New Generation of Compositional Aquivion®-Type Membranes with Nanodiamonds for Hydrogen Fuel Cells: Design and Performance
Abstract
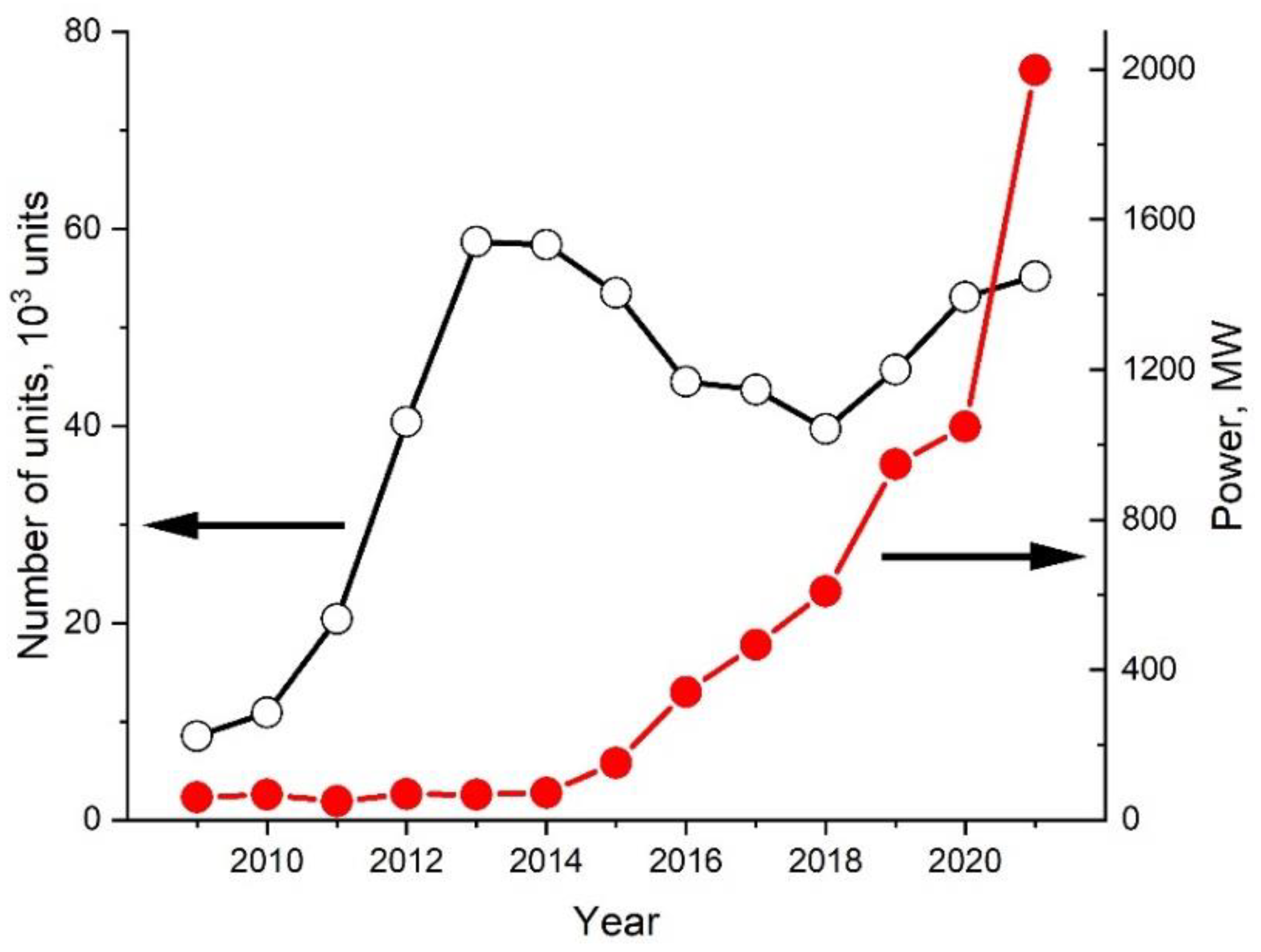
:1. Introduction
2. Experimental
2.1. Samples Preparation
2.1.1. Preparation of Aquivion®-Type Membranes with and without Nanodiamonds
2.1.2. MEA Preparation
- (1)
- Aquivion®-type membranes in an SO3H+ form with an EW of 897 ± 3 g-eq/mol and different DND contents (0, 0.5, and 2.6% wt.) were used as the proton-conducting membrane;
- (2)
- Platinized carbon black (Pt/C) containing 40% Pt, a commercial product of the E-TEK brand, was used as an electrocatalyst;
- (3)
- A perfluorinated short-side-chain (Aquivion®-type) ionomer solution in an SO3H+ form with 4.2 wt.% and an EW of 790 g-eq/mol in an n-propanol/water/ethanol mixture;
- (4)
- N-propanol; and
- (5)
- Deionized water (R > 18 MΩ cm).
2.2. Methods of Characterization
2.2.1. Proton Conductivity Measurements
2.2.2. Scanning Electron Microscopy
2.2.3. Stress–Strain Mechanical Tests
2.2.4. Small-Angle Neutron Scattering
2.2.5. Thermogravimetric Analysis
2.2.6. Electrochemical Measurements
Samples activation
Measurements
- (1)
- open circuit voltage (OCV) vs. time;
- (2)
- voltammograms (VAC); and
- (3)
- the dependences of the current density in the potentiostatic mode at a voltage E = 0.65 V vs. time.
