Preparation and Characterization of Chitosan/LDH Composite Membranes for Drug Delivery Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Membranes
2.3. Characterization
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shukla, A.K.; Alam, J.; Alhoshan, M. Recent Advancements in Polyphenylsulfone Membrane Modification Methods for Separation Applications. Membranes 2022, 12, 247. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, A.; Drabik, M.; Lipko, A.; Grzeczkowicz, A.; Stachowiak, R.; Marszalik, A.; Granicka, L.H. Composite Membrane Dressings System with Metallic Nanoparticles as an Antibacterial Factor in Wound Healing. Membranes 2022, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Radu, E.R.; Voicu, S.I. Functionalized Hemodialysis Polysulfone Membranes with Improved Hemocompatibility. Polymers 2022, 14, 1130. [Google Scholar] [CrossRef] [PubMed]
- Serbanescu, O.S.; Pandele, A.M.; Miculescu, F.; Voicu, S.I. Synthesis and Characterization of Cellulose Acetate Membranes with Self-Indicating Properties by Changing the Membrane Surface Color for Separation of Gd(III). Coatings 2020, 10, 468. [Google Scholar] [CrossRef]
- Muhulet, A.; Tuncel, C.; Miculescu, F.; Pandele, A.; Bobirica, C.; Orbeci, C.; Bobirica, L.; Palla-Papavlu, A.; Voicu, S.I. Synthesis and characterization of polysulfone–TiO2 decorated MWCNT composite membranes by sonochemical method. Appl. Phys. A 2020, 126, 233. [Google Scholar] [CrossRef]
- Pandele, A.M.; Iovu, H.; Orbeci, C.; Tuncel, C.; Miculescu, F.; Nicolescu, A.; Deleanu, C.; Voicu, S.I. Surface modified cellulose acetate membranes for the reactive retention of tetracycline. Sep. Purif. Technol. 2020, 249, 117145. [Google Scholar] [CrossRef]
- Serbanescu, O.S.; Voicu, S.I.; Thakur, V.K. Polysulfone functionalized membranes: Properties and challenges. Mater. Today Chem. 2020, 17, 100302. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Cellulose Composites with Graphene for Tissue Engineering Applications. Materials 2020, 13, 5347. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef]
- Jeon, G.; Yang, S.Y.; Kim, J.K. Functional nanoporous membranes for drug delivery. J. Mater. Chem. 2012, 22, 14814–14834. [Google Scholar] [CrossRef] [Green Version]
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, M.; Rajendran, R.; Soliman, I.E.; Ashour, M.M.; Beherei, H.H.; Tohamy, K.M.; Thomas, S.; Kalarikkal, N.; Arthanareeswaran, G.; Das, D.B. Nanoparticle- and Nanoporous-Membrane-Mediated Delivery of Therapeutics. Pharmaceutics 2019, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Rosli, N.A.; Loh, K.S.; Wong, W.Y.; Lee, T.K.; Ahmad, A. Hybrid Composite Membrane of Phosphorylated Chitosan/Poly (Vinyl Alcohol)/Silica as a Proton Exchange Membrane. Membranes 2021, 11, 675. [Google Scholar] [CrossRef]
