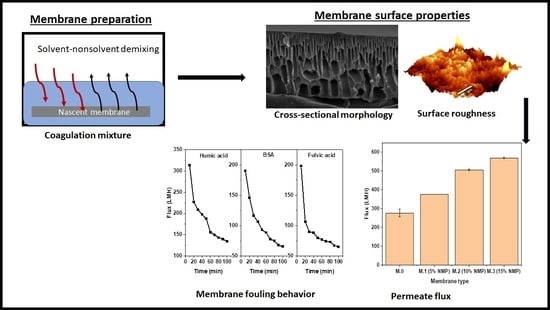
Delayed Solvent–Nonsolvent Demixing Preparation and Performance of a Highly Permeable Polyethersulfone Ultrafiltration Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
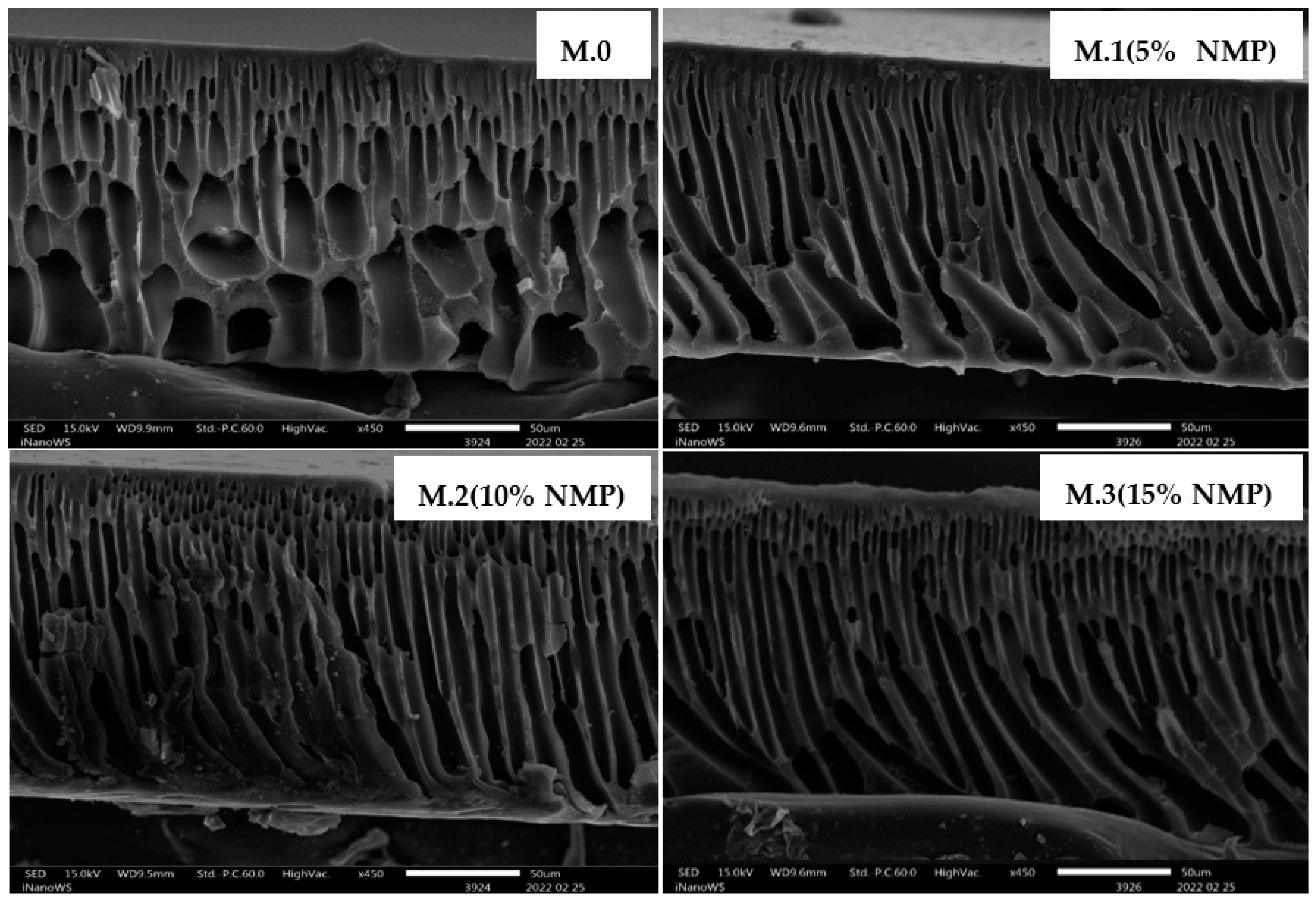
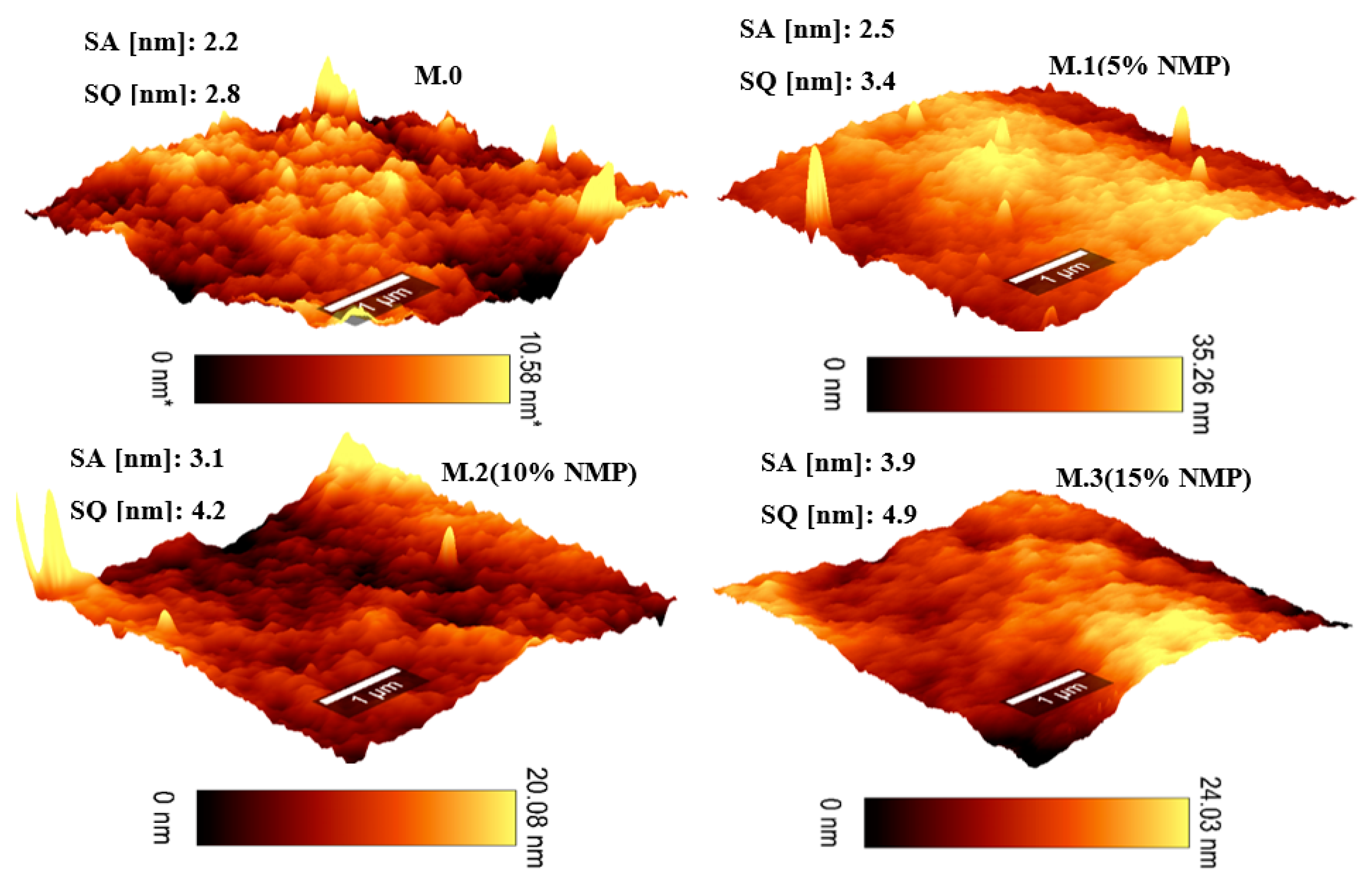
3.1. Morphological Studies
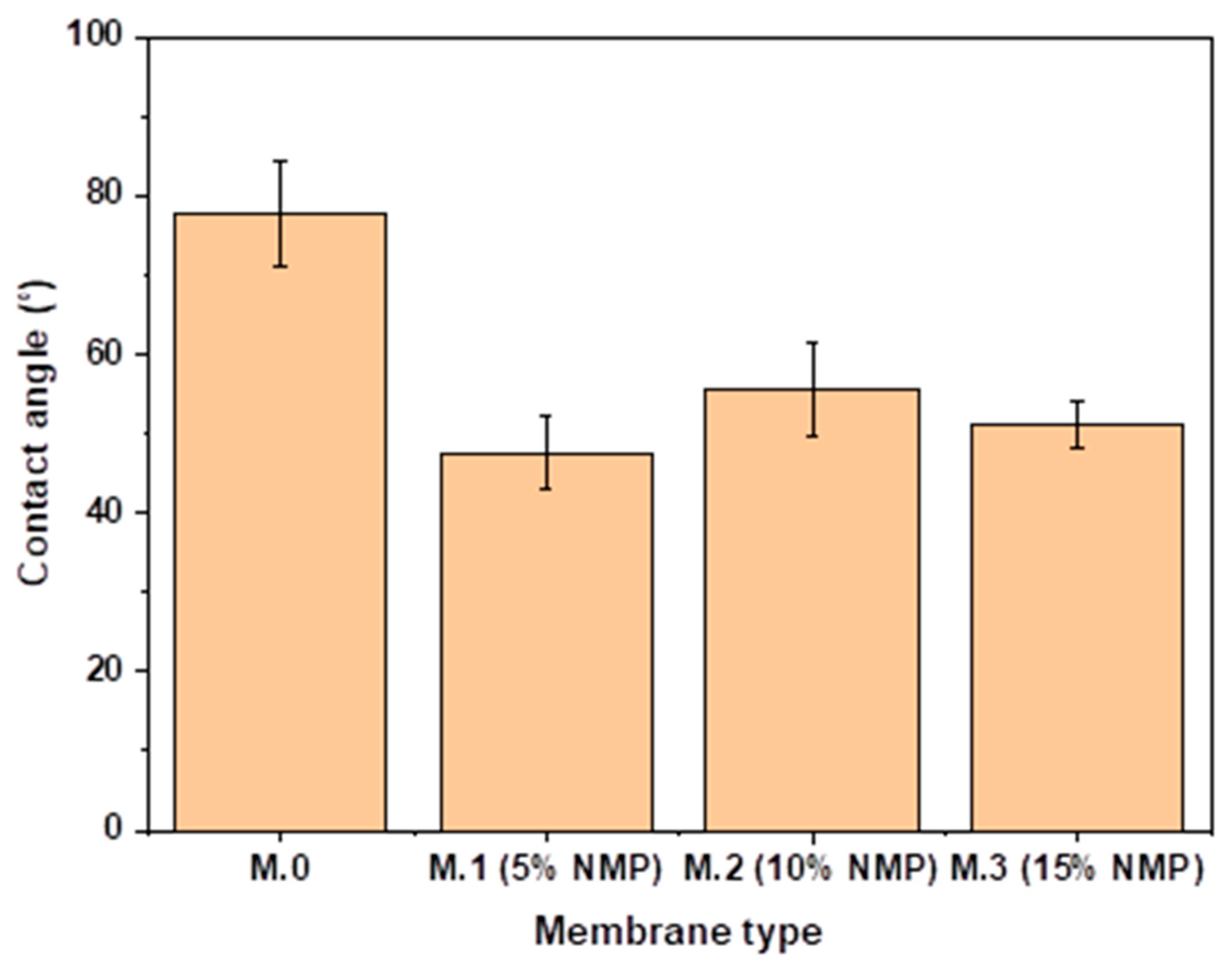
3.2. Contact Angle (Wettability)
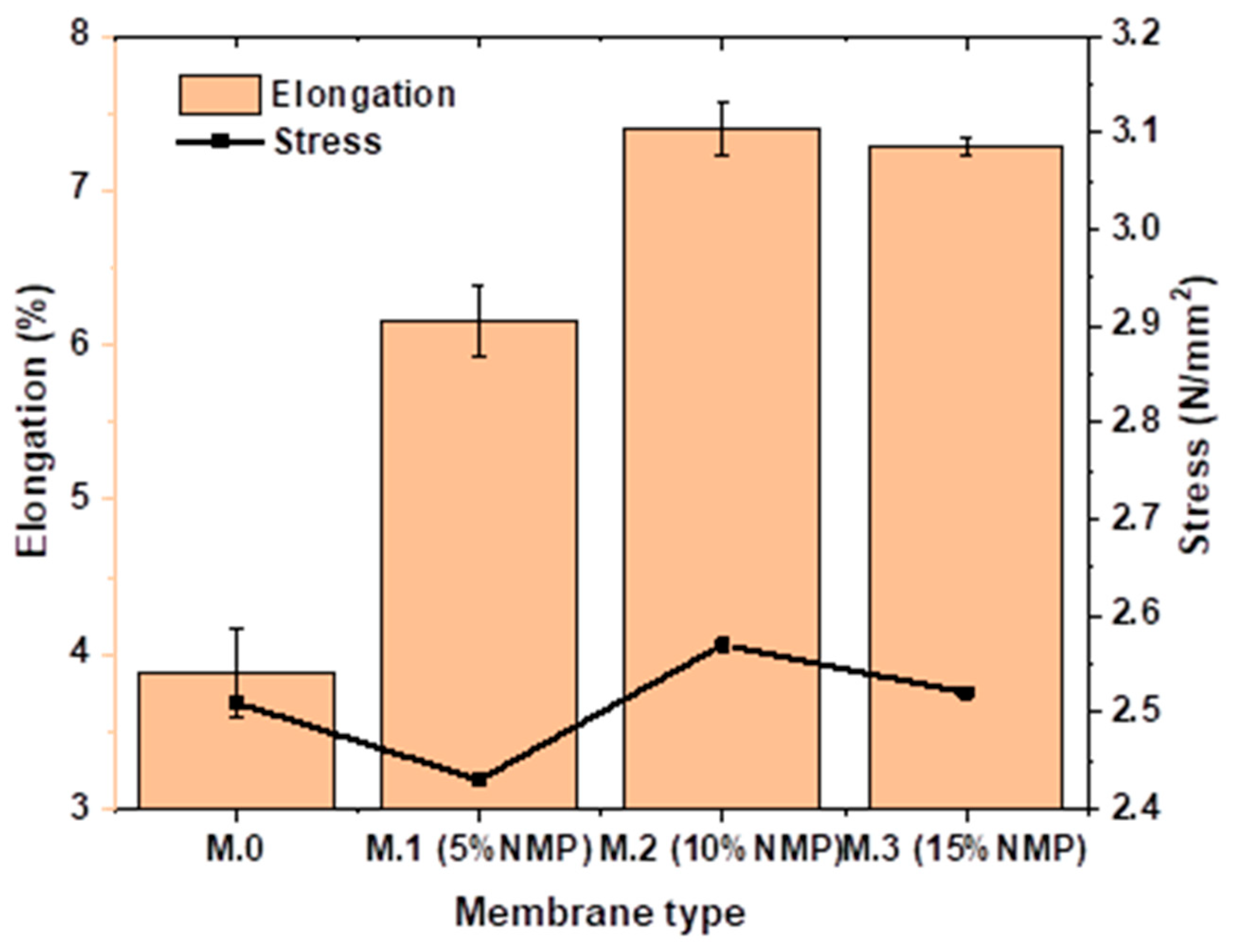
3.3. Mechanical Properties
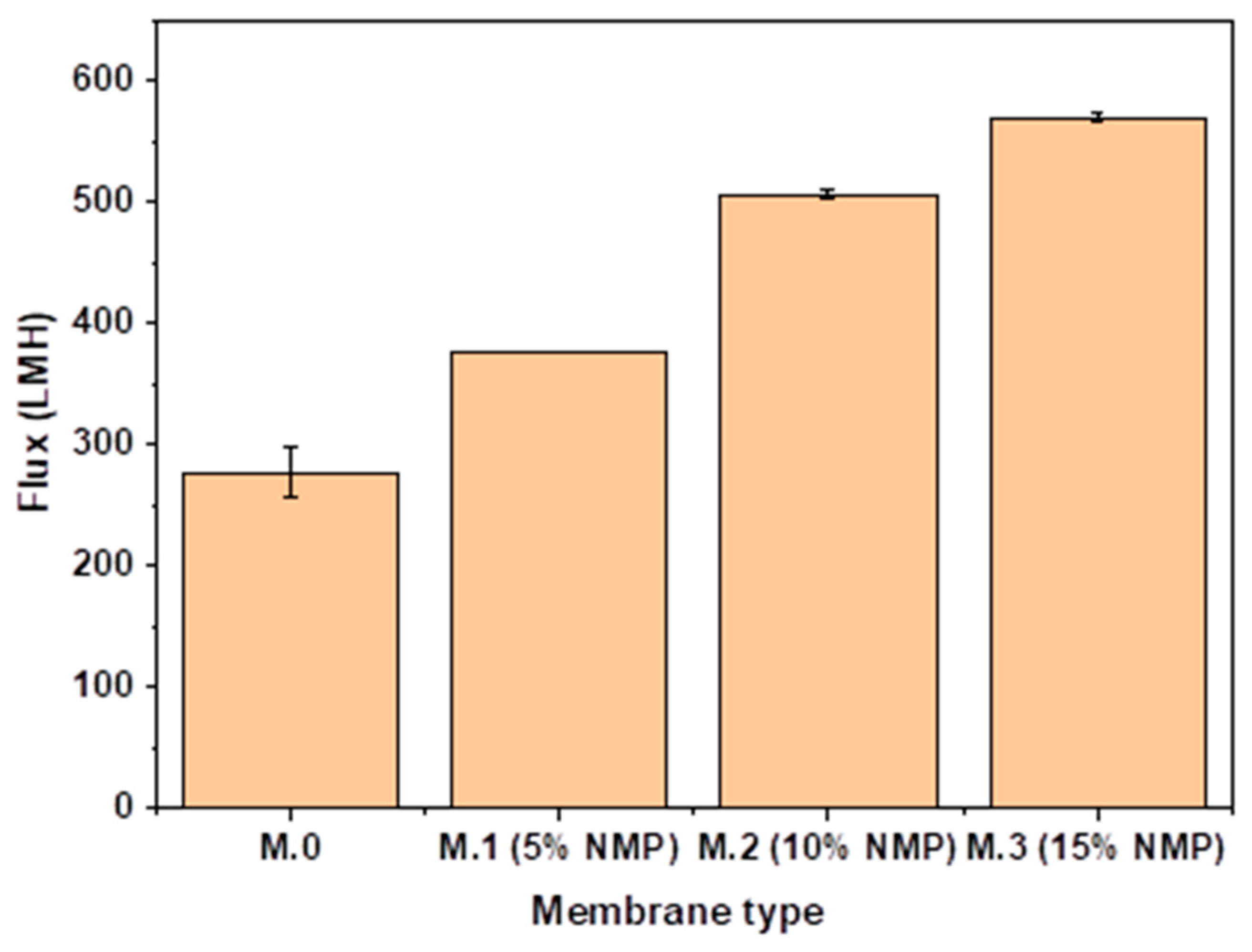
3.4. Performance: Flux and Rejection
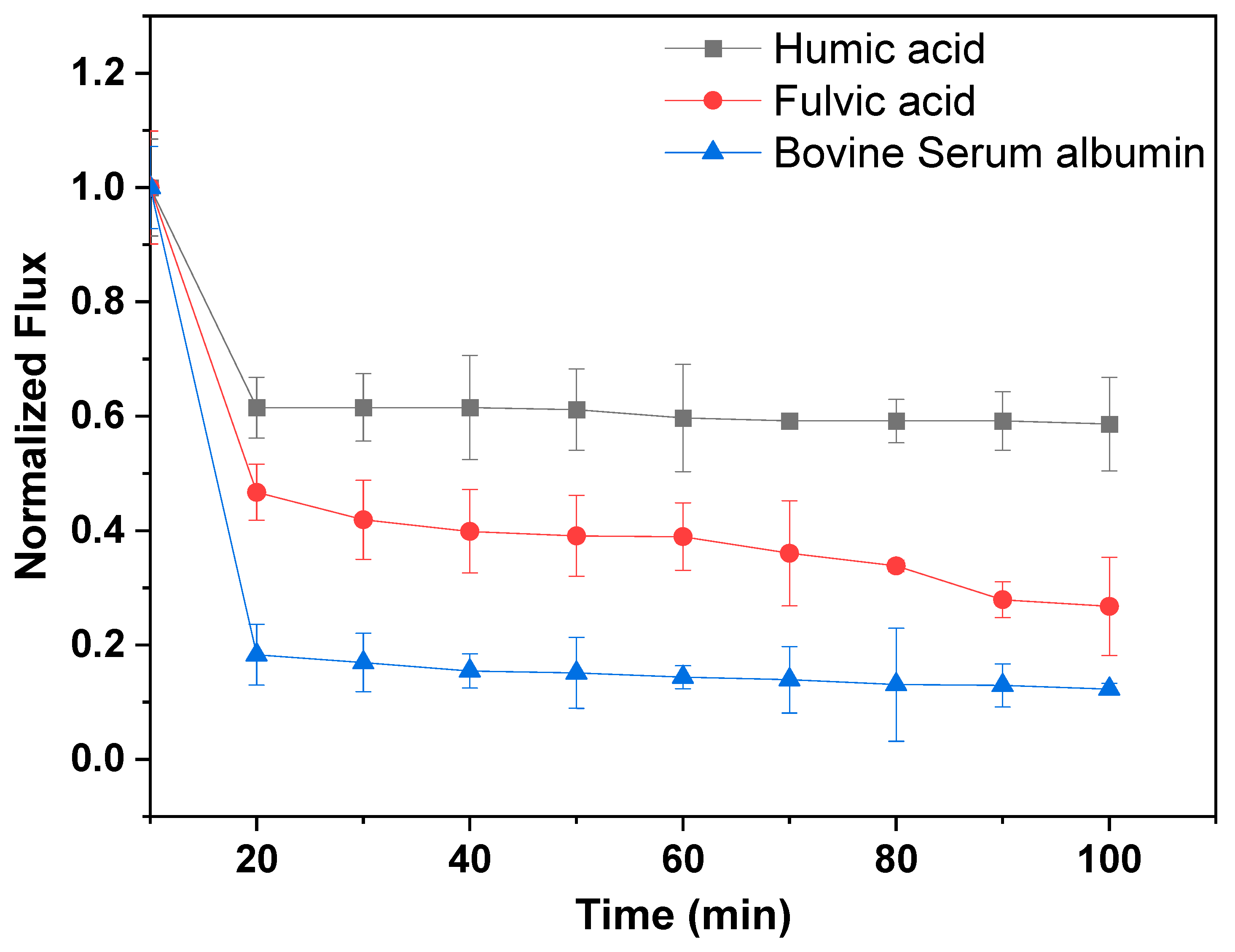
3.5. Fouling Trends and Antifouling Tests
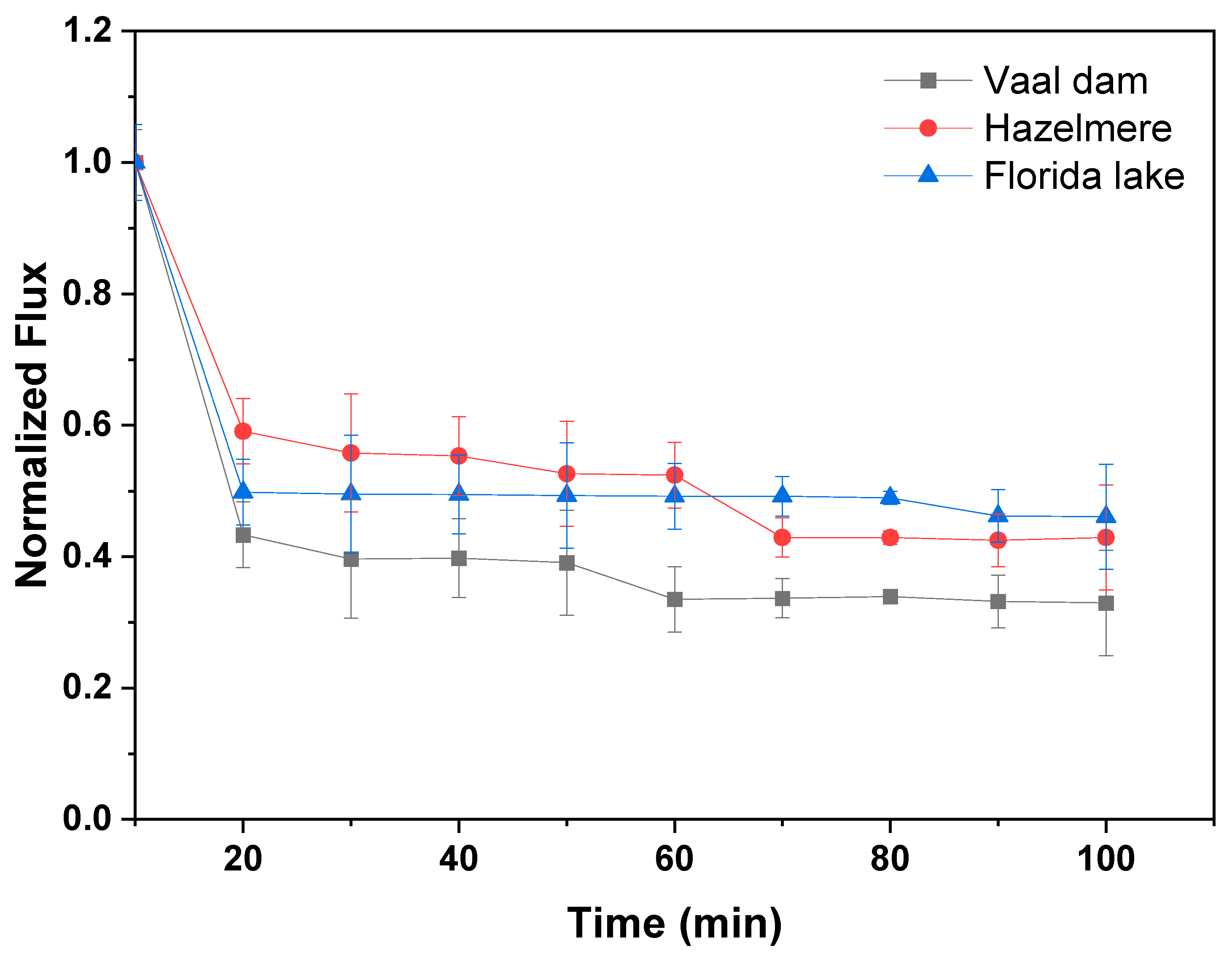
3.5.1. Normalized Flux and Fouling
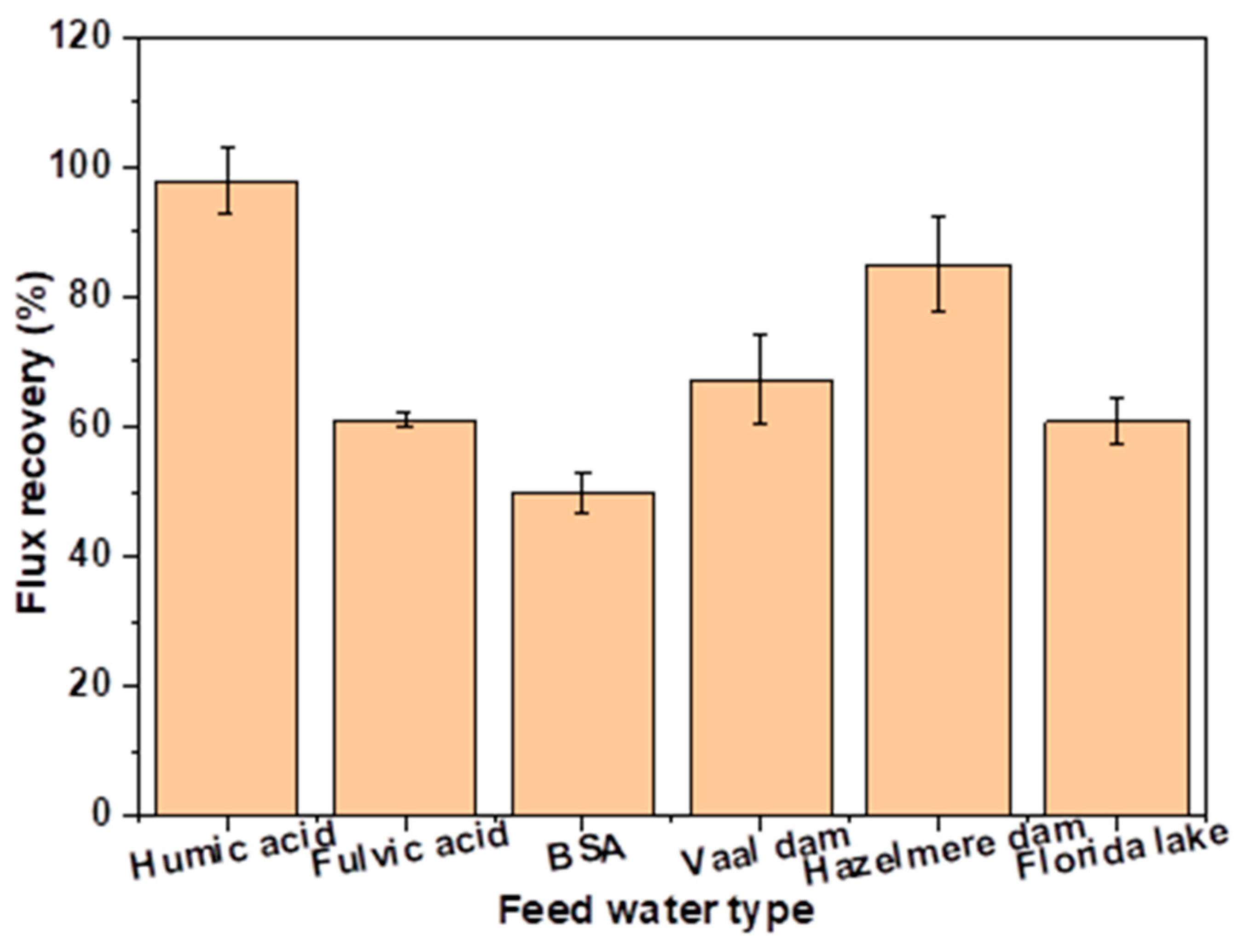
3.5.2. Flux Recovery
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Li, X.; Jiang, L.; Li, H. Application of ultrafiltration technology in water treatment. In Proceedings of the InIOP Conference Series: Earth and Environmental Science, Banda Aceh, Indonesia, 26–27 September 2018; IOP Publishing: Bristol, UK, 2018; Volume 186, p. 012009. [Google Scholar]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A review on reverse osmosis and nanofiltration membranes for water purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef] [Green Version]
- Sheng, C.; Nnanna, A.A.; Liu, Y.; Vargo, J.D. Removal of Trace Pharmaceuticals from Water using coagulation and powdered activated carbon as pretreatment to ultrafiltration membrane system. Sci. Total Environ. 2016, 550, 1075–1083. [Google Scholar] [CrossRef]
- Alosaimi, E.H.; Hassan, H.M.; Alsohaimi, I.H.; Chen, Q.; Melhi, S.; Younes, A.A.; El-Shwiniy, W.H. Fabrication of sulfonated polyethersulfone ultrafiltration membranes with an excellent antifouling performance by impregnating with polysulfopropyl acrylate coated ZnO nanoparticles. Environ. Technol. Innov. 2022, 25, 102210. [Google Scholar] [CrossRef]