3. Results and Discussion
3.1. Proton Conductivity of the Compositional Membranes
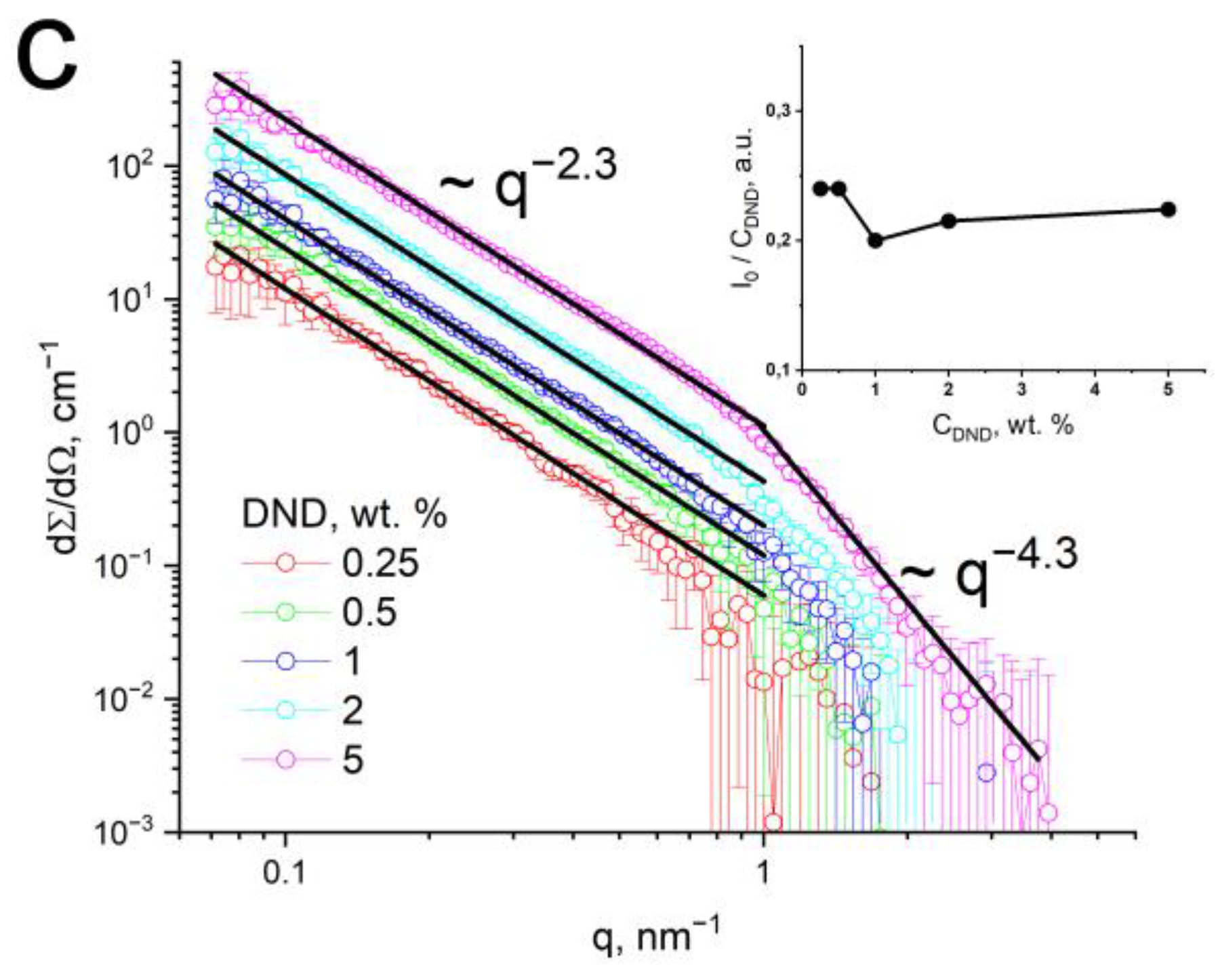
3.2. Structural Studies by SANS
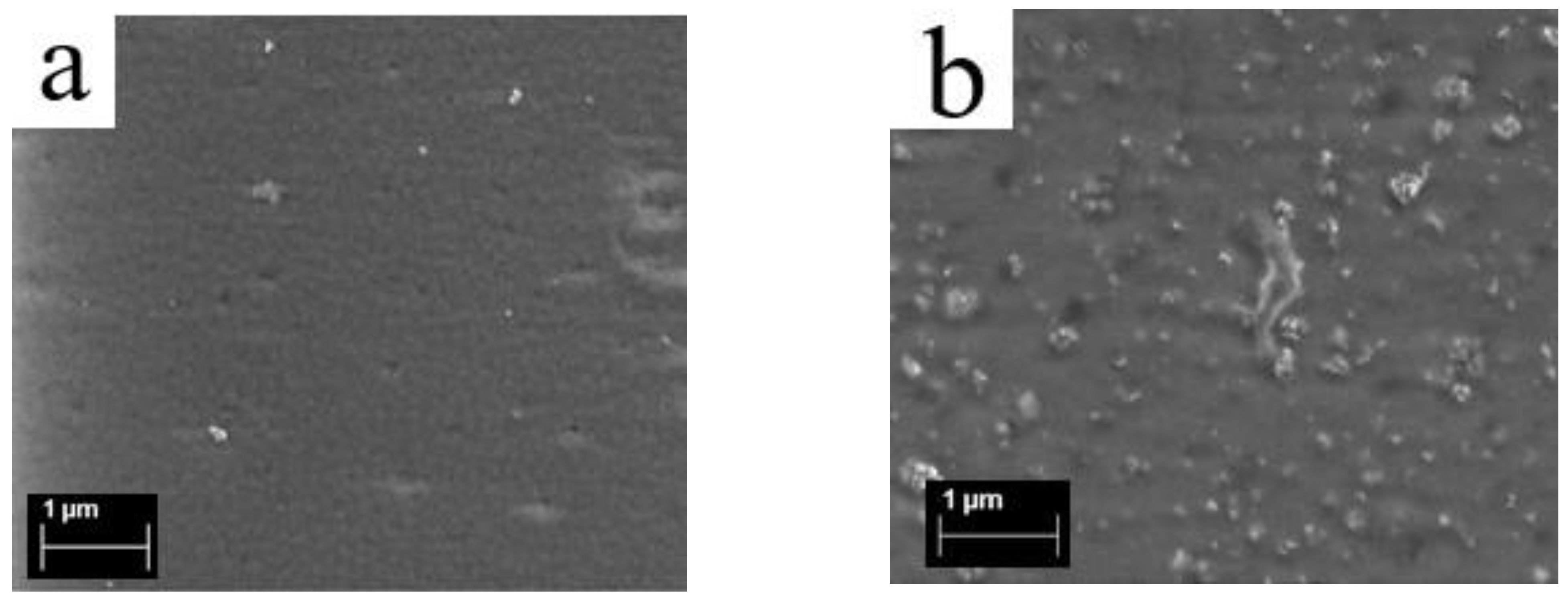
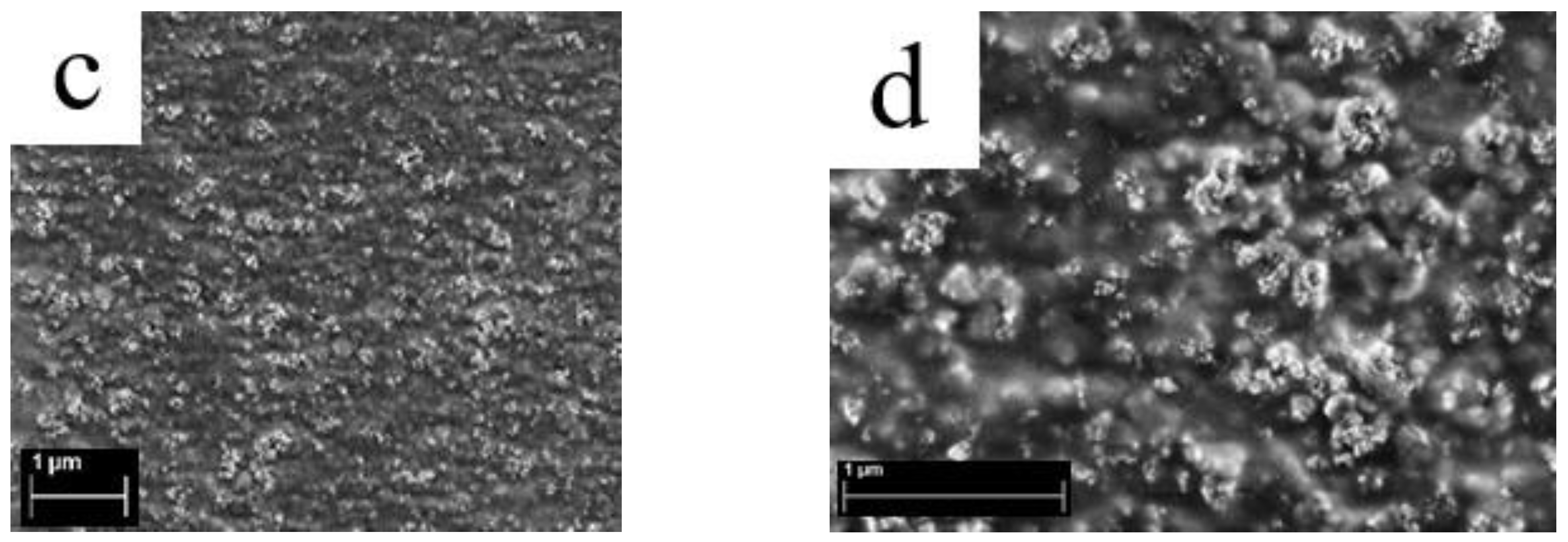
3.3. Surface Structure of Membranes from Scanning Electron Microscopy
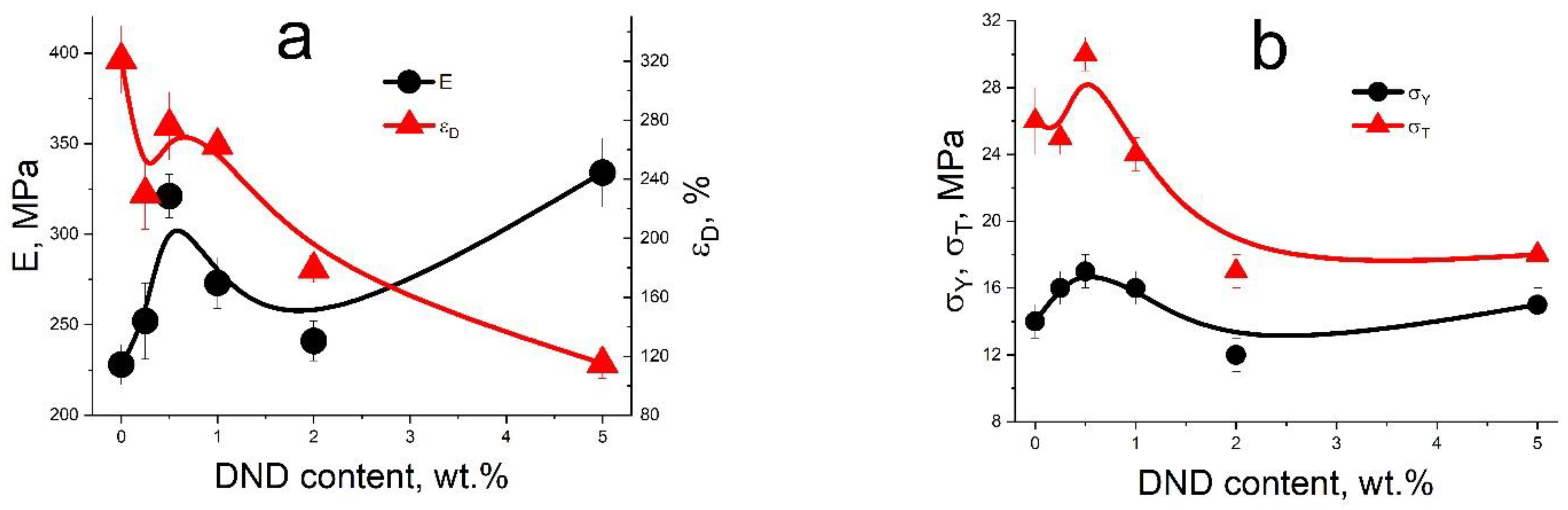
3.4. Mechanical Tests of Compositional Membranes
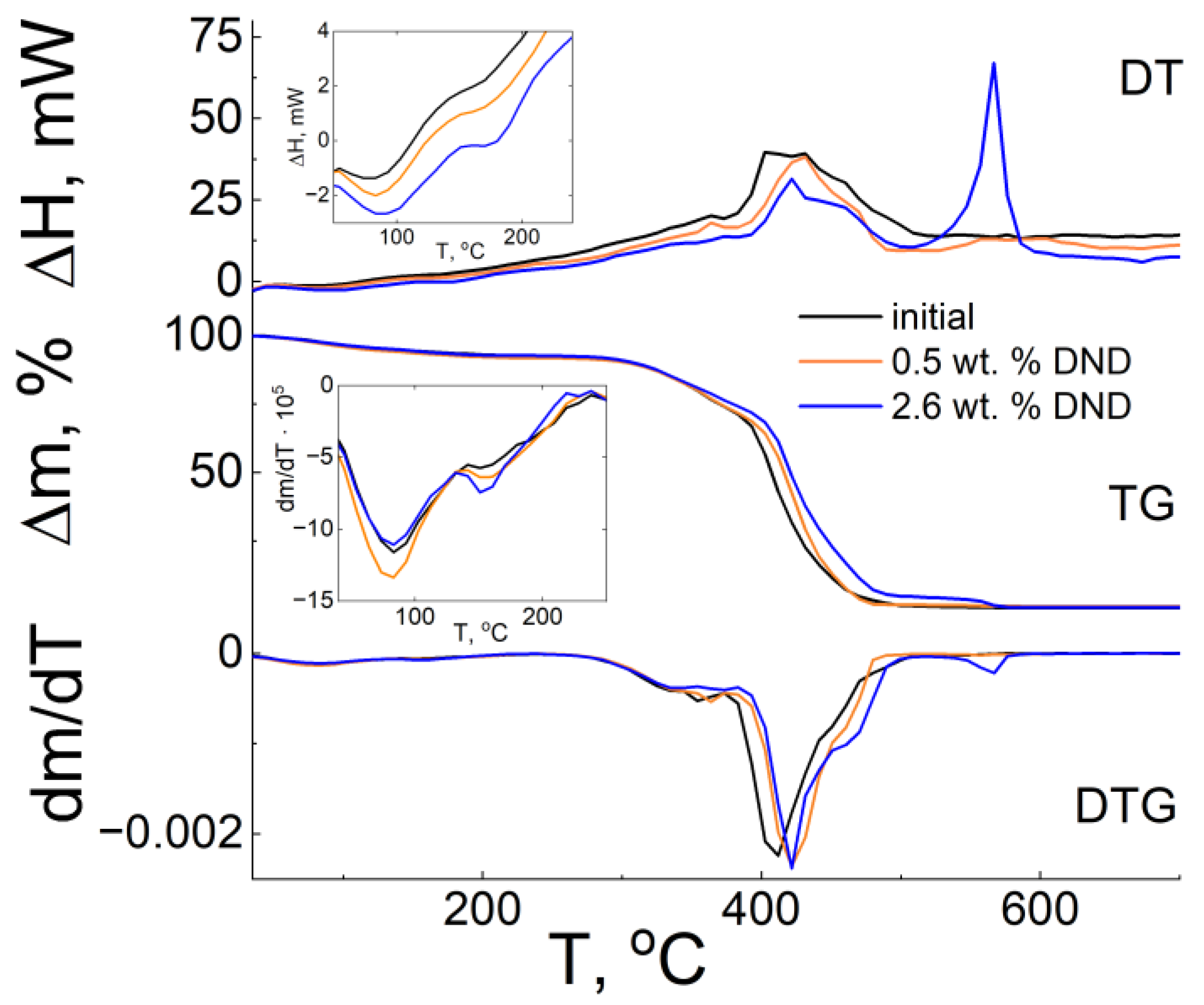
3.5. Thermogravimetric Analysis (TGA)
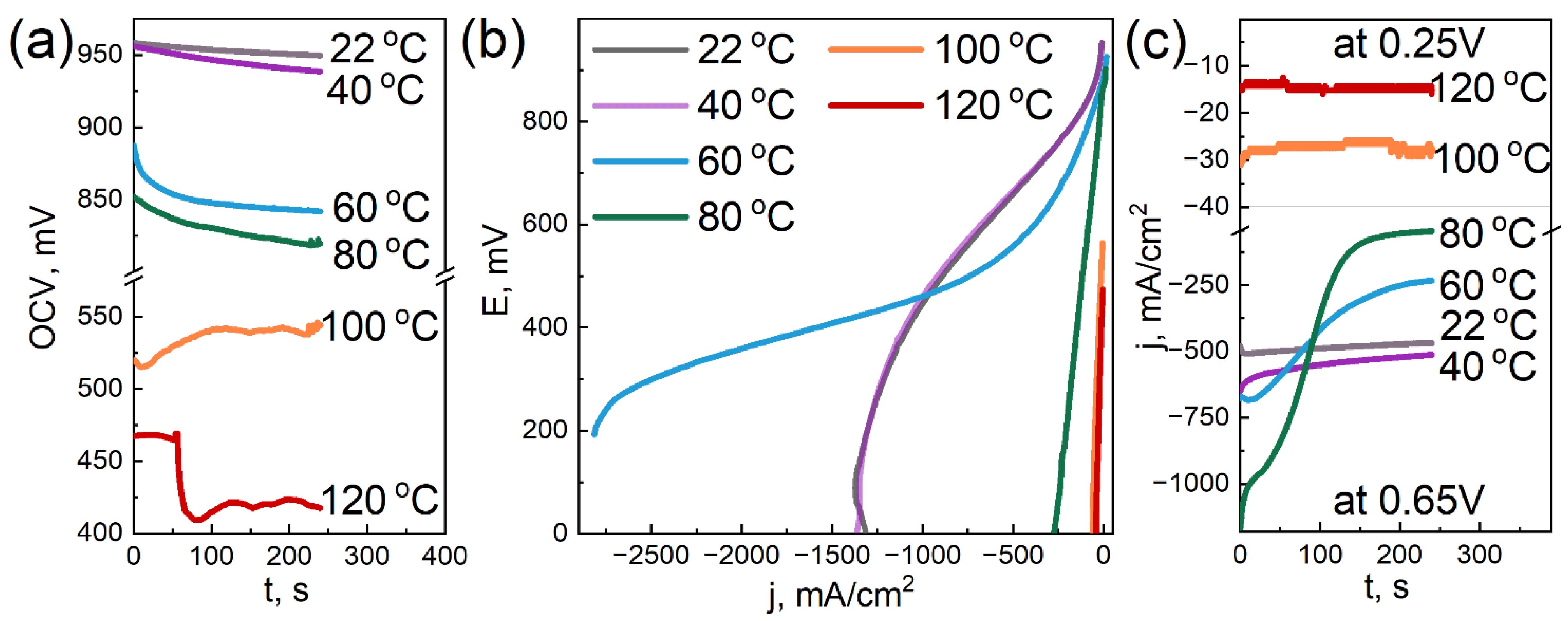
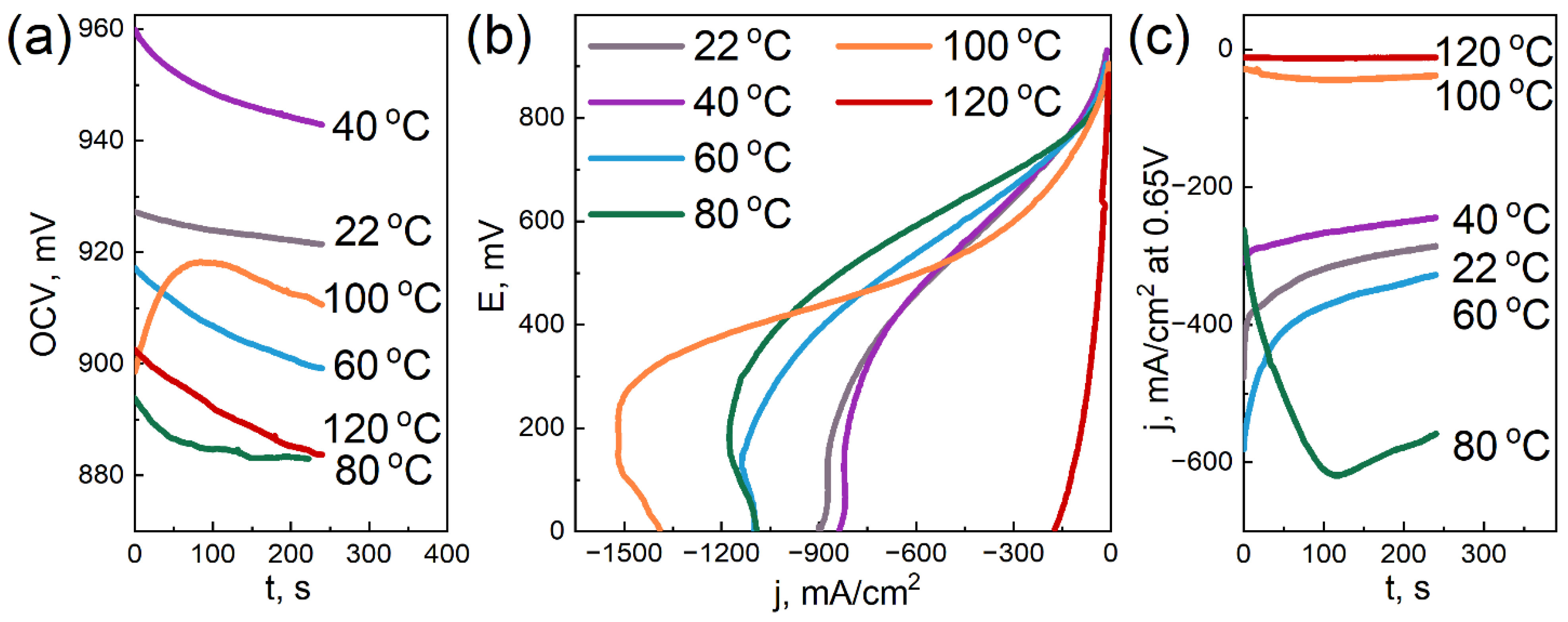
3.6. Electrochemical Tests
4. Conclusions
- the addition of DNDs had a significant effect on the electrochemical performance of the membrane at elevated temperatures;
- the compositional membrane with a 0.5 wt.% DND demonstrated the best water-retaining capacity;
- at 120 °C, the membranes with 0.5 and 2.6 wt.% DNDs showed the lowest ohmic loss due to moisture loss compared to the sample without DNDs; and
- the sample without DNDs demonstrated a sharp drop in current density to very low values, already at 80 °C, while the compositional samples with DNDs showed greater resistance to elevated temperatures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, J.M.; Edwards, P.P.; Dobson, P.J.; Owen, G.P. Decarbonising energy: The developing international activity in hydrogen technologies and fuel cells. J. Energy Chem. 2020, 51, 405–415. [Google Scholar] [CrossRef] [PubMed]
- World Energy Outlook 2019; International Energy Agency: Paris, France, 2019.
- Van Hoecke, L.; Laffineur, L.; Campe, R.; Perreault, P.; Verbruggen, S.W.; Lenaerts, S. Challenges in the use of hydrogen for maritime applications. Energy Environ. Sci. 2021, 14, 815–843. [Google Scholar] [CrossRef]
- Alent’ev, A.Y.; Volkov, A.V.; Vorotyntsev, I.V.; Maksimov, A.L.; Yaroslavtsev, A.B. Membrane technologies for decarbonization. Membr. Membr. Technol. 2021, 3, 255–273. [Google Scholar] [CrossRef]
- Ivanchev, S.S.; Myakin, S.V. Polymer membranes for fuel cells: Manufacture, structure, modification, properties. Russ. Chem. Rev. 2010, 79, 101–117. [Google Scholar] [CrossRef]
- Yakubson, K.I. Prospects for Production and Use of Hydrogen as one of Directions of the Development of Low-Carbon Economy in the Russian Federation. Russ. J. Appl. Chem. 2020, 93, 1775–1795. [Google Scholar] [CrossRef]
