Stability of Filled PDMS Pervaporation Membranes in Bio-Ethanol Recovery from a Real Fermentation Broth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Hydrolysate Fermentation
2.4. Membrane Testing
2.5. Membrane Characterization
2.6. ZIF-8 Stability in Fermented Hydrolysate
3. Results
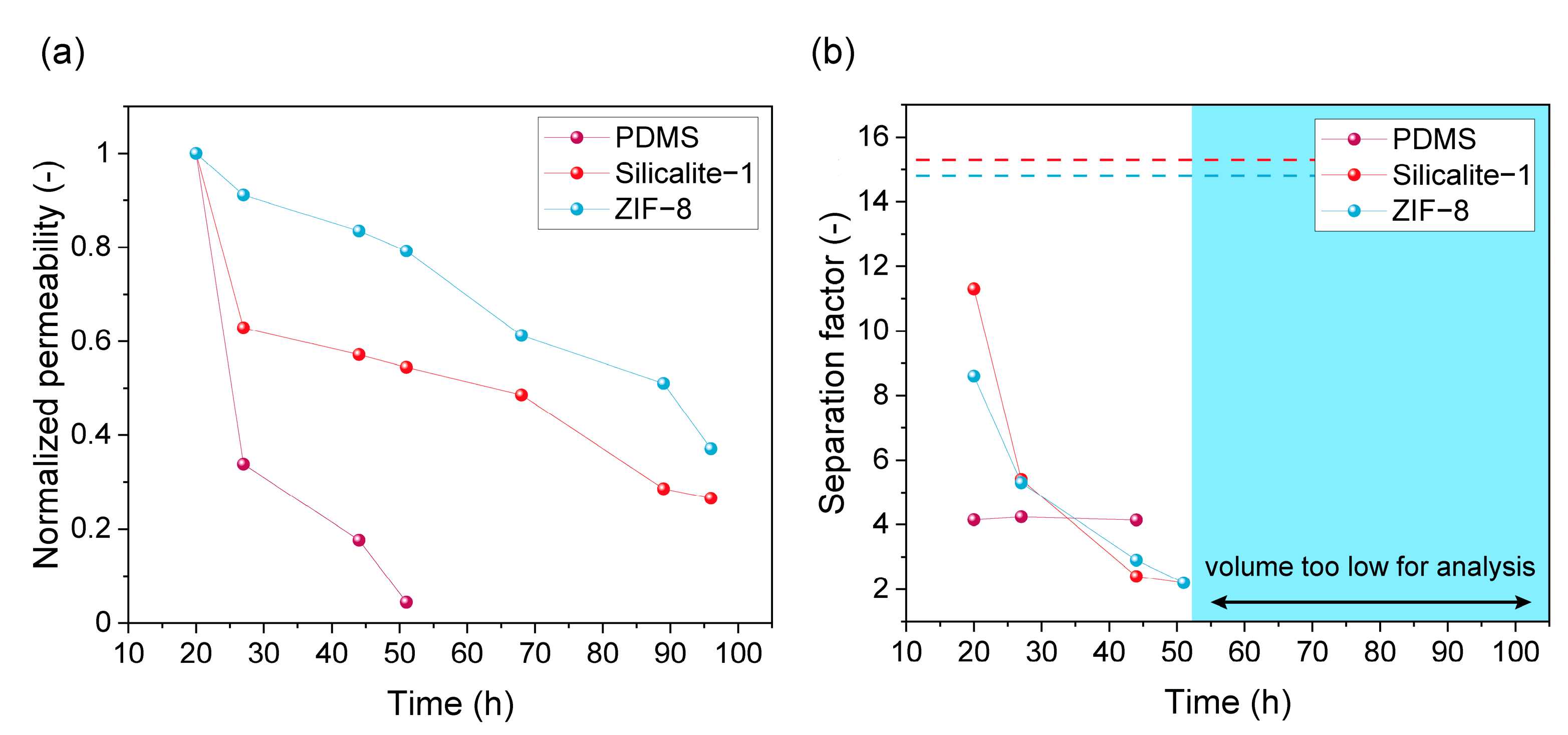
3.1. PV Performance in Fermented Wheat/Hay Straw Hydrolysate
3.2. ZIF-8 Stability
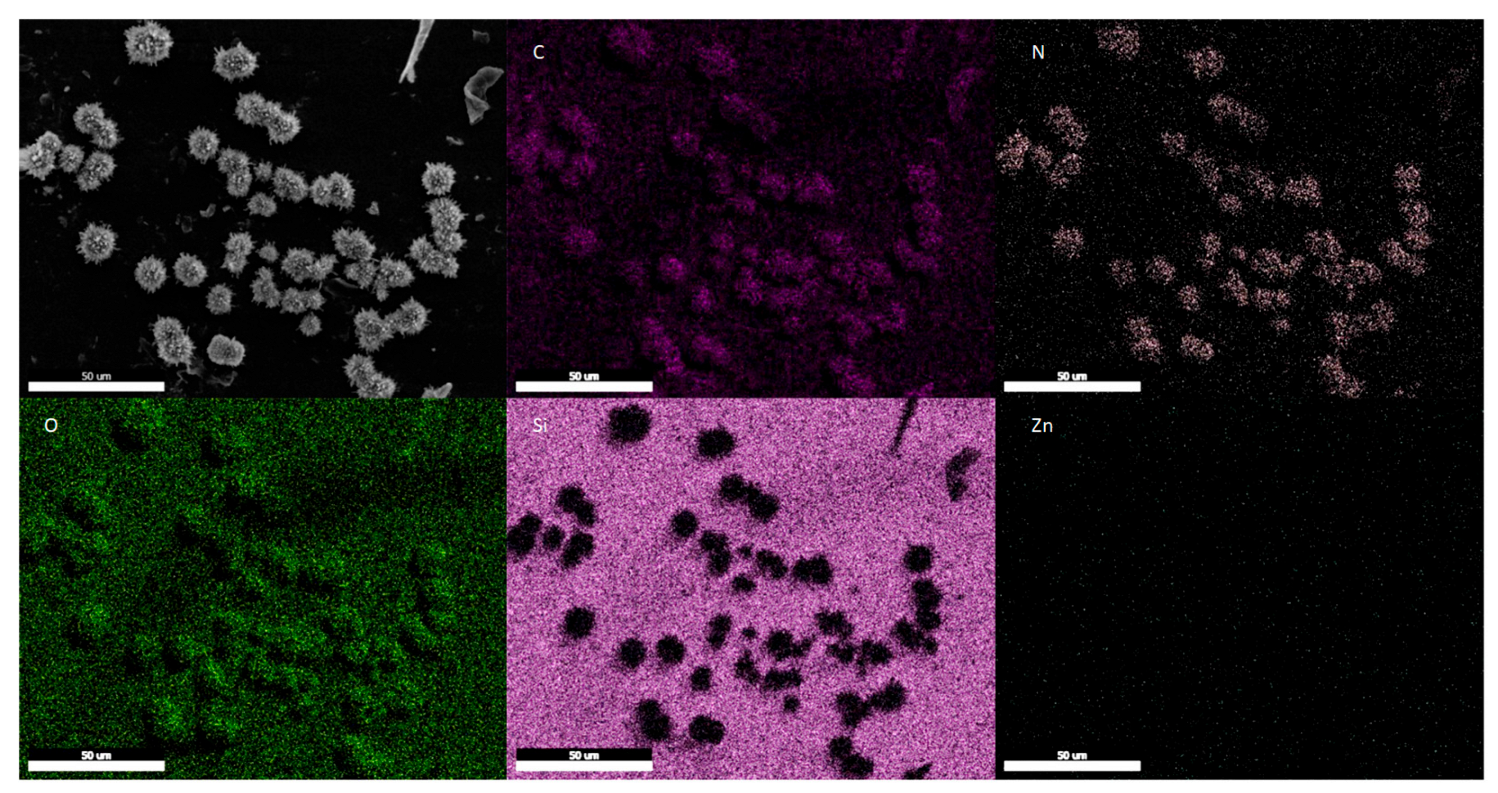
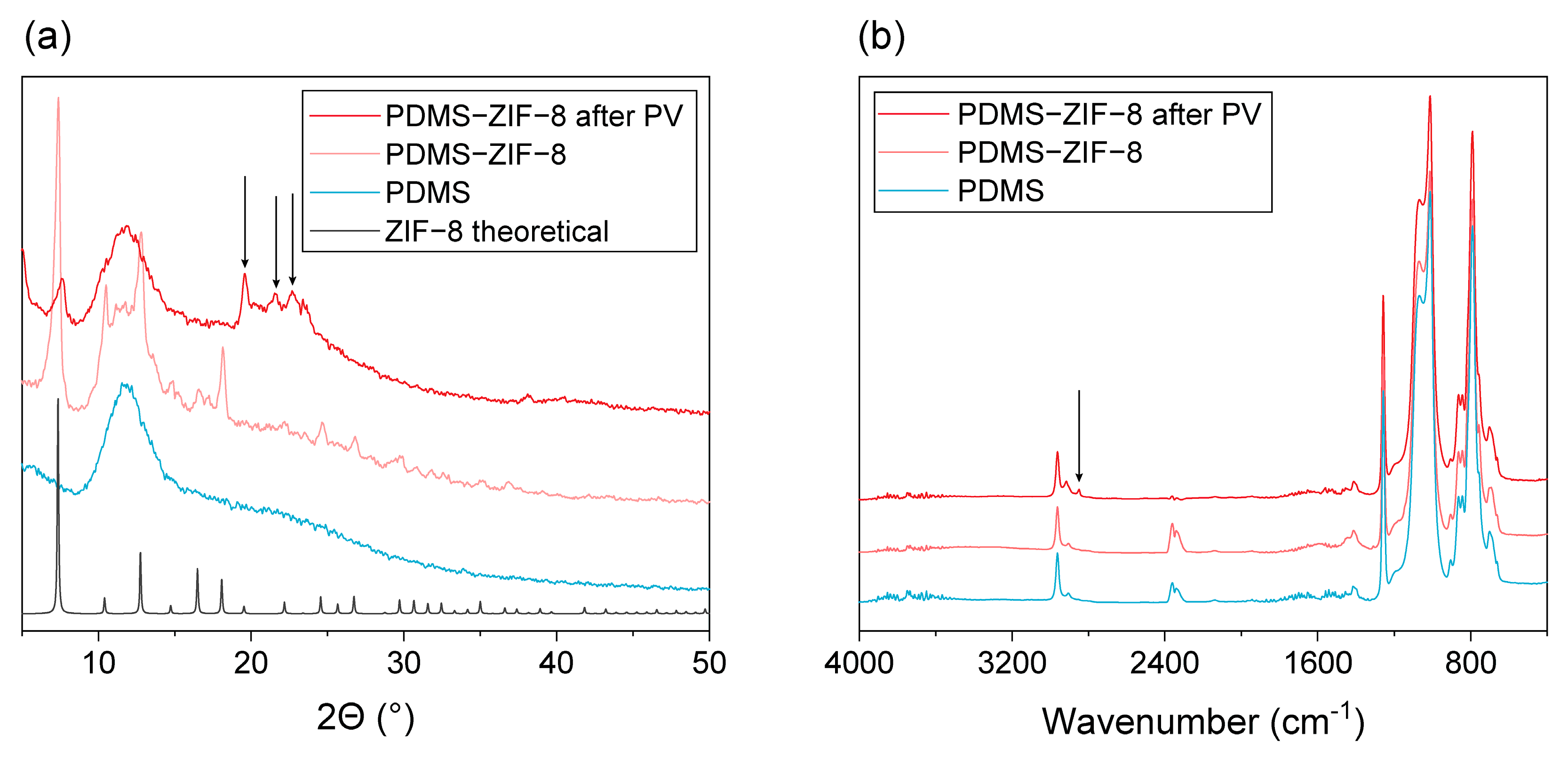
3.2.1. ZIF-8 Stability in the MMM
3.2.2. ZIF-8 Nanoparticle Stability in the Fermented Hydrolysate Broth
3.2.3. General Remarks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dürre, P. Fermentative Production of Butanol—The Academic Perspective. Curr. Opin. Biotechnol. 2011, 22, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lee, C.; Huo, M.; Yao, M. Comparison of Ethanol and Butanol as Additives in Soybean Biodiesel Using a Constant Volume Combustion Chamber. Energy Fuels 2011, 25, 1837–1846. [Google Scholar] [CrossRef]
- Cardona, C.A.; Sánchez, Ó.J. Fuel Ethanol Production: Process Design Trends and Integration Opportunities. Bioresour. Technol. 2007, 98, 2415–2457. [Google Scholar] [CrossRef] [PubMed]
- Aiba, S.; Shoda, M.; Nagatani, M. Kinetics of Product Inhibition in Alcohol Fermentation. Biotechnol. Bioeng. 1968, 10, 845–864. [Google Scholar] [CrossRef]
- Huang, H.-J.; Ramaswamy, S.; Tschirner, U.W.; Ramarao, B.V. A Review of Separation Technologies in Current and Future Biorefineries. Sep. Purif. Technol. 2008, 62, 1–21. [Google Scholar] [CrossRef]