- Gumbi, N.N.; Hu, M.; Mamba, B.B.; Li, J.; Nxumalo, E.N. Macrovoid-free PES/SPSf/O-MWCNT ultrafiltration membranes with improved mechanical strength, antifouling and antibacterial properties. J. Membr. Sci. 2018, 566, 288–300. [Google Scholar] [CrossRef]
- Wu, L.G.; Zhang, X.Y.; Wang, T.; Du, C.H.; Yang, C.H. Enhanced performance of polyvinylidene fluoride ultrafiltration membranes by incorporating TiO2/graphene oxide. Chem. Eng. Res. Des. 2019, 141, 492–501. [Google Scholar] [CrossRef]
- Kotp, Y.H. Removal of organic pollutants using polysulfone ultrafiltration membrane containing polystyrene silicomolybdate nanoparticles: Case study: Borg El Arab area. J. Water Process Eng. 2019, 30, 100553. [Google Scholar] [CrossRef]
- Ajdary, M.; Keyhanfar, F.; Moosavi, M.A.; Shabani, R.; Mehdizadeh, M.; Varma, R.S. Potential toxicity of nanoparticles on the reproductive system animal models: A review. J. Reprod. Immunol. 2021, 148, 103384. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M. Preparation and characterization of membranes formed by nonsolvent induced phase separation: A review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Strathmann, H.; Kock, K.; Amar, P.; Baker, R.W. The formation mechanism of asymmetric membranes. Desalination 1975, 16, 179–203. [Google Scholar] [CrossRef]
- Mat Nawi, N.I.; Chean, H.M.; Shamsuddin, N.; Bilad, M.R.; Narkkun, T.; Faungnawakij, K.; Khan, A.L. Development of hydrophilic PVDF membrane using vapour induced phase separation method for produced water treatment. Membranes 2020, 10, 121. [Google Scholar] [CrossRef]