- Filippov, S.P.; Yaroslavtsev, A.B. Hydrogen energy: Development prospects and materials. Russ. Chem. Rev. 2021, 90, 627–643. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, P.; Niu, M.; Maddy, J. The survey of key technologies in hydrogen energy storage. Int. J. Hydren Energy 2016, 41, 14535–14552. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H. Review the development of first-generation redox flow batteries: Iron-chromium system. ChemSusChem 2022, 15, e202101798. [Google Scholar] [CrossRef]
- Danilczuck, M.; Lancucki, L.; Schlick, S.; Hamrock, S.J.; Haugen, G.M. In-depth profiling of degradationin processes in a fuel cell: 2D spectral-spatial FTIR spectra of Nafion membranes. ASC Macro Lett. 2012, 1, 280–285. [Google Scholar] [CrossRef]
- Banergjee, S.; Curtin, D.E. Nafion® perfluorinated membranes in fuel cell. J. Fluor. Chem. 2004, 125, 1211–1216. [Google Scholar] [CrossRef]
- Hiesgen, R.; Aleksandrova, E.; Meichsner, G.; Wehl, I.; Roduner, E.; Friedrich, K.A. High-resolution imaging of ion conductivity of Nafion® membranes with electrochemical atomic force microscopy. Electrochim. Acta 2009, 55, 423–429. [Google Scholar] [CrossRef]
- Xie, T. Tunable polymer multi-shape memory effect. Nature 2010, 464, 267–270. [Google Scholar] [CrossRef]
- Komarov, P.V.; Khalatur, P.G.; Khokhlov, A.R. Large-scale atomistic and quantum-mechanical simulations of a Nafion membrane: Morphology, proton solvation and charge transport. Beilstein J. Nanotechnol. 2013, 4, 567–587. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Li, J.; Cho, C. Experimental investigation and discussion on the mechanical endurance limit of Nafion membrane used in proton membrane fuel cell. Energies 2014, 7, 6401–6411. [Google Scholar] [CrossRef]
- Kreuer, K.D.; Schuster, M.; Obliers, B.; Diat, O.; Traub, U.; Fuchs, A.; Klock, U.; Paddison, S.J.; Maier, J. Short-side-chain proton conducting perfluorosulfonic acid ionomers: Why they perform better in PEM fuel cells. J. Power Source 2008, 178, 499–509. [Google Scholar] [CrossRef]
- Arico, A.S.; Di Blasi, A.; Brunaccini, G.; Sergi, F.; Dispenza, G.; Andaloro, L.; Ferraro, M.; Antonucci, V.; Asher, P.; Buche, S. High temperature operation of a solid polymer electrolyte fuel cell stack based on a new ionomer membrane. Fuel Cells 2010, 10, 1013–1023. [Google Scholar] [CrossRef]
- Jeon, Y.; Hwang, H.-k.; Park, J.; Hwang, H.; Shul, Y.-G. Temperature-dependent performance of the polymer electrolyte membrane fuel cell using short-side-chain perfluorosulfonic acid ionomer. Int. J. Hydrogen Energy 2014, 39, 11690–11699. [Google Scholar] [CrossRef]