- Ong, Y.K.; Shi, G.M.; Le, N.L.; Tang, Y.P.; Zuo, J.; Nunes, S.P.; Chung, T.-S. Recent Membrane Development for Pervaporation Processes. Prog. Polym. Sci. 2016, 57, 1–31. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W. Pervaporation Membrane Materials: Recent Trends and Perspectives. J. Memb. Sci. 2021, 636, 119557. [Google Scholar] [CrossRef]
- Tang, J.; Sirkar, K.K.; Majumdar, S. Permeation and Sorption of Organic Solvents and Separation of Their Mixtures through an Amorphous Perfluoropolymer Membrane in Pervaporation. J. Memb. Sci. 2013, 447, 345–354. [Google Scholar] [CrossRef]
- Li, Y.; Verbiest, T.; Vankelecom, I. Improving the Flux of PDMS Membranes via Localized Heating through Incorporation of Gold Nanoparticles. J. Memb. Sci. 2013, 428, 63–69. [Google Scholar] [CrossRef]
- Chapman, P.D.; Oliveira, T.; Livingston, A.G.; Li, K. Membranes for the Dehydration of Solvents by Pervaporation. J. Memb. Sci. 2008, 318, 5–37. [Google Scholar] [CrossRef]
- Zimmerman, C.M.; Singh, A.; Koros, W.J. Tailoring Mixed Matrix Composite Membranes for Gas Separations. J. Memb. Sci. 1997, 137, 145–154. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary Perspective: Polymers and Mixed Matrix Membranes for Gas and Vapor Separation: A Review and Prospective Opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Bowen, T.C.; Meier, R.G.; Vane, L.M. Stability of MFI Zeolite-Filled PDMS Membranes during Pervaporative Ethanol Recovery from Aqueous Mixtures Containing Acetic Acid. J. Memb. Sci. 2007, 298, 117–125. [Google Scholar] [CrossRef]
- Zhan, X.; Lu, J.; Tan, T.; Li, J. Mixed Matrix Membranes with HF Acid Etched ZSM-5 for Ethanol/Water Separation: Preparation and Pervaporation Performance. Appl. Surf. Sci. 2012, 259, 547–556. [Google Scholar] [CrossRef]