- Xu, J.; Tang, Y.; Wang, Y.; Shan, B.; Yu, L.; Gao, C. Effect of coagulation bath conditions on the morphology and performance of PSf membrane blended with a capsaicin-mimic copolymer. J. Membr. Sci. 2014, 455, 121–130. [Google Scholar] [CrossRef]
- Kajau, A.; Motsa, M.; Mamba, B.B.; Mahlangu, O. Leaching of CuO Nanoparticles from PES Ultrafiltration Membranes. ACS Omega 2021, 6, 31797–31809. [Google Scholar] [CrossRef] [PubMed]
- Van Oss, C.J.; Good, R.J.; Chaudhury, M.K. The role of van der Waals forces and hydrogen bonds in “hydrophobic interactions” between biopolymers and low energy surfaces. J. Colloid Interface Sci. 1986, 111, 378–390. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem. Rev. 1988, 88, 927–941. [Google Scholar] [CrossRef]
- Li, S.; Cui, Z.; Zhang, L.; He, B.; Li, J. The effect of sulfonated polysulfone on the compatibility and structure of polyethersulfone-based blend membranes. J. Membr. Sci. 2016, 513, 1. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, X.Y.; Chung, T.S.; Weber, M.; Staudt, C.; Maletzko, C. Removal of organic micro-pollutants (phenol, aniline and nitrobenzene) via forward osmosis (FO) process: Evaluation of FO as an alternative method to reverse osmosis (RO). Water Res. 2016, 91, 104–114. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Xiang, J.; Hu, X.; Cheng, P.; Zhang, L.; Du, W.; Wang, S.; Tang, N. Effects of coagulation-bath conditions on polyphenylsulfone ultrafiltration membranes. Chin. J. Chem. Eng. 2021, 34, 332–340. [Google Scholar] [CrossRef]