- Sun, X.; Yin, L.; Zhu, H.; Zhu, J.; Hu, J.; Luo, X.; Huang, H.; Fu, Y. Enhanced Antimicrobial Cellulose/Chitosan/ZnO Biodegradable Composite Membrane. Membranes 2022, 12, 239. [Google Scholar] [CrossRef]
- Wu, Y.; Rashidpour, A.; Almajano, M.P.; Metón, I. Chitosan-Based Drug Delivery System: Applications in Fish Biotechnology. Polymers 2020, 12, 1177. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Thakur, V.K.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef]
- Woraphatphadung, T.; Sajomsang, W.; Rojanarata, T.; Ngawhirunpat, T.; Tonglairoum, P.; Opanasopit, P. Development of Chitosan-Based pH-Sensitive Polymeric Micelles Containing Curcumin for Colon-Targeted Drug Delivery. AAPS PharmSciTech 2018, 19, 991–1000. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef]
- Sivanesan, I.; Gopal, J.; Muthu, M.; Shin, J.; Mari, S.; Oh, J. Green Synthesized Chitosan/Chitosan Nanoforms/Nanocomposites for Drug Delivery Applications. Polymers 2021, 13, 2256. [Google Scholar] [CrossRef]
- Aibani, N.; Rai, R.; Patel, P.; Cuddihy, G.; Wasan, E.K. Chitosan Nanoparticles at the Biological Interface: Implications for Drug Delivery. Pharmaceutics 2021, 13, 1686. [Google Scholar] [CrossRef]
- Mikušová, V.; Mikuš, P. Advances in Chitosan-Based Nanoparticles for Drug Delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, D.R.; Tavano, L.; Mitjans, M.; Pérez, L.; Infante, M.R.; Vinardell, M.P. In vitro antitumor activity of methotrexate via pH-sensitive chitosan nanoparticles. Biomaterials 2013, 34, 2758–2772. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Chakraborty, S.; Bhattacharya, S.; Mishra, R.; Kundu, P.P. pH-sensitive chitosan/alginate core-shell nanoparticles for efficient and safe oral insulin delivery. Int. J. Biol. Macromol. 2015, 72, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Andalib, F.; Mohammadikish, M.; Divsalar, A.; Sahebi, U. Hollow microcapsule with pH-sensitive chitosan/polymer shell for in vitro delivery of curcumin and gemcitabine. Eur. Polym. J. 2022, 162, 110887. [Google Scholar] [CrossRef]
- Qin, Y.; Li, P. Antimicrobial Chitosan Conjugates: Current Synthetic Strategies and Potential Applications. Int. J. Mol. Sci. 2020, 21, 499. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; de Mello, J.M.M.; Dalcanton, F.; Macuvele, D.L.P.; Padoin, N.; Fiori, M.A.; Soares, C.; Riella, H.G. Mechanical, Thermal and Antimicrobial Properties of Chitosan-Based-Nanocomposite with Potential Applications for Food Packaging. J. Polym. Environ. 2020, 28, 1216–1236. [Google Scholar] [CrossRef]