- Giancola, S.; Zatoń, M.; Reyes-Carmona, A.; Dupont, M.; Donnadio, A.; Cavaliere, S.; Roziere, J.; Jones, D.J. Composite short side chain PFSA membranes for PEM water electrolysis. J. Membr. Sci. 2019, 570, 69–76. [Google Scholar] [CrossRef]
- Primachenko, O.N.; Marinenko, E.A.; Odinokov, A.S.; Kononova, S.V.; Kulvelis, Y.V.; Lebedev, V.T. State of the art and prospects in the development of proton conducting perfluorinated membranes with short side chains: A review. Polym. Adv. Technol. 2021, 32, 1386–1408. [Google Scholar] [CrossRef]
- Thoennes, M.; Busse, A.; Eckstein, L. Forecast of performance parameters of automotive fuel cell systems—Delphi study results. Fuel Cells 2014, 14, 781–791. [Google Scholar] [CrossRef]
- Halim, R.; Kirstein, L.; Merk, O.; Martinez, L. Decarbonization pathways for international maritime transport: A model-based policy impact assessment. Sustainability 2018, 10, 2243. [Google Scholar] [CrossRef]
- Siracusano, S.; Oldani, C.; Navarra, M.A.; Tonella, S.; Mazzapioda, L.; Briguglio, N.; Arico, A.S. Chemically stabilized extruded and recast short side chain Aquivion® proton exchange membranes for high current density operation in water electrolysis. J. Membr. Sci. 2019, 578, 136–148. [Google Scholar] [CrossRef]
- The Fuel Cell Industry Reviews 2014, 2017, 2021. Available online: https://fuelcellindustryreview.com/ (accessed on 19 July 2022).
- Wong, C.Y.; Wong, W.Y.; Ramya, K.; Khali, M.; Loh, K.S.; Daud, K.L.; Lim, W.R.W.; Walvekar, R.; Kadhum, A.A.H. Additives in proton exchange membranes for low and high-temperature fuel cell applications: A review. Int. J. Hydrogen Energy 2019, 44, 6116–6135. [Google Scholar] [CrossRef]
- Bakangura, E.; Wu, L.; Ge, L.; Yang, Z.; Xu, T. Mixed matrix proton exchange membranes for fuel cells: State of the art and perspectives. Prog. Polym. Sci. 2016, 57, 103–152. [Google Scholar] [CrossRef]
- Simari, C.; Stallworth, P.; Peng, J.; Coppola, L.; Greenbaum, S.; Nicotera, I. Graphene oxide and sulfonated-derivative: Proton transport properties and electrochemical behavior of Nafion-based nanocomposites. Electrochim. Acta 2019, 297, 240–249. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Q.; Zheng, X.; Yin, Y.; Wu, H.; Jiang, Z. Incorporating phosphoric acid-functionalized polydopamine into Nafion polymer by in situ sol-gel method for enhanced proton conductivity. J. Membr. Sci. 2019, 570, 236–244. [Google Scholar] [CrossRef]
- Ying, Y.P.; Kamarudin, S.K.; Masdar, M.S. Silica-related membranes in fuel cell applications: An overview. Int. J. Hydrogen Energy 2018, 43, 16068–16084. [Google Scholar] [CrossRef]