- Vatanpour, V.; Esmaeili, M.; Farahani, M.H. Fouling reduction and retention increment of polyethersulfone nanofiltration membranes embedded by amine-functionalized multi-walled carbon nanotubes. J. Membr. Sci. 2014, 466, 70–81. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Alhoshan, M.; Dass, L.A.; Muthumareeswaran, M.R. Development of a nanocomposite ultrafiltration membrane based on polyphenylsulfone blended with graphene oxide. Sci. Rep. 2017, 7, 41976. [Google Scholar] [CrossRef] [Green Version]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Hackett, C.; Abolhassani, M.; Greenlee, L.F.; Thompson, A.K. Ultrafiltration Membranes Functionalized with Copper Oxide and Zwitterions for Fouling Resistance. Membranes 2022, 12, 544. [Google Scholar] [CrossRef]
- Guha, R.; Xiong, B.; Geitner, M.; Moore, T.; Wood, T.K.; Velegol, D.; Kumar, M. Reactive micromixing eliminates fouling and concentration polarization in reverse osmosis membranes. J. Membr. Sci. 2017, 542, 8–17. [Google Scholar] [CrossRef]
- Mahlangu, O.T.; Nackaerts, R.; Thwala, J.M.; Mamba, B.B.; Verliefde, A.R. Hydrophilic fouling-resistant GO-ZnO/PES membranes for wastewater reclamation. J. Membr. Sci. 2017, 524, 43–55. [Google Scholar] [CrossRef]
- Förch, R.; Schönherr, H.; Jenkins, A.T. (Eds.) Surface Design: Applications in Bioscience and Nanotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Ahmad, A.L.; Ramli, W.K.; Fernando, W.J.; Daud, W.R. Effect of ethanol concentration in water coagulation bath on pore geometry of PVDF membrane for Membrane Gas Absorption application in CO2 removal. Sep. Purif. Technol. 2012, 88, 11–18. [Google Scholar] [CrossRef]
- Ji, Y.; Qian, W.; Yu, Y.; An, Q.; Liu, L.; Zhou, Y.; Gao, C. Recent developments in nanofiltration membranes based on nanomaterials. Chin. J. Chem. Eng. 2017, 25, 1639–1652. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, S.; Liu, Z. Progress and challenges of carbon nanotube membrane in water treatment. Crit. Rev. Environ. Sci. Technol. 2016, 46, 999–1046. [Google Scholar] [CrossRef]
