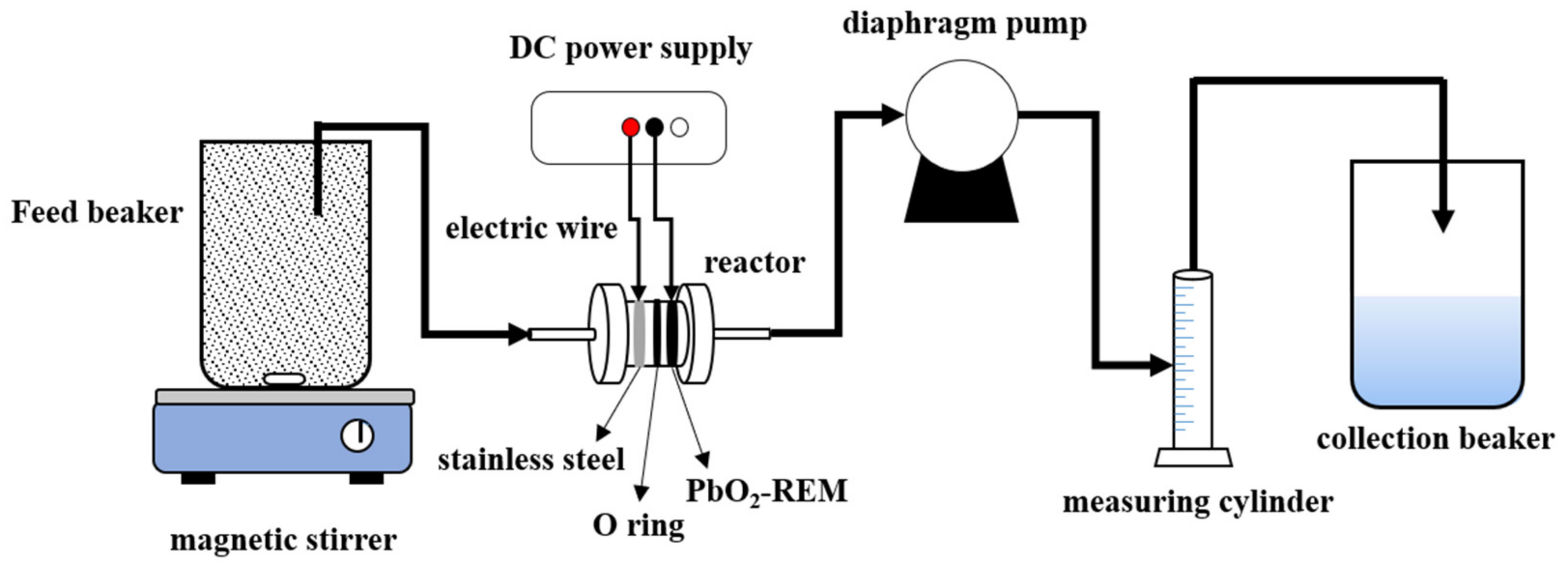
2.4. Experimental Apparatu and Methods
2.5. FLUENT Model
3. Results and Discussion
3.1. Structure and Morphology of PbO2-REM
3.1.1. Surface Morphology and Microstructure
3.1.2. Element and Pore Size Distribution
3.2. Antifouling Ability of PbO2-REM
3.3. Regeneration of PbO2-REM
3.3.1. Regeneration in FW Mode
3.3.2. Regeneration in BW Mode
3.3.3. Repeated Regeneration in e-BW Mode
3.4. Mechanism of Antifouling and Regeneration of PbO2-REM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, J.; Liao, Z.P.; Zhang, M.; Ni, L.J.; Qi, J.W.; Wang, C.H.; Sun, X.Y.; Wang, L.J.; Wang, S.B.; Li, J.S. Sequential ultrafiltration-catalysis membrane for excellent removal of multiple pollutants in water. Environ. Sci. Technol. 2021, 55, 2652. [Google Scholar] [CrossRef] [PubMed]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, K.; Cui, T.; Huang, F.; Zhang, Y.; Han, W. Membrane separation coupled with electrochemical advanced oxidation processes for organic wastewater treatment: A Short Review. Membrane 2020, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Katsoufidou, K.; Yiantsios, S.G.; Karabelas, A.J. Experimental study of ultrafiltration membrane fouling by sodium alginate and flux recovery by backwashing. J. Membr. Sci. 2007, 300, 137. [Google Scholar] [CrossRef]
- Sun, M.; Wang, X.X.; Winter, L.R.; Zhao, Y.M.; Ma, W.; Hedtke, T.; Kim, J.H.; Elimelech, M. Electrified membranes for water treatment applications. ACS EST Engg. 2021, 1, 725. [Google Scholar] [CrossRef]
- Zhu, X.B.; Jassby, D. Electroactive membranes for water treatment: Enhanced treatment functionalities, energy considerations, and future challenges. Acc. Chem. Res. 2019, 52, 1177. [Google Scholar] [CrossRef]
- Li, N.; He, M.T.; Duan, X.G.; Yan, B.B.; Chen, G.Y.; Wang, S.B. Catalytic membrane-based oxidation-filtration systems for organic wastewater purification: A review. J. Hazard. Mater. 2021, 414, 125478. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, F.X.; Hu, X.M.; Hua, T. Electrochemical advanced oxidation processes coupled with membrane filtration for degrading antibiotic residues: A review on its potential applications, advances, and challenges. Sci. Total. Environ. 2021, 784, 146912. [Google Scholar] [CrossRef]
- Chaplin, B.P. The prospect of electrochemical technologies advancing worldwide water treatment. Acc. Chem. Res. 2019, 52, 596. [Google Scholar] [CrossRef]
- Chaplin, B.P. Critical review of electrochemical advanced oxidation processes for water treatment applications. Environ. Sci. Processes Impacts 2014, 16, 1182. [Google Scholar] [CrossRef]
- Zaky, A.M.; Chaplin, B.P. Porous substoichiometric TiO2 anodes as reactive electrochemical membranes for water treatment. Environ. Sci. Technol. 2013, 47, 6554–6563. [Google Scholar] [CrossRef] [PubMed]
- Trellu, C.; Chaplin, B.P.; Coetsier, C.; Esmilaire, R.; Cerneaux, S.; Causserand, C.; Cretin, M. Electro-oxidation of organic pollutants by reactive electrochemical membranes. Chemosphere 2018, 208, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Z.L.; Song, C.W.; Li, L.; Wang, H.; Pan, Y.Q.; Wang, C.L.; Li, J.X.; Wang, T.H.; Feng, X.S. Membrane technology coupled with electrochemical advanced oxidation processes for organic wastewater treatment: Recent advances and future prospects. Chem. Eng. J. 2019, 376, 120909. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Wei, K.J.; Xu, A.L.; Han, W.Q.; Sun, X.Y.; Li, J.S.; Shen, J.Y.; Wang, L.J. Pesticide tailwater deeply treated by tubular porous electrode reactor (TPER): Purpose for discharging and cost saving. Chemosphere 2017, 185, 86. [Google Scholar] [CrossRef]
