The Role of the Gravitational Field in Generating Electric Potentials in a Double-Membrane System for Concentration Polarization Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membrane System
2.2. Mathematical Model of Membrane Potential
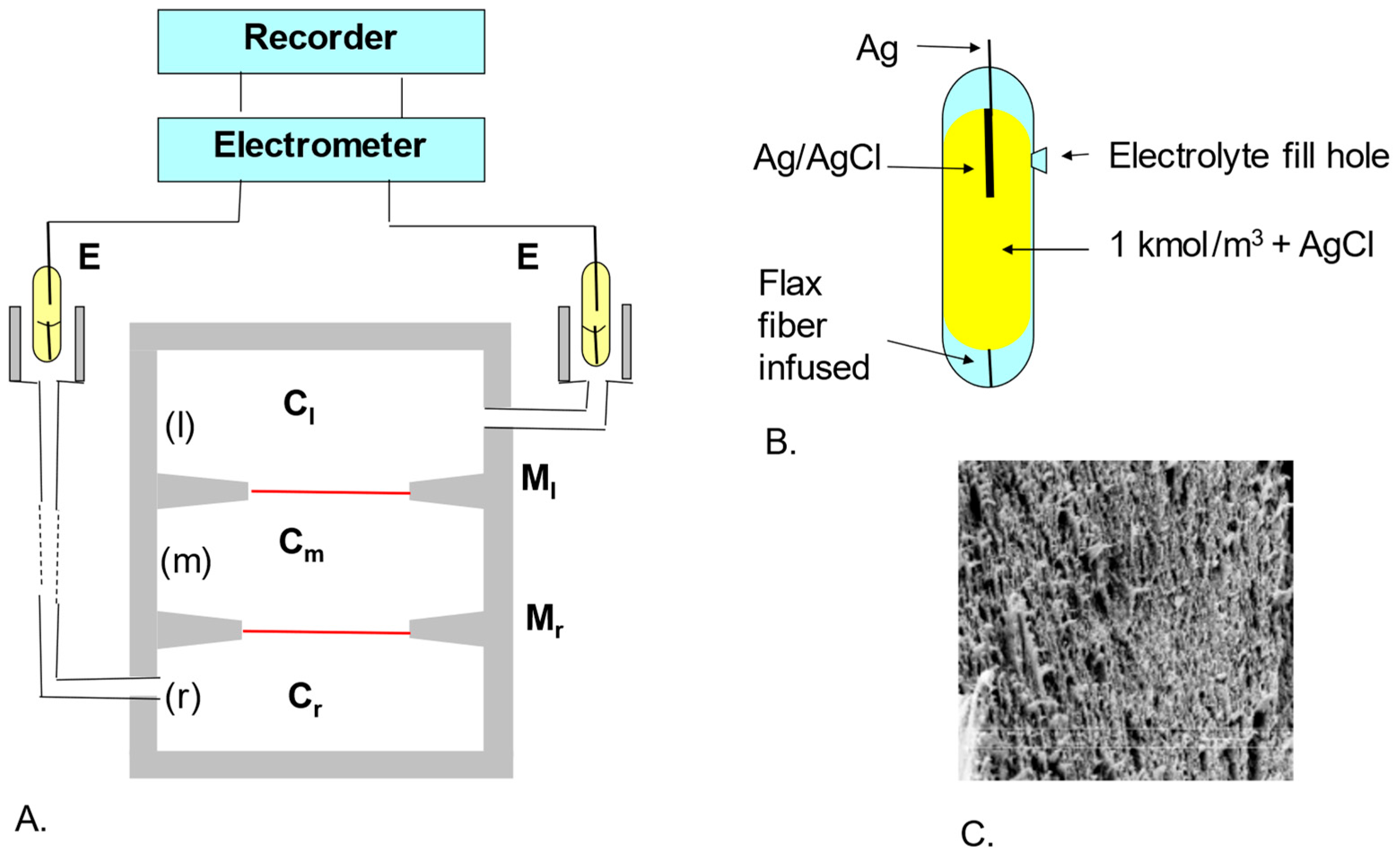
2.3. Measurement System
3. Results and Discussion
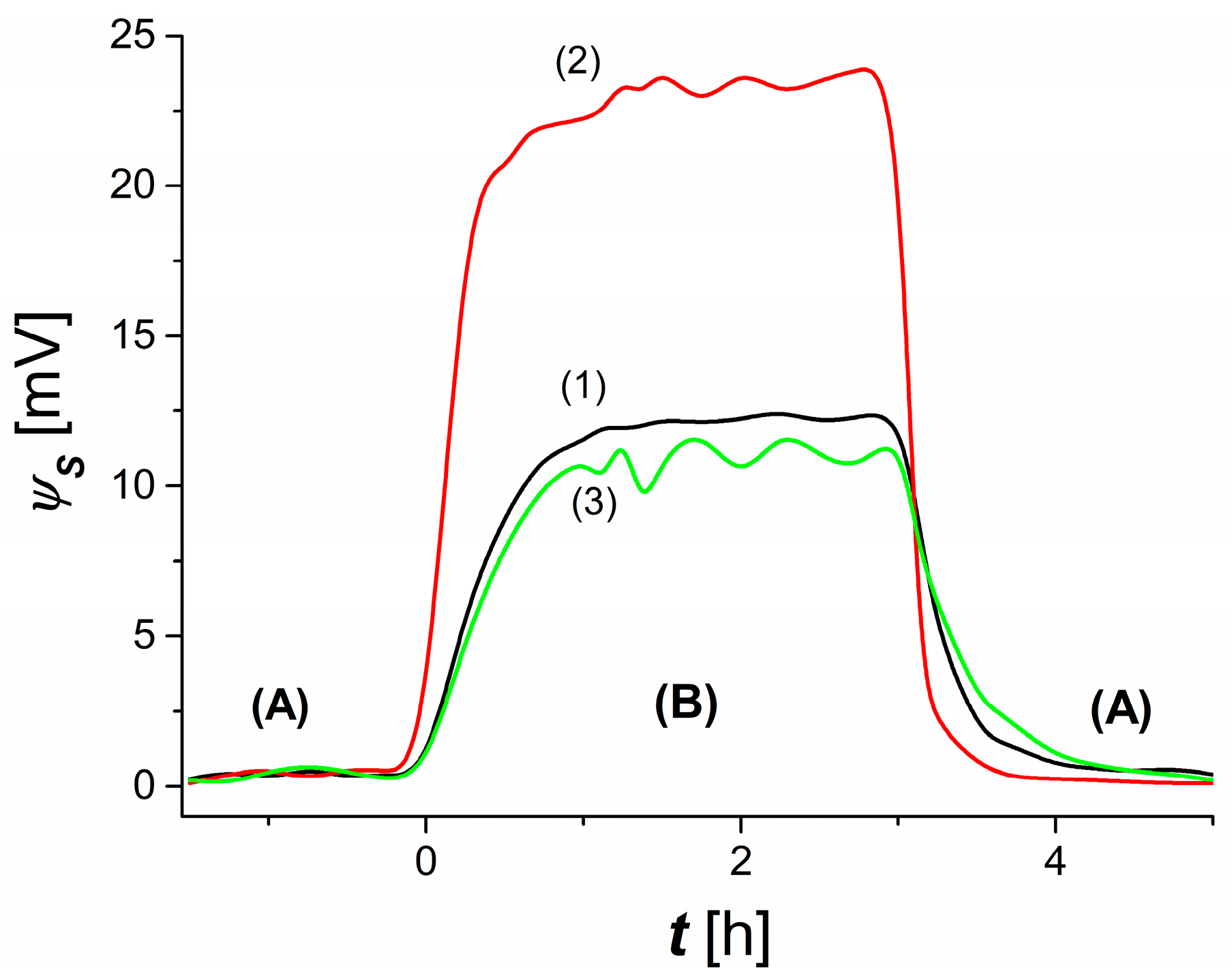
3.1. Time Dependence of Membrane Potential
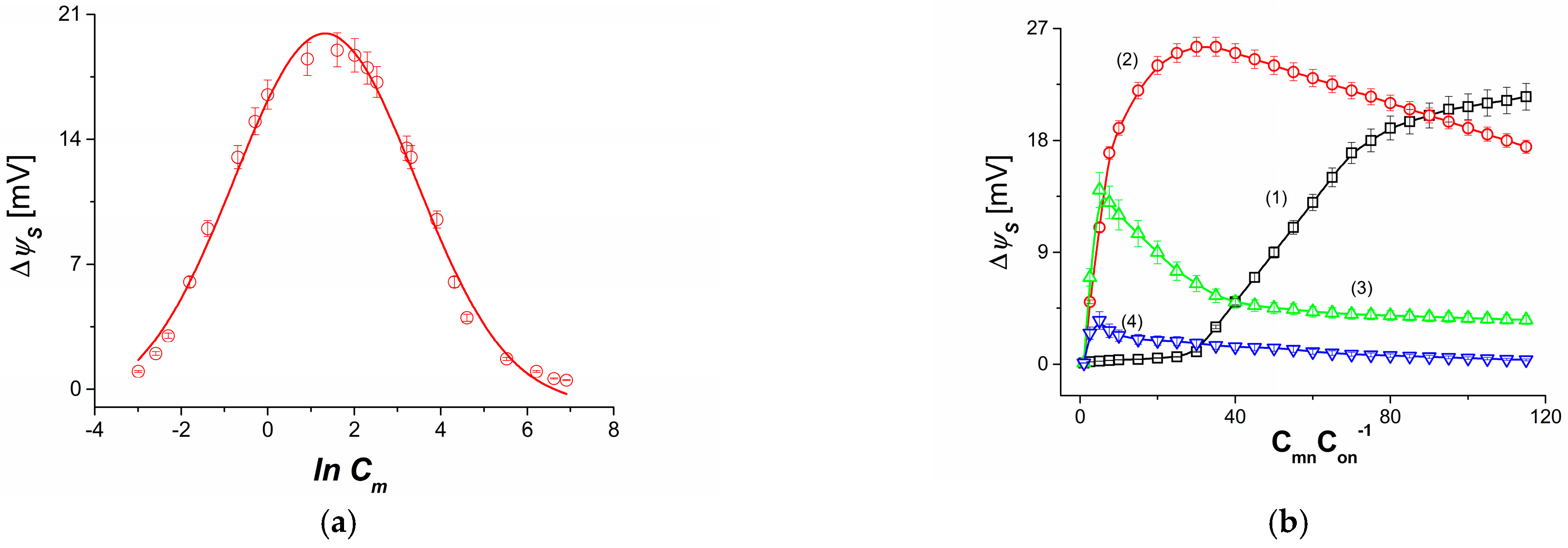