- Ihsanullah. Carbon nanotube membranes for water purification: Developments, challenges, and prospects for the future. Sep. Purif. Technol. 2019, 209, 307–337. [Google Scholar] [CrossRef]
- Machodi, M.J.; Daramola, M.O. Synthesis of PES and PES/chitosan membranes for synthetic acid mine drainage treatment. Water SA 2020, 46, 114–122. [Google Scholar]
- Zoka, L.; Khoo, Y.S.; Lau, W.J.; Matsuura, T.; Narbaitz, R.; Ismail, A.F. Flux Increase Occurring When an Ultrafiltration Membrane Is Flipped from a Normal to an Inverted Position—Experiments and Theory. Membranes 2022, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Santosh, V.; Babu, P.V.; Gopinath, J.; Rao, N.; Sainath, A.V.; Reddy, A.V. Development of hydroxyl and carboxylic acid functionalized CNTs–polysulphone nanocomposite fouling-resistant ultrafiltration membranes for oil–water separation. Bull. Mater. Sci. 2020, 43, 1–2. [Google Scholar] [CrossRef]
- Makwana, D.; Polisetti, V.; Castaño, J.; Ray, P.; Bajaj, H.C. Mg-Fe layered double hydroxide modified montmorillonite as hydrophilic nanofiller in polysulfone-polyvinylpyrrolidone blend ultrafiltration membranes: Separation of oil-water mixture. Appl. Clay Sci. 2020, 192, 105636. [Google Scholar] [CrossRef]
- Moslehyani, A.; Ismail, A.F.; Othman, M.H.; Matsuura, T. Design and performance study of hybrid photocatalytic reactor-PVDF/MWCNT nanocomposite membrane system for treatment of petroleum refinery wastewater. Desalination 2015, 363, 99–111. [Google Scholar] [CrossRef]