- Xu, T.; Gao, C.; Feng, X.; Huang, M.; Yang, Y.; Shen, X.; Tang, X. Cinnamon and clove essential oils to improve physical, thermal and antimicrobial properties of chitosan-gum arabic polyelectrolyte complexed films. Carbohydr. Polym. 2019, 217, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Jøraholmen, M.W.; Bhargava, A.; Julin, K.; Johannessen, M.; Škalko-Basnet, N. The Antimicrobial Properties of Chitosan Can Be Tailored by Formulation. Mar. Drugs 2020, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek-Szczepańska, B.; Wekwejt, M.; Mazur, O.; Zasada, L.; Pałubicka, A.; Olewnik-Kruszkowska, E. The Physicochemical and Antibacterial Properties of Chitosan-Based Materials Modified with Phenolic Acids Irradiated by UVC Light. Int. J. Mol. Sci. 2021, 22, 6472. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-García, M.; Ríos-Romo, R.A.; Melgarejo-Mancilla, A.; Díaz-Ramírez, M.; Hernández-Hernández, H.M.; Amaro-Reyes, A.; Pierro, P.D.; Regalado-González, C. Rheological and Antimicrobial Properties of Chitosan and Quinoa Protein Filmogenic Suspensions with Thyme and Rosemary Essential Oils. Foods 2020, 9, 1616. [Google Scholar] [CrossRef]
- Pandele, A.M.; Ionita, M.; Crica, L.; Dinescu, S.; Costache, M.; Iovu, H. Synthesis, characterization, and in vitro studies of graphene oxide/chitosan–polyvinyl alcohol films. Carbohydr. Polym. 2014, 102, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Yarger, M.S.; Steinmiller, E.M.P.; Choi, K.-S. Electrochemical Synthesis of Zn−Al Layered Double Hydroxide (LDH) Films. Inorg. Chem. 2008, 47, 5859–5865. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Forano, C.; Hibino, T.; Leroux, F.; Taviot-Guého, C. Chapter 13.1 Layered Double Hydroxides. In Developments in Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 1021–1095. [Google Scholar]
- Ameena Shirin, V.K.; Sankar, R.; Johnson, A.P.; Gangadharappa, H.V.; Pramod, K. Advanced drug delivery applications of layered double hydroxide. J. Control Release 2021, 330, 398–426. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Wang, Y.; Ping, Q.; Liao, Z. Zn–Al–NO3-layered double hydroxides with intercalated diclofenac for ocular delivery. Int. J. Pharm. 2011, 404, 250–256. [Google Scholar] [CrossRef]
- Kuthati, Y.; Kankala, R.K.; Lee, C.-H. Layered double hydroxide nanoparticles for biomedical applications: Current status and recent prospects. Appl. Clay Sci. 2015, 112–113, 100–116. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Zauska, L.; Bova, S.; Benova, E.; Bednarcik, J.; Balaz, M.; Zelenak, V.; Hornebecq, V.; Almasi, M. Thermosensitive Drug Delivery System SBA-15-PEI for Controlled Release of Nonsteroidal Anti-Inflammatory Drug Diclofenac Sodium Salt: A Comparative Study. Materials 2021, 14, 1880. [Google Scholar] [CrossRef] [PubMed]
- Brogden, R.N.; Heel, R.C.; Pakes, G.E.; Speight, T.M.; Avery, G.S. Diclofenac sodium: A review of its pharmacological properties and therapeutic use in rheumatic diseases and pain of varying origin. Drugs 1980, 20, 24–48. [Google Scholar] [CrossRef]