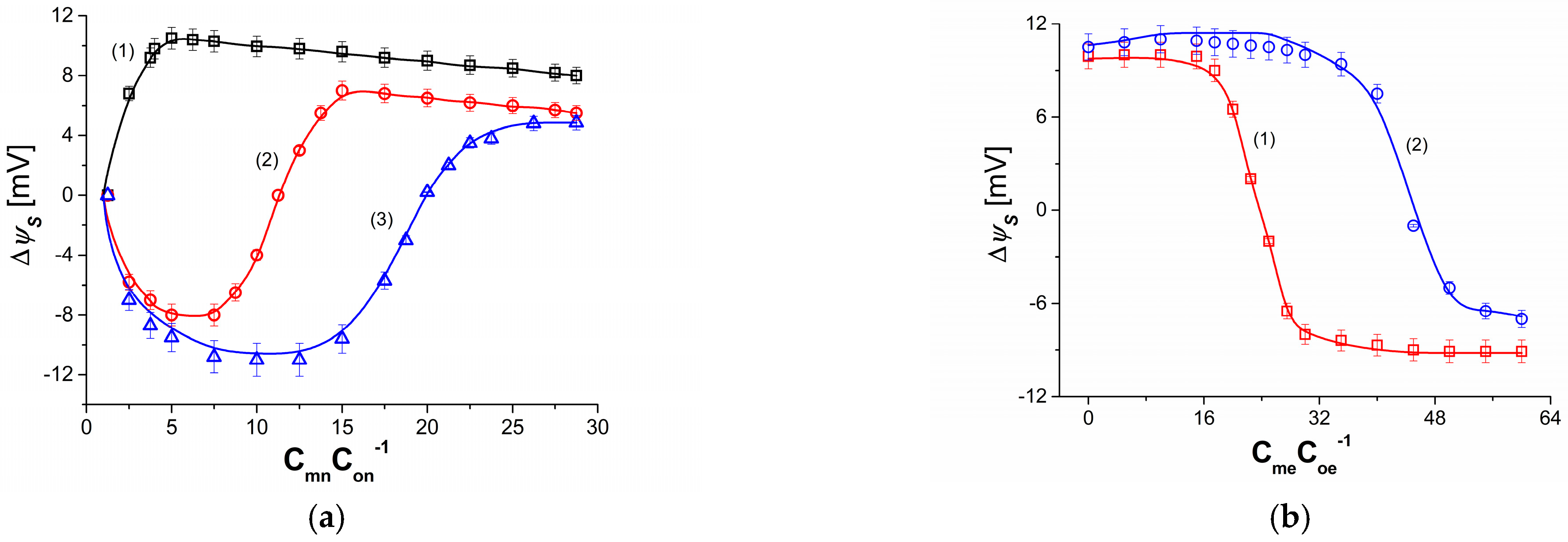
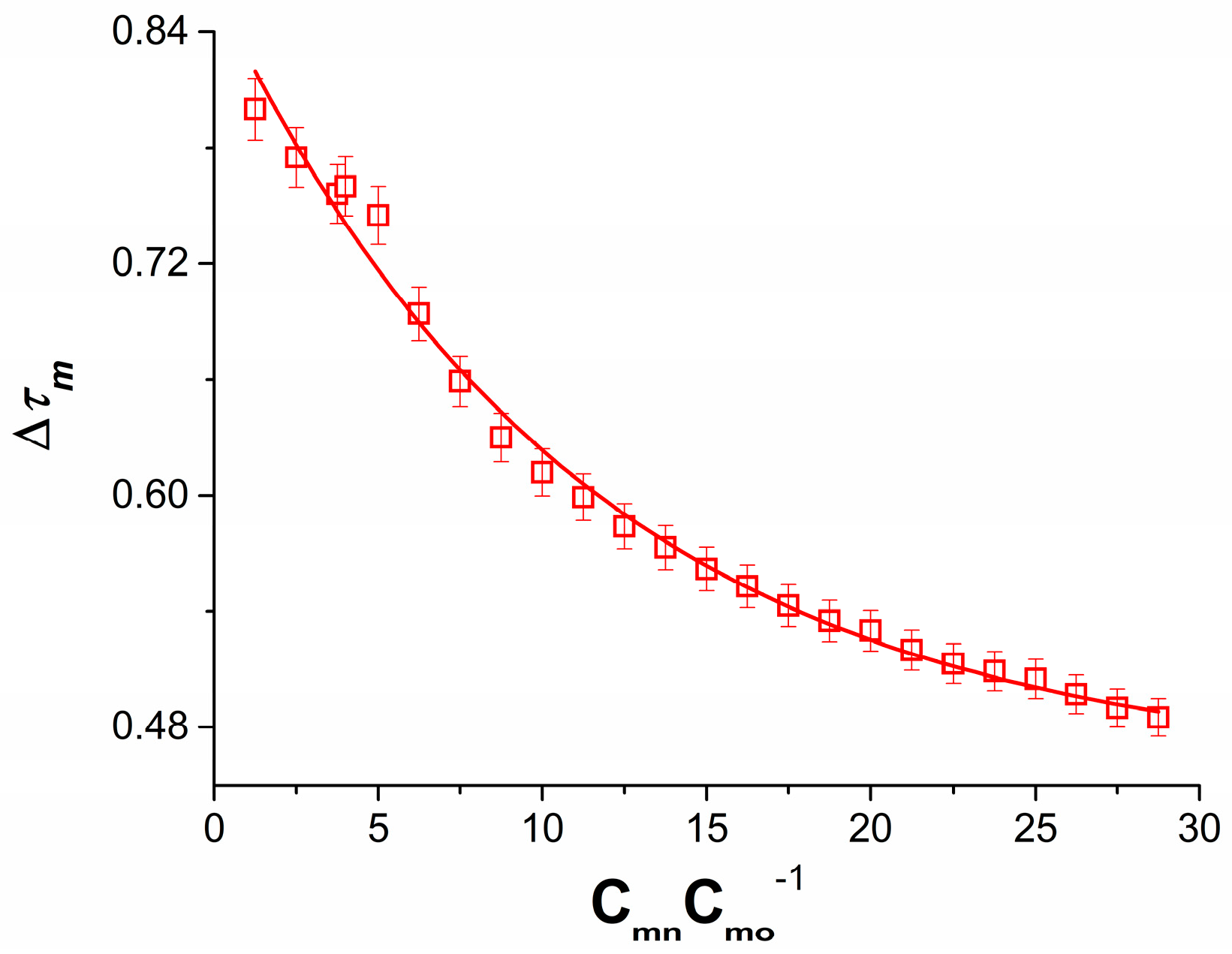
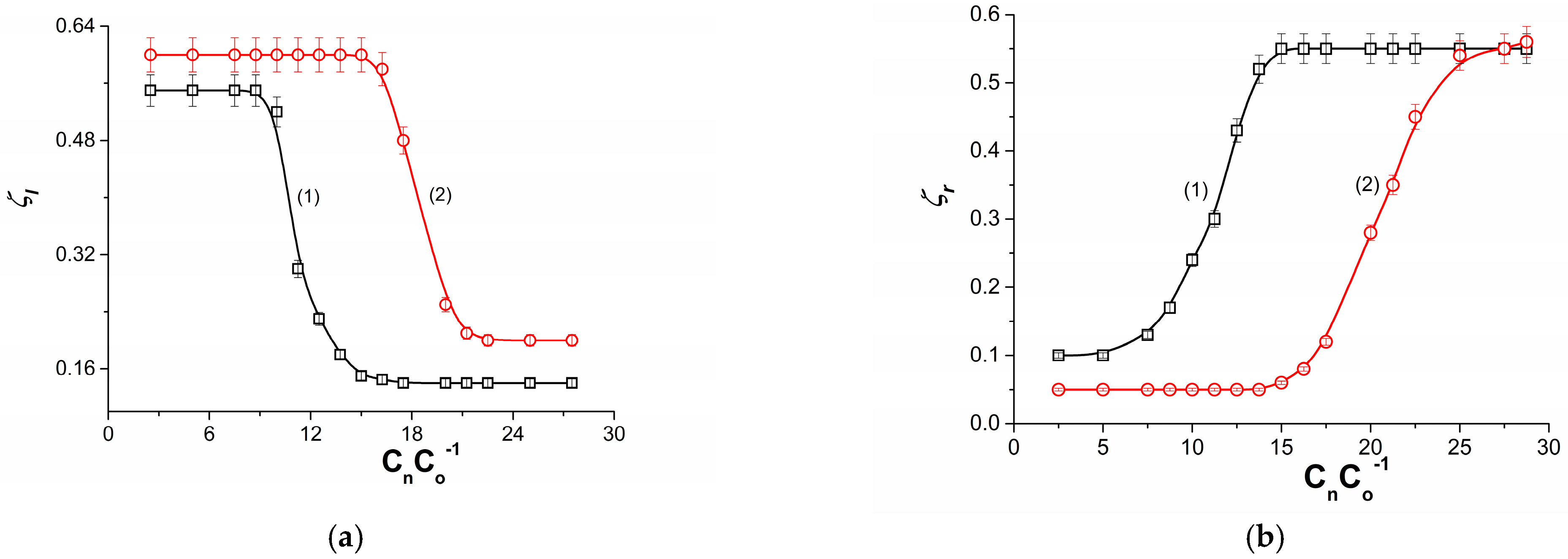
3.2. Concentration Dependence of Membrane Potential
4. Conclusions
- Initially, the double-membrane system is osmotically, diffusively and electrically symmetric under conditions of absence of concentration boundary layers (CBLs) and conditions of symmetric formation due to molecular diffusion of CBL complexes on both sides of each membrane. The system loses the symmetry of CBLs formation when hydrodynamic instabilities appear in the area of one of the complexes (non-convective state) leading, after exceeding the critical value of the concentration Rayleigh number, to a convective state.