- Park, J.-S.; Shin, M.-S.; Kim, C.-S. Proton exchange membranes for fuel cell operation at low relative humidity and intermediate temperature: An updated review. Curr. Opin. Electrochem. 2017, 5, 43–55. [Google Scholar] [CrossRef]
- Pethaiah, S.S.; Kalaignan, G.P.; Ulaganathan, M.; Arunkumar, J. Preparation of durable nanocatalyzed MEA for PEM fuel cell applications. Ionics 2011, 17, 361–366. [Google Scholar] [CrossRef]
- Kim, D.J.; Jo, M.J.; Nam, S.Y. A review of polymer-nanocomposite electrolyte membranes for fuel cell application. J. Ind. Eng. Chem. 2015, 21, 36–52. [Google Scholar] [CrossRef]
- Sgambetterra, M.; Brutti, S.; Allodi, V.; Mariotto, G.; Panero, S.; Navarra, M.A. Critical filler concentration in sulfated titania-added Nafion™ membranes for fuel cell applications. Energies 2016, 9, 272. [Google Scholar] [CrossRef]
- Porozhnyy, M.V.; Shkirskaya, S.A.; Butylskii, D.Y.; Dotsenko, V.V.; Safronova, E.Y.; Yaroslavtsev, A.B.; Deabate, S.; Huguet, P.; Nikonenko, V.V. Physicochemical and electrochemical characterization of Nafion-type membranes with embedded silica nanoparticles: Effect of functionalization. Electrochim. Acta 2021, 370, 137689. [Google Scholar] [CrossRef]
- Dideikin, A.T.; Aleksenskii, A.E.; Baidakova, M.V.; Brunkov, P.N.; Brzhezinskaya, M.; Davydov, V.Y.; Levitskii, V.S.; Kidalov, S.V.; Kukushkina, Y.A.; Kirilenko, D.A. Rehybridization of carbon on facets of detonation diamond nanocrystals and forming hydrosols of individual particles. Carbon 2017, 122, 737–745. [Google Scholar] [CrossRef]
- Aleksenskiy, A.E.; Eydelman, E.D.; Vul’, A.Y. Deagglomeration of detonation nanodiamonds. Nanosci. Nanotechnol. Lett. 2011, 3, 68–74. [Google Scholar] [CrossRef]
- Kulvelis, Y.V.; Primachenko, O.N.; Odinokov, A.S.; Shvidchenko, A.V.; Bairamukov, V.Y.; Gofman, I.V.; Lebedev, V.T.; Ivanchev, S.S.; Vul, A.Y.; Kuklin, A.I. Composite proton-conducting membranes with nanodiamonds. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 140–146. [Google Scholar] [CrossRef]
- Kulvelis, Y.V.; Primachenko, O.N.; Gofman, I.V.; Odinokov, A.S.; Shvidchenko, A.V.; Yudina, E.B.; Marinenko, E.A.; Lebedev, V.T.; Vul, A.Y. Modification of the mechanism of proton conductivity of the perfluorinated membrane copolymer by nanodiamonds. Russ. Chem. Bull. Int. Ed. 2021, 70, 1713–1717. [Google Scholar] [CrossRef]
- Primachenko, O.N.; Kulvelis, Y.V.; Lebedev, V.T.; Odinokov, A.S.; Bayramukov, V.Y.; Marinenko, E.A.; Gofman, I.V.; Shvidchenko, A.V.; Vul, A.Y.; Ivanchev, S.S. Perfluorinated proton-conducting membrane composites with functionalized nanodiamonds. Membr. Membr. Technol. 2020, 2, 1–9. [Google Scholar] [CrossRef]