- Das, S.; Subuddhi, U. Controlled and targeted delivery of diclofenac sodium to the intestine from pH-Responsive chitosan/poly(vinyl alcohol) interpenetrating polymeric network hydrogels. Polym. Sci. Ser. A 2016, 58, 154–166. [Google Scholar] [CrossRef]
- Bogdan, M.; Caira, M.R.; Bogdan, D.; Morari, C.; Fărcaş, S.I. Evidence of a Bimodal Binding between Diclofenac-Na and β-Cyclodextrin in Solution. J. Incl. Phenom. Macrocycl. Chem. 2004, 49, 225–229. [Google Scholar] [CrossRef]
- Todd, P.A.; Sorkin, E.M. Diclofenac sodium. A reappraisal of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs 1988, 35, 244–285. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Anbu, J.; Maharajan, G.; Pillai, K.S. Targeted delivery of diclofenac sodium via gelatin magnetic microspheres formulated for intra-arterial administration. J. Drug Target. 2008, 16, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Mortazavi, S.A.; Rabbani, S.; Torshabi, M.; Talimi, R.; Haeri, A. Fast dissolving nanofibrous mats for diclofenac sodium delivery: Effects of electrospinning polymer and addition of super-disintegrant. J. Drug Deliv. Sci. Technol. 2022, 73, 103356. [Google Scholar] [CrossRef]
- Paul, A.; Augustine, R.; Hasan, A.; Zahid, A.A.; Thomas, S.; Agatemor, C.; Ghosal, K. Halloysite nanotube and chitosan polymer composites: Physicochemical and drug delivery properties. J. Drug Deliv. Sci. Technol. 2022, 72, 103380. [Google Scholar] [CrossRef]
- Dandekar, A.A.; Kale, M.; Garimella, H.T.; Banga, A.K. Effect of compromised skin barrier on delivery of diclofenac sodium from brand and generic formulations via microneedles and iontophoresis. Int. J. Pharm. 2022, 628, 122271. [Google Scholar] [CrossRef]
- Chopra, L.; Thakur, K.K.; Chohan, J.S.; Sharma, S.; Ilyas, R.A.; Asyraf, M.R.M.; Zakaria, S.Z.S. Comparative Drug Release Investigations for Diclofenac Sodium Drug (DS) by Chitosan-Based Grafted and Crosslinked Copolymers. Materials 2022, 15, 2404. [Google Scholar] [CrossRef] [PubMed]
- Barkhordari, S.; Alizadeh, A. Fabrication of pH-sensitive chitosan/layered double hydroxide (LDH)/Fe3O4 nanocomposite hydrogel beads for controlled release of diclofenac. Polym. Bull. 2022, 79, 5533–5548. [Google Scholar] [CrossRef]
- Lerner, D.A.; Bégu, S.; Aubert-Pouëssel, A.; Polexe, R.; Devoisselle, J.-M.; Azaïs, T.; Tichit, D. Synthesis and Properties of New Multilayer Chitosan@layered Double Hydroxide/Drug Loaded Phospholipid Bilayer Nanocomposite Bio-Hybrids. Materials 2020, 13, 3565. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Chithra Sekhar, V. Fabrication of functionalized layered double hydroxide/chitosan nanocomposite with dual responsive drug release for the targeted therapy of breast cancer. Eur. Polym. J. 2020, 139, 109993. [Google Scholar] [CrossRef]