- Within the framework of the Kedem–Katchalsky formalism, an ion transport model was developed that includes membrane and solution transport parameters and takes into account hydrodynamic (convective) instabilities for a double-membrane system. It is shown that the transition from the non-convective state to the convective state or vice versa can be controlled by a dimensionless concentration polarization factor or concentration Rayleigh number.
- The higher NaCl concentration in the solution causes the higher density of the solution in the intermembrane than in the outer compartment and induces convectional movements around the lower membrane. This entails the appearance of a positive gravielectric effect. In contrast, higher ethanol concentration in the solution causes a lower density of the solution in the intermembrane than in the outer compartment and induces convectional movements around the upper membrane, leading to a negative gravielectric effect. Such behavior of the double-membrane system indicates its regulator properties, due to its arbitrary switching from “−”, “0” or “+” states. The double-membrane electrochemical system considered in this paper is a source of electromotive force. In addition, the considered double-membrane system can be a model of an electrochemical gravireceptor.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
List of Symbols
| Ml, Mr | membranes |
| CBLs | concentration boundary layers |
| = A or B | configuration A or B |
| /Ml/ | complexes membranes/CBLs in configuration A or B |
| /Mr/ | complexes membranes/CBLs in configuration A or B |
| , , , | CBLs in configuration A or B |
| , , , | thickness of CBLs in configurations A or B |
| , | concentrations of solutions outside CBLs |
| concentration of solution at /Ml boundary | |
| concentration of solution at boundary | |
| concentration of the solution at the border of /Mr | |
| concentration of the solution at the border of | |
| concentration Rayleigh number | |
| volume flux (m s−1); | |
| solute flux (mol m−2s−1) | |
| electric charge flux (A) | |
| hydraulic conductivity coefficient (m3 N−1s−1 | |
| reflection coefficient | |
| electroosmotic permeability coefficient (N A−1) | |
| solute permeability coefficient (mol N−1s−1) | |
| γ | Van’t Hoff coefficient |
| R | gas constant (J mol−1K−1) |
| T | absolute temperature (K) |
| electrical conductivity (Ω−1m−2) | |
| , | transfer number of anion (a) and cation (c) |
| ion number | |
| valence | |
| F | Faraday’s constant. (C mol−1) |
| average concentration of the solution (mol m−3) | |
| potential difference measured with two reversible electrodes (V) | |
| , | electrical potential difference across the and layers (V) |
| , | electrical potential difference across the Ml and Mr membranes (V) |
| , , | electrical potential difference across the and layers (V) |
| , | ionic current through layer and (A) |
| , | ionic current through membrane Ml and Mr (A) |
| ionic current through layer (mol m−2s−1) | |
| soute flux through layer (mol m−2s−1) | |
| solute flux through /Ml/ complex (mol m−2s−1) | |
| solute flux through layer (mol m−2s−1) | |
| volume flux through layer (m s−1) | |
| volume flux through /Ml/ complex (m s−1) | |
| volume flux through layer (m s−1) |
References
- Lipton, B.H.; Bhaerman, S. Spontaneous Evolution—Our Positive Future and a Way to Get There from Here; Hay House Inc.: Carlsbad, CA, USA, 2018. [Google Scholar]
- Bruinsma, R.; Aleksander, S. Theory of electrohydrodynamic instabilities in electrolytic cells. J. Chem. Phys. 1990, 92, 3074–3080. [Google Scholar] [CrossRef]