- Amid, M.; Nabian, N.; Delavar, M. Performance evaluation and modeling study of PC blended membranes incorporated with SDS-modified and unmodified halloysite nanotubes in the separation of oil from water. J. Environ. Chem. Eng. 2021, 9, 105237. [Google Scholar] [CrossRef]
- Zeng, G.; He, Y.; Yu, Z.; Zhan, Y.; Ma, L.; Zhang, L. Preparation and characterization of a novel PVDF ultrafiltration membrane by blending with TiO2-HNTs nanocomposites. Appl. Surf. Sci. 2016, 371, 624–632. [Google Scholar] [CrossRef]
- Hwang, T.; Oh, J.S.; Yim, W.; Nam, J.D.; Bae, C.; Kim, H.I.; Kim, K.J. Ultrafiltration using graphene oxide surface-embedded polysulfone membranes. Sep. Purif. Technol. 2016, 166, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Brohi, R.D.; Wang, L.; Talpur, H.S.; Wu, D.; Khan, F.A.; Bhattarai, D.; Rehman, Z.U.; Farmanullah, F.; Huo, L.J. Toxicity of nanoparticles on the reproductive system in animal models: A review. Front. Pharmacol. 2017, 8, 606. [Google Scholar] [CrossRef]
- Ajdary, M.; Moosavi, M.A.; Rahmati, M.; Falahati, M.; Mahboubi, M.; Mandegary, A.; Jangjoo, S.; Mohammadinejad, R.; Varma, R.S. Health concerns of various nanoparticles: A review of their in vitro and in vivo toxicity. Nanomaterials 2018, 8, 634. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Jeong, J.S.; Kim, S.Y.; Lee, S.J.; Shin, Y.J.; Im, W.J.; Kim, S.H.; Park, K.; Jeong, E.J.; Nam, S.Y.; et al. Safety assessment of cerium oxide nanoparticles: Combined repeated-dose toxicity with reproductive/developmental toxicity screening and biodistribution in rats. Nanotoxicology 2020, 14, 696–710. [Google Scholar] [CrossRef]
- Malkoske, T.A.; Bérubé, P.R.; Andrews, R.C. Coagulation/flocculation prior to low pressure membranes in drinking water treatment: A review. Environ. Sci. Water Res. Technol. 2020, 6, 2993–3023. [Google Scholar] [CrossRef]