- Radu, I.-C.; Vasile, E.; Damian, C.; Iovu, H.; Stanescu, P.; Zaharia, C. Influence of the Double Bond LDH Clay on the Exfoliation / Intercalation Mechanism of Polyacrylamide Nanocomposite Hydrogels. Mater. Plast. 2018, 55, 263–268. [Google Scholar] [CrossRef]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef]
- Namazi, H.; Dadkhah, A. Convenient method for preparation of hydrophobically modified starch nanocrystals with using fatty acids. Carbohydr. Polym. 2010, 79, 731–737. [Google Scholar] [CrossRef]
- Shigemasa, Y.; Matsuura, H.; Sashiwa, H.; Saimoto, H. Evaluation of different absorbance ratios from infrared spectroscopy for analyzing the degree of deacetylation in chitin. Int. J. Biol. Macromol. 1996, 18, 237–242. [Google Scholar] [CrossRef]
- Dimzon, I.K.D.; Knepper, T.P. Degree of deacetylation of chitosan by infrared spectroscopy and partial least squares. Int. J. Biol. Macromol. 2015, 72, 939–945. [Google Scholar] [CrossRef]
- Debnath, T.; Ghosh, S.; Potlapuvu, U.; Kona, L.; Kamaraju, S.; Sarkar, S.; Latha, G.; Chelluri, L. Proliferation and Differentiation Potential of Human Adipose-Derived Stem Cells Grown on Chitosan Hydrogel. PLoS ONE 2015, 10, e0120803. [Google Scholar] [CrossRef]
- Lagat, M.K.; Were, S.; Ndwigah, F.; Kemboi, V.J.; Kipkoech, C.; Tanga, C.M. Antimicrobial Activity of Chemically and Biologically Treated Chitosan Prepared from Black Soldier Fly (Hermetia illucens) Pupal Shell Waste. Microorganisms 2021, 9, 2417. [Google Scholar] [CrossRef] [PubMed]
- Barkhordari, S.; Yadollahi, M. Carboxymethyl cellulose capsulated layered double hydroxides/drug nanohybrids for Cephalexin oral delivery. Appl. Clay Sci. 2016, 121–122, 77–85. [Google Scholar] [CrossRef]
- Asasutjarit, R.; Theerachayanan, T.; Kewsuwan, P.; Veeranodha, S.; Fuongfuchat, A.; Ritthidej, G.C. Development and Evaluation of Diclofenac Sodium Loaded-N-Trimethyl Chitosan Nanoparticles for Ophthalmic Use. AAPS PharmSciTech 2015, 16, 1013–1024. [Google Scholar] [CrossRef]
- Zhou, J.; Li, R.; Liu, S.; Li, Q.; Zhang, L.; Zhang, L.; Guan, J. Structure and magnetic properties of regenerated cellulose/Fe3O4 nanocomposite films. J. Appl. Polym. Sci. 2009, 111, 2477–2484. [Google Scholar] [CrossRef]
- Liang, X.X.; Omer, A.M.; Hu, Z.-H.; Wang, Y.G.; Yu, D.; Ouyang, X.-K. Efficient adsorption of diclofenac sodium from aqueous solutions using magnetic amine-functionalized chitosan. Chemosphere 2019, 217, 270–278. [Google Scholar] [CrossRef]
- Yeh, J.-T.; Chen, C.-L.; Huang, K.S.; Nien, Y.H.; Chen, J.L.; Huang, P.Z. Synthesis, characterization, and application of PVP/chitosan blended polymers. J. Appl. Polym. Sci. 2006, 101, 885–891. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, S.; Xia, M.; Wang, F. Rapid and efficient removal of diclofenac sodium from aqueous solution via ternary core-shell CS@PANI@LDH composite: Experimental and adsorption mechanism study. J. Hazard. Mater. 2021, 402, 123815. [Google Scholar] [CrossRef]