- Batko, K.M.; Ślęzak, A.A. Evaluation of the global S-entropy production in membrane transport of aqueous solutions of hydrochloric acid and ammonia. Entropy 2020, 22, 1021. [Google Scholar] [CrossRef] [PubMed]
- Baker, R. Membrane Technology and Application; John Wiley & Sons: New York, NY, USA, 2012. [Google Scholar]
- Radu, E.R.; Voicu, S.I.; Thakur, V.K. Polymeric membranes for biomedical applications. Polymers 2023, 15, 619. [Google Scholar] [CrossRef] [PubMed]
- Dorotkiewicz-Jach, A.; Markowitz, P.; Rachuna, J.; Arabski, M.; Drulis-Kawa, Z. The impact of agarose immobilization on the activity of lytic Pseudomonas araginosa phages combined with chemicals. Appl. Microbiol. Biotechnol. 2023, 107, 897–913. [Google Scholar] [CrossRef]
- Anton-Sales, I.; D’Antin, J.C.; Fernández-Engroba, J.; Charoenrook, V.; Laromaine, A.; Roig, A.; Michael, R. Bacterial nanocellulose as a corneal bandage material: A comparison with amniotic membrane. Biomater. Sci. 2020, 8, 2921–2930. [Google Scholar] [CrossRef]
- Bizzarri, M.; Monici, M.; van Loon, J.J.W.A. How microgravity affects the biology of living systems. Biomededical Res. Int. 2015, 2015, 863075. [Google Scholar] [CrossRef]
- Regel, L. Materials Science in Space: Theory, Experiments, Technology; Halsted Press: New York, NY, USA, 1987. [Google Scholar]
- Barry, P.H.; Diamond, J.M. Effects of unstirred layers on membrane phenomena. Physiol. Rev. 1984, 64, 763–872. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, I.; Zaltzman, B. Electroosmotical induced convection at a permselective membrane. Phys. Rev. E 2000, 62, 2238–2251. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rjal, R.; Prigozhin, L.; Rubinstein, I.; Zaltzman, B. Teorell instability in concentration polarization. Phys. Rev. E 2015, 92, 022305. [Google Scholar] [CrossRef]
- Larchet, C.; Nouri, S.; Auclair, B.; Dammak, L.; Nikonenko, V. Application of chronopotentiometry to determine the thickness of diffusion layer adjacent to an ion-exchange membrane under natural convection. Adv. Colloid Interface Sci. 2008, 139, 45–61. [Google Scholar] [CrossRef]
- Dworecki, K. Interferometric investigation of near-membrane diffusion layer. J. Biol. Phys. 1995, 21, 37–49. [Google Scholar] [CrossRef]
- Ślęzak, A.; Jasik-Ślęzak, J.; Grzegorczyn, S.; Ślęzak-Prochazka, I. Nonlinear effects in osmotic volume flows of electrolyte solutions through double-membrane system. Transp. Porous Media 2012, 92, 337–356. [Google Scholar] [CrossRef]
- Ślęzak, A. A model equation for the gravielectric effect in electrochemical cells. Biophys. Chem. 1990, 38, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Batko, K.M.; Ślęzak-Prochazka, I.; Ślęzak, A.; Bajdur, W.M.; Ščurek, R. Modelling of the electrical membranę potential for concentration polarization conditions. Entropy 2022, 24, 138. [Google Scholar] [CrossRef]
- Normand, C.; Pomeau, Y.; Velarde, M.Y. Convective instability: A physict’s approach. Rev. Mod. Phys. 1977, 49, 581–624. [Google Scholar] [CrossRef]
- Puthenveettal, B.A.; Arakeri, J.H. Convection due to an unstable density difference across a permeable membrane. J. Fluid Mech. 2008, 609, 139–170. [Google Scholar] [CrossRef]
- Ramareddy, G.V.; Puthenveettil, B.A. The Pe ∼ 1 regime of convection across a horizontal permeable membrane. J. Fluid Mech. 2011, 679, 476–504. [Google Scholar] [CrossRef]
- Lebon, G.; Jou, D.; Casas-Vasquez, J. Understanding Non-Equilibrium Thermodynamics Foundations, Applications, Frontiers; Springer: Berlin, Germany, 2008. [Google Scholar]