- Lobanova, M.S.; Postnov, V.N.; Novikov, A.G.; Mel’nikova, N.A.; Murin, I.V. Aquivion-based composite membranes with nanosized additives. Mosc. Univ. Chem. Bull. 2020, 75, 121–124. [Google Scholar] [CrossRef]
- Kulvelis, Y.V.; Lebedev, V.T.; Primachenko, O.N.; Shvidchenko, A.V.; Kuklin, A.I. Structure of Compositional Perfluorinated Membranes with Nanodiamonds. In Proceedings of the Neutron Scattering in Condensed Matter Research (RNICS-2021), Ekaterinburg, Russia, 27 September–1 October 2021; Book of Abstracts, 108. ISBN 978-5-9500855-7-4. (In Russian). [Google Scholar]
- Williams, O.A.; Hees, J.; Dieker, C.; Jager, W.; Kirste, L.; Nebel, C.E. Size-Dependent Reactivity of Diamond Nanoparticles. ACS Nano 2010, 4, 4824–4830. [Google Scholar] [CrossRef]
- Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Xu, F.; Mu, S. Nanoceramic oxide hybrid electrolyte membranes for proton exchange membrane fuel cells. J. Nanosci. Nanotechnol. 2014, 14, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Ivanchev, S.S.; Primachenko, O.N.; Khajkin, S.J.; Likhomanov, V.S.; Barabanov, V.G.; Odinokov, A.S. Method of Producing Copolymer of Tetrafluoroethylene with 2-Fluorosulphonyl Perfluoroethylvinyl ether—Proton-Conducting Membrane Precursor—by Emulsion Copolymerization. RU Patent No. 2545182, 27 March 2015. Bull. No. 9. [Google Scholar]
- Ivanchev, S.S.; Odinokov, A.S.; Primachenko, O.N.; Tyulmankov, V.P.; Marinenko, E.A. Method of Obtaining Perfluoro-3-Oxapentenesulfonyl Fluoride Copolymer and Tetrafluoroethylene as Precursor of Perfluorinated Proton-Conducting Membranes. RU Patent No. 2671812, 7 November 2018. Bull. No. 31. [Google Scholar]
- Primachenko, O.N.; Odinokov, A.S.; Marinenko, E.A.; Kulvelis, Y.V.; Barabanov, V.G.; Kononova, S.V. Influence of sulfonyl fluoride monomers on the mechanism of emulsion copolymerization with the preparation of proton-conducting membrane precursors. J. Fluor. Chem. 2021, 244, 109736. [Google Scholar] [CrossRef]
- Primachenko, O.N.; Odinokov, A.S.; Barabanov, V.G.; Tyul’mankov, V.P.; Marinenko, E.A.; Gofman, I.V.; Ivanchev, S.S. Relationship between the morphology, nanostructure and strength properties of Aquivion® type perfluorinated proton-conducting membranes prepared by casting from solution. Russ. J. Appl. Chem. 2018, 91, 101–104. [Google Scholar] [CrossRef]