- Van Gestel, T.; Kruidhof, H.; Blank, D.H.A.; Bouwmeester, H.J.M. ZrO2 and TiO2 Membranes for Nanofiltration and Pervaporation: Part 1. Preparation and Characterization of a Corrosion-Resistant ZrO2 Nanofiltration Membrane with a MWCO. J. Memb. Sci. 2006, 284, 128–136. [Google Scholar] [CrossRef]
- Li, Y.; Wee, L.H.; Martens, J.A.; Vankelecom, I.F.J. ZIF-71 as a Potential Filler to Prepare Pervaporation Membranes for Bio-Alcohol Recovery. J. Mater. Chem. A 2014, 2, 10034–10040. [Google Scholar] [CrossRef]
- Liu, S.; Liu, G.; Zhao, X.; Jin, W. Hydrophobic-ZIF-71 Filled PEBA Mixed Matrix Membranes for Recovery of Biobutanol via Pervaporation. J. Memb. Sci. 2013, 446, 181–188. [Google Scholar] [CrossRef]
- Fan, H.; Wang, N.; Ji, S.; Yan, H.; Zhang, G. Nanodisperse ZIF-8/PDMS Hybrid Membranes for Biobutanol Permselective Pervaporation. J. Mater. Chem. A 2014, 2, 20947–20957. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular Chemistry: Secondary Building Units as a Basis for the Design of Highly Porous and Robust Metal-Organic Carboxylate Frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular Synthesis and the Design of New Materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Cirujano, F.G.; Luz, I.; Soukri, M.; Van Goethem, C.; Vankelecom, I.F.J.; Lail, M.; De Vos, D.E. Boosting the Catalytic Performance of Metal–Organic Frameworks for Steroid Transformations by Confinement within a Mesoporous Scaffold. Angew. Chemie Int. Ed. 2017, 56, 13302–13306. [Google Scholar] [CrossRef] [PubMed]
- Alaerts, L.; Kirschhock, C.E.A.; Maes, M.; van der Veen, M.A.; Finsy, V.; Depla, A.; Martens, J.A.; Baron, G.V.; Jacobs, P.A.; Denayer, J.F.M.; et al. Selective Adsorption and Separation of Xylene Isomers and Ethylbenzene with the Microporous Vanadium(IV) Terephthalate MIL-47. Angew. Chemie Int. Ed. 2007, 46, 4293–4297. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Shi, Q.; Yan, H.; Ji, S.; Dong, J.; Zhang, G. Simultaneous Spray Self-Assembly of Highly Loaded ZIF-8-PDMS Nanohybrid Membranes Exhibiting Exceptionally High Biobutanol-Permselective Pervaporation. Angew. Chemie Int. Ed. 2014, 53, 5578–5582. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Si, Z.; Cai, D.; Wang, Z.; Qin, P.; Tan, T. The In-Situ Synthesis of a High-Flux ZIF-8/Polydimethylsiloxane Mixed Matrix Membrane for n-Butanol Pervaporation. Sep. Purif. Technol. 2020, 236, 116263. [Google Scholar] [CrossRef]
- Diestel, L.; Bux, H.; Wachsmuth, D.; Caro, J. Pervaporation Studies of N-Hexane, Benzene, Mesitylene and Their Mixtures on Zeolitic Imidazolate Framework-8 Membranes. Microporous Mesoporous Mater. 2012, 164, 288–293. [Google Scholar] [CrossRef]
- Xu, Y.M.; Chung, T.-S. High-Performance UiO-66/Polyimide Mixed Matrix Membranes for Ethanol, Isopropanol and n-Butanol Dehydration via Pervaporation. J. Memb. Sci. 2017, 531, 16–26. [Google Scholar] [CrossRef]
- Hu, F.-H.; Chi, L.-T.; Syu, G.-B.; Yu, T.-Y.; Lin, M.-P.; Chen, J.-J.; Yu, W.-Y.; Kang, D.-Y. Mixed-Linker MOF-303 Membranes for Pervaporation. J. Membr. Sci. Lett. 2023, 3, 100053. [Google Scholar] [CrossRef]
- Böddeker, K.W.; Bengtson, G.; Bode, E. Pervaporation of Low Volatility Aromatics from Water. J. Memb. Sci. 1990, 53, 143–158. [Google Scholar] [CrossRef]
- Lipnizki, F.; Hausmanns, S.; Field, R.W. Influence of Impermeable Components on the Permeation of Aqueous 1-Propanol Mixtures in Hydrophobic Pervaporation. Proc. J. Membr. Sci. 2004, 228, 129–138. [Google Scholar] [CrossRef]