- Gebru, K.A.; Das, C. Removal of bovine serum albumin from wastewater using fouling resistant ultrafiltration membranes based on the blends of cellulose acetate, and PVP-TiO2 nanoparticles. J. Environ. Manag. 2017, 200, 283–294. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, T.; Liu, Z.; Su, Y.; Yu, H.; Ma, J.; Yang, Y.; Jiang, Z. Synthesis and properties of sulfonated biphenyl poly (ether sulfone) and its mixed-matrix membranes containing carbon nanotubes for gas separation. J. Appl. Polym. Sci. 2017, 134, 44995. [Google Scholar] [CrossRef]
- Yamamura, H.; Okimoto, K.; Kimura, K.; Watanabe, Y. Hydrophilic fraction of natural organic matter causing irreversible fouling of microfiltration and ultrafiltration membranes. Water Res. 2014, 54, 123–136. [Google Scholar] [CrossRef] [PubMed]

| Membrane | PES wt.% | NMP wt.% | PEG wt.% | Sol/Nsol v/v.% |
|---|---|---|---|---|
| M.0 | 20 | 44 | 36 | 0/100 |
| M.1 (5% NMP) | 20 | 44 | 36 | 5/95 |
| M.2 (10% NMP) | 20 | 44 | 36 | 10/90 |
| M.3 (15% NMP) | 20 | 44 | 36 | 15/85 |
| Membrane | Thickness (µm) | Porosity (%) |
|---|---|---|
| M.0 | 221 ± 4.9 | 77 ± 2.5 |
| M.1 (5% NMP) | 229 ± 3.4 | 73 ± 3.6 |
| M.2 (10% NMP) | 214 ± 4.1 | 67 ± 4.1 |
| M.3 (15% NMP) | 211 ± 4.7 | 70 ± 2.7 |
| Membrane | Pure Water Flux (LMH) | Reference |
|---|---|---|
| M.0 | 276 @ 3 bar | This work |
| M.1 (5% NMP) | 376 @ 3 bar | This work |
| M.2 (10% NMP) | 505 @ 3 bar | This work |
| M.3 (15% NMP) | 568 @ 3 bar | This work |
| SPES-ZnO-g-PSPA | 420 @ 1 bar | [4] |
| M-MWCNT | 578 @ 1 bar | [5] |
| PES | 102 @ 3 bar | [30] |
| PES | 170 @ 3 bar | [31] |
| COOHC1.3PS85PV13.2 | 71 @ 3 bar | [32] |
| PSF-PVP/LDH-M | 592 @ 1 bar | [33] |
| PVDF-HNTs | 315 @ 1 bar | [34] |