- Kuklin, A.I.; Ivankov, A.I.; Soloviov, D.V.; Rogachev, A.V.; Kovalev, Y.S.; Soloviev, A.G.; Islamov, A.K.; Balasoiu, M.; Vlasov, A.V.; Kutuzov, S.A. High-throughput SANS experiment on two-detector system of YuMO spectrometer. J. Phys. Conf. Ser. 2018, 994, 012016. [Google Scholar] [CrossRef]
- Soloviev, A.G.; Solovjeva, T.M.; Ivankov, O.I.; Soloviov, D.V.; Rogachev, A.V.; Kuklin, A.I. SAS program for two-detector system: Seamless curve from both detectors. J. Phys. Conf. Ser. 2017, 848, 012020. [Google Scholar] [CrossRef]
- Kulvelis, Y.V.; Ivanchev, S.S.; Lebedev, V.T.; Primachenko, O.N.; Likhomanov, V.S.; Torok, G. Structure characterization of perfluorosulfonic short side chain polymer membranes. RSC Adv. 2015, 5, 73820–73826. [Google Scholar] [CrossRef]
- Nallet, F. De l’intensité à la structure en physico-chimie des milieux dispersés. J. Phys. IV 1999, 9, Pr.1-95–Pr.1-107. [Google Scholar] [CrossRef]
- Svergun, D.I.; Feigin, L.A. Structure Analysis by Small-Angle X-ray and Neutron Scattering; Plenum: New York, NY, USA, 1987. [Google Scholar]
- Nechitailov, A.A.; Glebova, N.V.; Tomasov, A.A.; Krasnova, A.O.; Zelenina, N.K. Study of the heterogeneity of a mixed-conducting electrochemical electrode. Tech. Phys. 2019, 64, 899–907. [Google Scholar] [CrossRef]
- Stenina, I.A.; Yaroslavtsev, A.B. Ionic mobility in ion-exchange membranes. Membranes 2021, 11, 198. [Google Scholar] [CrossRef]
- Vul, A.Y.; Eidelman, E.D.; Aleksenskiy, A.E.; Shvidchenko, A.V.; Dideikin, A.T.; Yuferev, V.S.; Lebedev, V.T.; Kul’velis, Y.V.; Avdeev, M.V. Transition sol-gel in nanodiamond hydrosols. Carbon 2017, 114, 242–249. [Google Scholar] [CrossRef]
- Lebedev, V.; Kulvelis, Y.; Kuklin, A.; Vul, A. Neutron Study of Multilevel Structures of Diamond Gels. Cond. Matter 2016, 1, 10. [Google Scholar] [CrossRef]
- Avdeev, M.V.; Tomchuk, O.V.; Ivankov, O.I.; Alexenskii, A.E.; Dideikin, A.T.; Vul, A.Y. On the structure of concentrated detonation nanodiamond hydrosols with a positive ζ potential: Analysis of small-angle neutron scattering. Chem. Phys. Lett. 2016, 658, 58–62. [Google Scholar] [CrossRef]
- Likhomanov, V.S.; Primachenko, O.N.; Ivanchev, S.S. Thermodynamic properties of water in perfluorinated membranes of Nafion and Aquivion types, prepared by emulsion polymerization. Russ. J. Appl. Chem. 2014, 87, 1314–1318. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, Y.J.; Hong, W.H.; Choi, Y.S.; Lee, H.K. Influence of morphology on the transport properties of perfluorosulfonate ionomers/polypyrrole composite membrane. Macromolecules 2005, 38, 2289–2295. [Google Scholar] [CrossRef]