- Huang, E.; White, T.; Wang, B.B.; Shi, H.H.; Liu, J.Y. Disinfection of Escherichia coli by a Reactive electrochemical membrane system involving activated carbon fiber cloth (ACFC). Water 2019, 11, 430. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Xu, J.L.; Lin, H.; Xie, R.Z.; Wang, K.; Lv, S.H.; Liao, J.B.; Liu, X.H.; Chen, J.; Yang, Z.F. Developing a low-pressure and super stable electrochemical tubular reactive filter: Outstanding efficiency for wastewater purification. Electrochim. Acta 2020, 335, 135634. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Wei, K.J.; Han, W.Q.; Sun, X.Y.; Li, J.S.; Shen, J.Y.; Wang, L.J. Improved electrochemical oxidation of tricyclazole from aqueous solution by enhancing mass transfer in a tubular porous electrode electrocatalytic reactor. Electrochim. Acta 2016, 189, 1. [Google Scholar] [CrossRef]
- Li, D.; Tang, J.Y.; Zhou, X.Z.; Li, J.S.; Sun, X.Y.; Shen, J.Y.; Wang, L.J.; Han, W.Q. Electrochemical degradation of pyridine by Ti/SnO2-Sb tubular porous electrode. Chemosphere 2016, 149, 49–56. [Google Scholar] [CrossRef]
- Li, X.H.; Pletcher, D.; Walsh, F.C. Electrodeposited lead dioxide layers. Chem. Soc. Rev. 2011, 40, 3879. [Google Scholar] [CrossRef] [Green Version]
- Zaky, A.M.; Chaplin, B.P. Mechanism of p-substituted phenol oxidation at a Ti4O7 reactive electrochemical membrane. Environ. Sci. Technol. 2014, 48, 5857–5867. [Google Scholar] [CrossRef]
- Zhao, G.H.; Zhang, Y.G.; Lei, Y.Z.; Lv, B.Y.; Gao, J.X.; Zhang, Y.A.; Li, D.M. Fabrication and electrochemical treatment application of a novel lead dioxide anode with superhydrophobic surfaces, high oxygen evolution potential, and oxidation capability. Environ. Sci. Technol. 2010, 44, 1754. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.H.; Dai, Q.Z.; Lei, L.C.; Ma, C.A.; Wang, D.H. Long life modified lead dioxide anode for organic wastewater treatment: Electrochemical characteristics and degradation mechanism. Environ. Sci. Technol. 2005, 39, 363. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Chaplin, B.P. Electrochemical impedance spectroscopy study of membrane fouling characterization at a conductive sub-stoichiometric TiO2 reactive electrochemical membrane: Transmission line model development. J. Membr. Sci. 2016, 511, 238. [Google Scholar] [CrossRef] [Green Version]
- Jing, Y.; Guo, L.; Chaplin, B.P. Electrochemical impedance spectroscopy study of membrane fouling and electrochemical regeneration at a sub-stoichiometric TiO2 reactive electrochemical membrane. J. Membr. Sci. 2016, 510, 510. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Hong, L.; Xue, H.M.; Han, W.Q.; Wang, L.J.; Sun, X.Y.; Li, J.S. Preparation and characterization of TiO2-NTs/SnO2-Sb electrodes by electrodeposition. J. Electroanal. Chem. 2010, 348, 119. [Google Scholar] [CrossRef]
- Zhou, X.Z.; Liu, S.Q.; Yu, H.X.; Xu, A.L.; Li, J.S.; Sun, X.Y.; Shen, J.Y.; Han, W.Q.; Wang, L.J. Electrochemical oxidation of pyrrole, pyrazole and tetrazole using a TiO2 nanotubes based SnO2-Sb/3D highly ordered macro-porous PbO2 electrode. J. Electroanal. Chem. 2018, 826, 181. [Google Scholar] [CrossRef]