- García, M.; Sanz, M.T.; Beltrán, S. Separation by Pervaporation of Ethanol from Aqueous Solutions and Effect of Other Components Present in Fermentation Broths. J. Chem. Technol. Biotechnol. 2009, 84, 1873–1882. [Google Scholar] [CrossRef]
- Van Goethem, C.; Verbeke, R.; Pfanmöller, M.; Koschine, T.; Dickmann, M.; Timpel-Lindner, T.; Egger, W.; Bals, S.; Vankelecom, I.F.J. The Role of MOFs in Thin-Film Nanocomposite (TFN) Membranes. J. Memb. Sci. 2018, 563, 938–948. [Google Scholar] [CrossRef]
- Chovau, S.; Gaykawad, S.; Straathof, A.J.J.; Van der Bruggen, B. Influence of Fermentation By-Products on the Purification of Ethanol from Water Using Pervaporation. Bioresour. Technol. 2011, 102, 1669–1674. [Google Scholar] [CrossRef]
- Naik, P.V.; Wee, L.H.; Meledina, M.; Turner, S.; Li, Y.; Van Tendeloo, G.; Martens, J.A.; Vankelecom, I.F.J. PDMS Membranes Containing ZIF-Coated Mesoporous Silica Spheres for Efficient Ethanol Recovery via Pervaporation. J. Mater. Chem. A 2016, 4, 12790–12798. [Google Scholar] [CrossRef]
- Naik, P.V.; Kerkhofs, S.; Martens, J.A.; Vankelecom, I.F.J. PDMS Mixed Matrix Membranes Containing Hollow Silicalite Sphere for Ethanol/Water Separation by Pervaporation. J. Memb. Sci. 2016, 502, 48–56. [Google Scholar] [CrossRef]
- Wee, L.H.; Lescouet, T.; Ethiraj, J.; Bonino, F.; Vidruk, R.; Garrier, E.; Packet, D.; Bordiga, S.; Farrusseng, D.; Herskowitz, M.; et al. Hierarchical Zeolitic Imidazolate Framework-8 Catalyst for Monoglyceride Synthesis. ChemCatChem 2013, 5, 3562–3566. [Google Scholar] [CrossRef]
- Offeman, R.D.; Ludvik, C.N. Poisoning of Mixed Matrix Membranes by Fermentation Components in Pervaporation of Ethanol. J. Memb. Sci. 2010, 367, 288–295. [Google Scholar] [CrossRef]