- Fernández-Sempere, J.; Ruiz-Beviá, F.; Salcedo-Díaz, R.; García-Algado, P. Diffusion studies in polarized reverse osmosis processes by holographic interferometry. Opt. Lasers Eng. 2008, 46, 877–887. [Google Scholar] [CrossRef]
- Kargol, M.; Ornal, B.; Kosztołowicz, T. A study on gravielectric polarization in 1-membrane system. Curr. Top. Biophys. 1994, 18, 105–111. [Google Scholar]
- Grzegorczyn, S.; Ślęzak, A. Kinetics of concentration boundary layers buildup in the system consisted of microbial cellulose biomembrane and electrolyte solutions. J. Membr. Sci. 2007, 304, 148–155. [Google Scholar] [CrossRef]
- Curran, P.F.; McIntosh, J.R. A model system for biological water transport. Nature 1962, 193, 347–348. [Google Scholar] [CrossRef]
- Przestalski, S.; Kargol, M. Graviosmotic volume flow through membrane systems. Stud. Biophys. 1972, 34, 7–14. [Google Scholar]
- Kargol, M.; Dworecki, K.; Przestalski, S. Graviosmotic flow amplification effect in a series membrane system. Stud. Biophys. 1979, 76, 137–142. [Google Scholar]
- Kargol, M.; Dworecki, K.; Przestalski, S. Interferometric investigation boundary layers in a graviosmotic system. Stud. Biophys. 1986, 113, 31–37. [Google Scholar]
- Kargol, M.; Dworecki, K. Interferometric studies of diffusive unstirred layers generated in graviosmotic systems. Curr. Top. Biophys. 1994, 18, 99–104. [Google Scholar]
- Kargol, M. The graviosmotic hypothesis of xylem transport of water in plants. Gen. Physiol. Biophys. 1992, 11, 469–487. [Google Scholar]
- Katchalsky, A.; Curran, P.F. Nonequilibrium Thermodynamics in Biophysics; Harvard University Press: Cambridge, MA, USA, 1965. [Google Scholar]
- Schmitt, R.W. Observational and laboratory insights into salt-finger convection. Prog. Oceanogr. 2003, 56, 419–433. [Google Scholar] [CrossRef]
- Oschlies, A.; Dietze, H.; Kahler, P. Salt-finger driven enhancement of upper ocean nutrient supply. Geophys. Res. Lett. 2003, 30, 2204. [Google Scholar] [CrossRef]
- Goswami, N.; van Loon, J.J.W.A.; Roessler, A.; Blaber, A.P.; White, O. Editorial: Gravitational physiology, aging and medicine. Front. Physiol. 2019, 10, 1338. [Google Scholar] [CrossRef]
- Topal, U.; Zamur, C. Microgravity, stem cells, and cancer: A new hope for cancer treatment. Stem Cells Int. 2021, 2021, 5566872. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batko, K.; Ślęzak-Prochazka, I.; Sokołowska, W.; Rak, M.; Płonka, W.; Ślęzak, A. The Role of the Gravitational Field in Generating Electric Potentials in a Double-Membrane System for Concentration Polarization Conditions. Membranes 2023, 13, 833. https://doi.org/10.3390/membranes13100833
Batko K, Ślęzak-Prochazka I, Sokołowska W, Rak M, Płonka W, Ślęzak A. The Role of the Gravitational Field in Generating Electric Potentials in a Double-Membrane System for Concentration Polarization Conditions. Membranes. 2023; 13(10):833. https://doi.org/10.3390/membranes13100833
Chicago/Turabian StyleBatko, Kornelia, Izabella Ślęzak-Prochazka, Weronika Sokołowska, Małgorzata Rak, Wiktoria Płonka, and Andrzej Ślęzak. 2023. "The Role of the Gravitational Field in Generating Electric Potentials in a Double-Membrane System for Concentration Polarization Conditions" Membranes 13, no. 10: 833. https://doi.org/10.3390/membranes13100833
APA StyleBatko, K., Ślęzak-Prochazka, I., Sokołowska, W., Rak, M., Płonka, W., & Ślęzak, A. (2023). The Role of the Gravitational Field in Generating Electric Potentials in a Double-Membrane System for Concentration Polarization Conditions. Membranes, 13(10), 833. https://doi.org/10.3390/membranes13100833