- Xu, A.L.; Dai, X.; Wei, K.J.; Han, W.Q.; Li, J.S.; Sun, X.Y.; Shen, J.Y.; Wang, L.J. Preparation and characterization of a TiO2-NT/SnO2-Sb tubular porous electrode with long service lifetime for wastewater treatment process. RSC ADV 2017, 7, 37806. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.Q.; Wang, Y.; Zhou, X.Z.; Han, W.Q.; Li, J.S.; Sun, X.Y.; Shen, J.S.; Wang, L.J. Improved degradation of the aqueous flutriafol using a nanostructure macroporous PbO2 as reactive electrochemical membrane. Electrochim. Acta 2017, 253, 357. [Google Scholar] [CrossRef]
- Comisso, N.; Cattarin, S.; Guerriero, P.; Mattarozzi, L.; Musiani, M.; Verlato, E. Oxygen bubble–templated anodic deposition of porous PbO2. Electrochem. Commun. 2015, 60, 144. [Google Scholar] [CrossRef]
- Li, X.W.; Li, J.X.; Gao, C.Y.; Chang, M. Surface modification of titanium membrane by chemical vapor deposition and its electrochemical self-cleaning. Appl. Surf. Sci. 2011, 258, 489. [Google Scholar] [CrossRef]
- Formoso, P.; Pantuso, E.; De Filpo, G.; Nicoletta, F.P. Electro-conductive membranes for permeation enhancement and fouling mitigation: A Short Review. Membrane 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Li, J.; Wang, H.; Song, X.; Wang, T.; He, B.; Liang, X.; Ngo, H.H. An electrocatalytic membrane reactor with self-cleaning function for industrial wastewater treatment. Angew. Chem. Int. Ed. Engl. 2011, 123, 2196–2198. [Google Scholar] [CrossRef]
- Han, W.Q.; Chen, Y.; Wang, L.J.; Sun, X.Y.; Li, J.S. Mechanism and kinetics of electrochemical degradation of isothiazolin-ones using Ti/SnO2–Sb/PbO2 anode. Desalination 2011, 276, 1. [Google Scholar] [CrossRef]
- Li, C.; Zhu, J.T.; Zhao, Z.K.; Wang, J.; Yang, Q.P.; Sun, H.F.; Jiang, B. An efficient and robust flow-through electrochemical Ti4O7 membrane system for simultaneous Cr(VI) reduction and Cr immobilization with membrane cleaning by a periodic polarity reversal strategy. Sep. Purif. Technol. 2022, 297, 121424. [Google Scholar] [CrossRef]
- Fu, W.C.; Wang, X.Y.; Zheng, J.J.; Liu, M.X.; Wang, Z.W. Antifouling performance and mechanisms in an electrochemical ceramic membrane reactor for wastewater treatment. J. Membr. Sci. 2019, 570–571, 355. [Google Scholar] [CrossRef]







| Cycle | Normalized Flux (%) | |
|---|---|---|
| Fouling | Regeneration | |
| 1st | 0.77 | 103.57 |
| 2nd | 0.81 | 111.29 |
| 3rd | 0.68 | 105.43 |
| 4th | 0.17 | 107.48 |
| 5th | 0.41 | 108.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, L.; Zhang, Y.; Han, W.; Wei, K. Membrane Fouling and Electrochemical Regeneration at a PbO2-Reactive Electrochemical Membrane: Study on Experiment and Mechanism. Membranes 2022, 12, 814. https://doi.org/10.3390/membranes12080814
Gu L, Zhang Y, Han W, Wei K. Membrane Fouling and Electrochemical Regeneration at a PbO2-Reactive Electrochemical Membrane: Study on Experiment and Mechanism. Membranes. 2022; 12(8):814. https://doi.org/10.3390/membranes12080814
Chicago/Turabian StyleGu, Liankai, Yonghao Zhang, Weiqing Han, and Kajia Wei. 2022. "Membrane Fouling and Electrochemical Regeneration at a PbO2-Reactive Electrochemical Membrane: Study on Experiment and Mechanism" Membranes 12, no. 8: 814. https://doi.org/10.3390/membranes12080814
APA StyleGu, L., Zhang, Y., Han, W., & Wei, K. (2022). Membrane Fouling and Electrochemical Regeneration at a PbO2-Reactive Electrochemical Membrane: Study on Experiment and Mechanism. Membranes, 12(8), 814. https://doi.org/10.3390/membranes12080814