- Qureshi, N.; Meagher, M.M.; Huang, J.; Hutkins, R.W. Acetone Butanol Ethanol (ABE) Recovery by Pervaporation Using Silicalite-Silicone Composite Membrane from Fed-Batch Reactor of Clostridium Acetobutylicum. J. Memb. Sci. 2001, 187, 93–102. [Google Scholar] [CrossRef]
- Leus, K.; Bogaerts, T.; De Decker, J.; Depauw, H.; Hendrickx, K.; Vrielinck, H.; Van Speybroeck, V.; Van Der Voort, P. Systematic Study of the Chemical and Hydrothermal Stability of Selected “Stable” Metal Organic Frameworks. Microporous Mesoporous Mater. 2016, 226, 110–116. [Google Scholar] [CrossRef]




| Contact Time (h) | pH |
|---|---|
| 0 | 4.96 |
| 3 | 5.46 |
| 18 | 5.68 |
| 168 | 5.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Goethem, C.; Naik, P.V.; Van de Velde, M.; Van Durme, J.; Verplaetse, A.; Vankelecom, I.F.J. Stability of Filled PDMS Pervaporation Membranes in Bio-Ethanol Recovery from a Real Fermentation Broth. Membranes 2023, 13, 863. https://doi.org/10.3390/membranes13110863
Van Goethem C, Naik PV, Van de Velde M, Van Durme J, Verplaetse A, Vankelecom IFJ. Stability of Filled PDMS Pervaporation Membranes in Bio-Ethanol Recovery from a Real Fermentation Broth. Membranes. 2023; 13(11):863. https://doi.org/10.3390/membranes13110863
Chicago/Turabian StyleVan Goethem, Cédric, Parimal V. Naik, Miet Van de Velde, Jim Van Durme, Alex Verplaetse, and Ivo F. J. Vankelecom. 2023. "Stability of Filled PDMS Pervaporation Membranes in Bio-Ethanol Recovery from a Real Fermentation Broth" Membranes 13, no. 11: 863. https://doi.org/10.3390/membranes13110863
APA StyleVan Goethem, C., Naik, P. V., Van de Velde, M., Van Durme, J., Verplaetse, A., & Vankelecom, I. F. J. (2023). Stability of Filled PDMS Pervaporation Membranes in Bio-Ethanol Recovery from a Real Fermentation Broth. Membranes, 13(11), 863. https://doi.org/10.3390/membranes13110863