| PVDF-TiO2/HNTs | 263 @ 1 bar | [35] |
| PSU/GO | 309 @ 1 bar | [36] |
| PES-HNTs | 454 @ 1 bar | [37] |
| Membrane | BSA Rejection (%) | Reference |
|---|---|---|
| M.3 (15%NMP) | 88% | This work |
| PES/SPSF/O-MWCNT | 100% | [5] |
| CA/PVP/TiO2 | 97% | [42] |
| SIMo/PS/PSf | 99% | [6] |
| PSF/GO | 95% | [7] |
| PVDF/CuXO/GO | 80% | [43] |
| PVDF/TiO2/GO | 89% | [6] |
| Parameter | Florida Lake | Vaal Dam | Hazelmere |
|---|---|---|---|
| DOC (mg/L) | 7.3 | 1.2 | 2.9 |
| UV254 (cm−1) | 0.2 | 0.7 | 0.2 |
| SUVA (L/mg·m) | 3.2 | 62.1 | 6.4 |
| Conductivity µS/cm | 615 | 498 | 418 |
| pH | 7.3 | 6.9 | 7.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tshindane, P.; Mamba, B.B.; Motsa, M.M.; Nkambule, T.T.I. Delayed Solvent–Nonsolvent Demixing Preparation and Performance of a Highly Permeable Polyethersulfone Ultrafiltration Membrane. Membranes 2023, 13, 39. https://doi.org/10.3390/membranes13010039
Tshindane P, Mamba BB, Motsa MM, Nkambule TTI. Delayed Solvent–Nonsolvent Demixing Preparation and Performance of a Highly Permeable Polyethersulfone Ultrafiltration Membrane. Membranes. 2023; 13(1):39. https://doi.org/10.3390/membranes13010039
Chicago/Turabian StyleTshindane, Pfano, Bhekie B. Mamba, Machawe M. Motsa, and Thabo T. I. Nkambule. 2023. "Delayed Solvent–Nonsolvent Demixing Preparation and Performance of a Highly Permeable Polyethersulfone Ultrafiltration Membrane" Membranes 13, no. 1: 39. https://doi.org/10.3390/membranes13010039
APA StyleTshindane, P., Mamba, B. B., Motsa, M. M., & Nkambule, T. T. I. (2023). Delayed Solvent–Nonsolvent Demixing Preparation and Performance of a Highly Permeable Polyethersulfone Ultrafiltration Membrane. Membranes, 13(1), 39. https://doi.org/10.3390/membranes13010039