- Malathi, M.; Surendra, U. Analysis of Characteristics of PEM Fuel Cell. IJITEE 2019, 8, 2368–2370. [Google Scholar] [CrossRef]


| DND Content, wt.% | Water Uptake, wt.% | Ion Exchange Capacity, mmol/g | Proton Conductivity, S/cm | |
|---|---|---|---|---|
| 20 °C | 50 °C | |||
| 0 | 30.3 | 1.12 | 0.131 | 0.178 |
| 0.25 | 31.1 | 1.12 | 0.133 | 0.203 |
| 0.5 | 32.8 | 1.12 | 0.136 | 0.234 |
| 1.0 | 32.9 | 1.10 | 0.130 | 0.210 |
| 2.0 | 32.2 | 1.07 | 0.120 | 0.207 |
| 2.6 | 33.2 | 1.06 | 0.127 | 0.204 |
| 5.0 | 31.9 | 1.04 | 0.115 | 0.191 |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| I0, cm−1 nm−3 | 3.6 ± 1.0 | R2, nm | 3.2 ± 0.3 |
| n | 1.5 ± 0.4 | C3 | −0.6 ± 0.3 |
| Rg, nm | 0.25 | R3, nm | 4.3 ± 0.4 |
| C1 | −1.2 ± 0.2 | C4 | 0.10 ± 0.07 |
| R1, nm | 1.83 ± 0.15 | R4, nm | 12.8 ± 1.3 |
| C2 | 0.8 ± 0.3 | B, cm−1 | 0.068 ± 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Primachenko, O.N.; Kulvelis, Y.V.; Odinokov, A.S.; Glebova, N.V.; Krasnova, A.O.; Antokolskiy, L.A.; Nechitailov, A.A.; Shvidchenko, A.V.; Gofman, I.V.; Marinenko, E.A.; et al. New Generation of Compositional Aquivion®-Type Membranes with Nanodiamonds for Hydrogen Fuel Cells: Design and Performance. Membranes 2022, 12, 827. https://doi.org/10.3390/membranes12090827
Primachenko ON, Kulvelis YV, Odinokov AS, Glebova NV, Krasnova AO, Antokolskiy LA, Nechitailov AA, Shvidchenko AV, Gofman IV, Marinenko EA, et al. New Generation of Compositional Aquivion®-Type Membranes with Nanodiamonds for Hydrogen Fuel Cells: Design and Performance. Membranes. 2022; 12(9):827. https://doi.org/10.3390/membranes12090827
Chicago/Turabian StylePrimachenko, Oleg N., Yuri V. Kulvelis, Alexei S. Odinokov, Nadezhda V. Glebova, Anna O. Krasnova, Lev A. Antokolskiy, Andrey A. Nechitailov, Alexander V. Shvidchenko, Iosif V. Gofman, Elena A. Marinenko, and et al. 2022. "New Generation of Compositional Aquivion®-Type Membranes with Nanodiamonds for Hydrogen Fuel Cells: Design and Performance" Membranes 12, no. 9: 827. https://doi.org/10.3390/membranes12090827
APA StylePrimachenko, O. N., Kulvelis, Y. V., Odinokov, A. S., Glebova, N. V., Krasnova, A. O., Antokolskiy, L. A., Nechitailov, A. A., Shvidchenko, A. V., Gofman, I. V., Marinenko, E. A., Yevlampieva, N. P., Lebedev, V. T., & Kuklin, A. I. (2022). New Generation of Compositional Aquivion®-Type Membranes with Nanodiamonds for Hydrogen Fuel Cells: Design and Performance. Membranes, 12(9), 827. https://doi.org/10.3390/membranes12090827