- Elanchezhiyan, S.S.; Meenakshi, S. Synthesis and characterization of chitosan/Mg-Al layered double hydroxide composite for the removal of oil particles from oil-in-water emulsion. Int. J. Biol. Macromol. 2017, 104, 1586–1595. [Google Scholar] [CrossRef]
- Zoromba, M.S.; Nour, M.A.; Eltamimy, H.E.; Abd El-Maksoud, S.A. Effect of modified layered double hydroxide on the flammability and mechanical properties of polypropylene. Sci. Eng. Compos. Mater. 2018, 25, 101–108. [Google Scholar] [CrossRef]
- Dey, S.C.; Al-Amin, M.; Rashid, T.U.; Sultan, M.Z.; Ashaduzzaman, M.; Sarker, M.; Shamsuddin, S.M. Preparation, characterization and performance evaluation of chitosan as an adsorbent for Remazol Red. Int. J. Latest Res. Eng. Technol. 2016, 2, 52–62. [Google Scholar]
- Bagre, A.C.; Jain, K.; Jain, N.K. Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: In vitro and in vivo assessment. Int. J. Pharmacol. 2013, 456, 31–40. [Google Scholar] [CrossRef]
- Corobea, M.S.; Albu, M.G.; Ion, R.; Cimpean, A.; Miculescu, F.; Antoniac, I.V.; Raditoiu, V.; Sirbu, I.; Stoenescu, M.; Voicu, S.I.; et al. Modification of titanium surface with collagen and doxycycline as a new approach in dental implants. J. Adhes. Sci. Technol. 2015, 29, 2537–2550. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug release study of the chitosan-based nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.E.; Czupiel, P.; Shoichet, M. Engineered polymeric nanoparticles to guide the cellular internalization and trafficking of small interfering ribonucleic acids. J. Control Release 2017, 259, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhou, G.; Xiao, F.; He, Q.; Zhang, J. Stimuli-responsive poly (ionic liquid) nanoparticles for controlled drug delivery. J. Mater. Chem. B 2020, 8, 7994–8001. [Google Scholar] [CrossRef]
- Abdo, G.G.; Zagho, M.M.; Khalil, A. Recent advances in stimuli-responsive drug release and targeting concepts using mesoporous silica nanoparticles. Emergent Mater. 2020, 3, 407–425. [Google Scholar]
- Gull, N.; Khan, S.M.; Butt, O.M.; Islam, A.; Shah, A.; Jabeen, S.; Khan, S.U.; Khan, A.; Khan, R.U.; Butt, M.T.Z. Inflammation targeted chitosan-based hydrogel for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2020, 162, 175–187. [Google Scholar] [CrossRef]
- Korkiatithaweechai, S.; Umsarika, P.; Praphairaksit, N.; Muangsin, N. Controlled Release of Diclofenac from Matrix Polymer of Chitosan and Oxidized Konjac Glucomannan. Mar. Drugs 2011, 9, 1649–1663. [Google Scholar] [CrossRef] [Green Version]
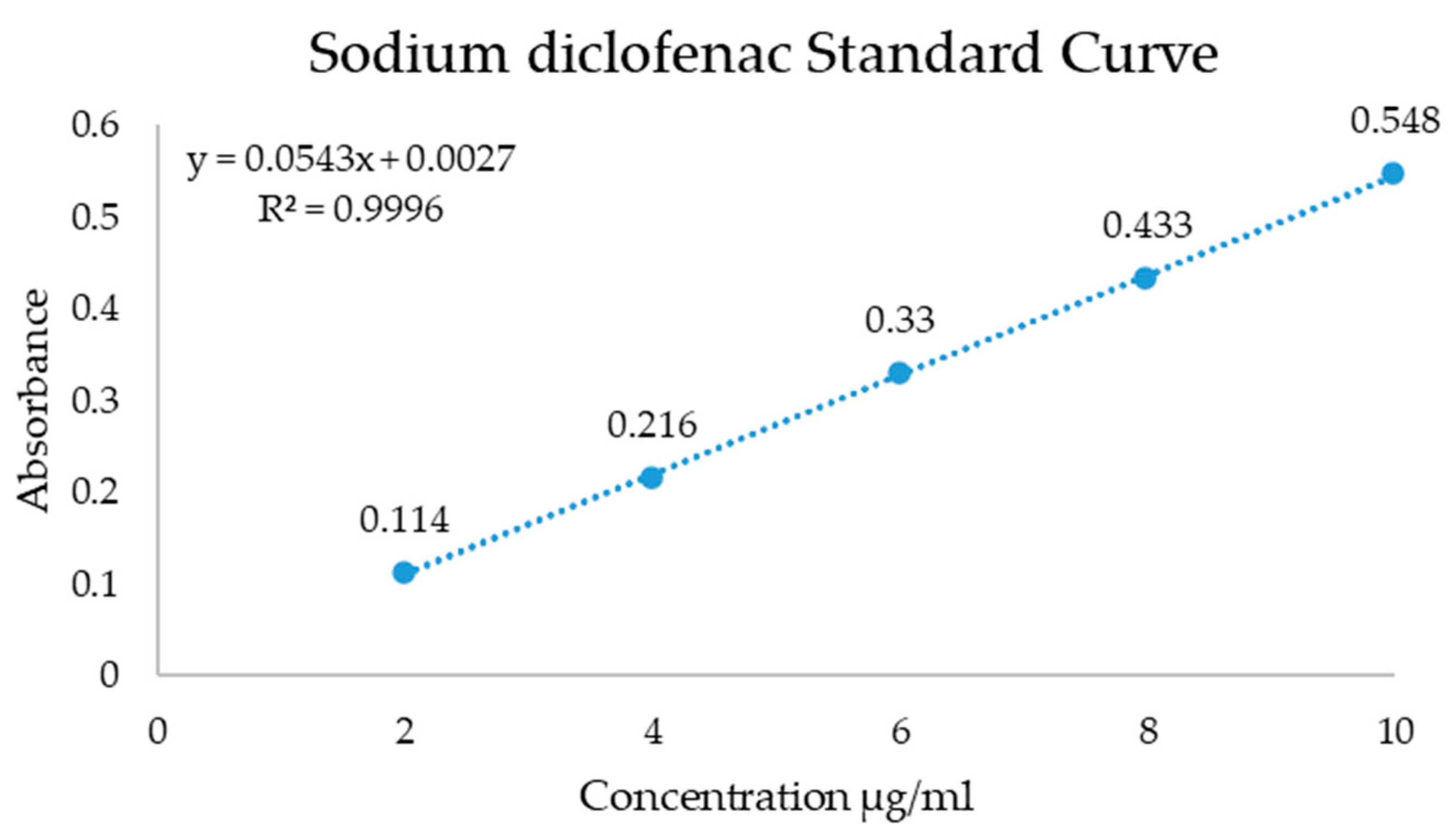
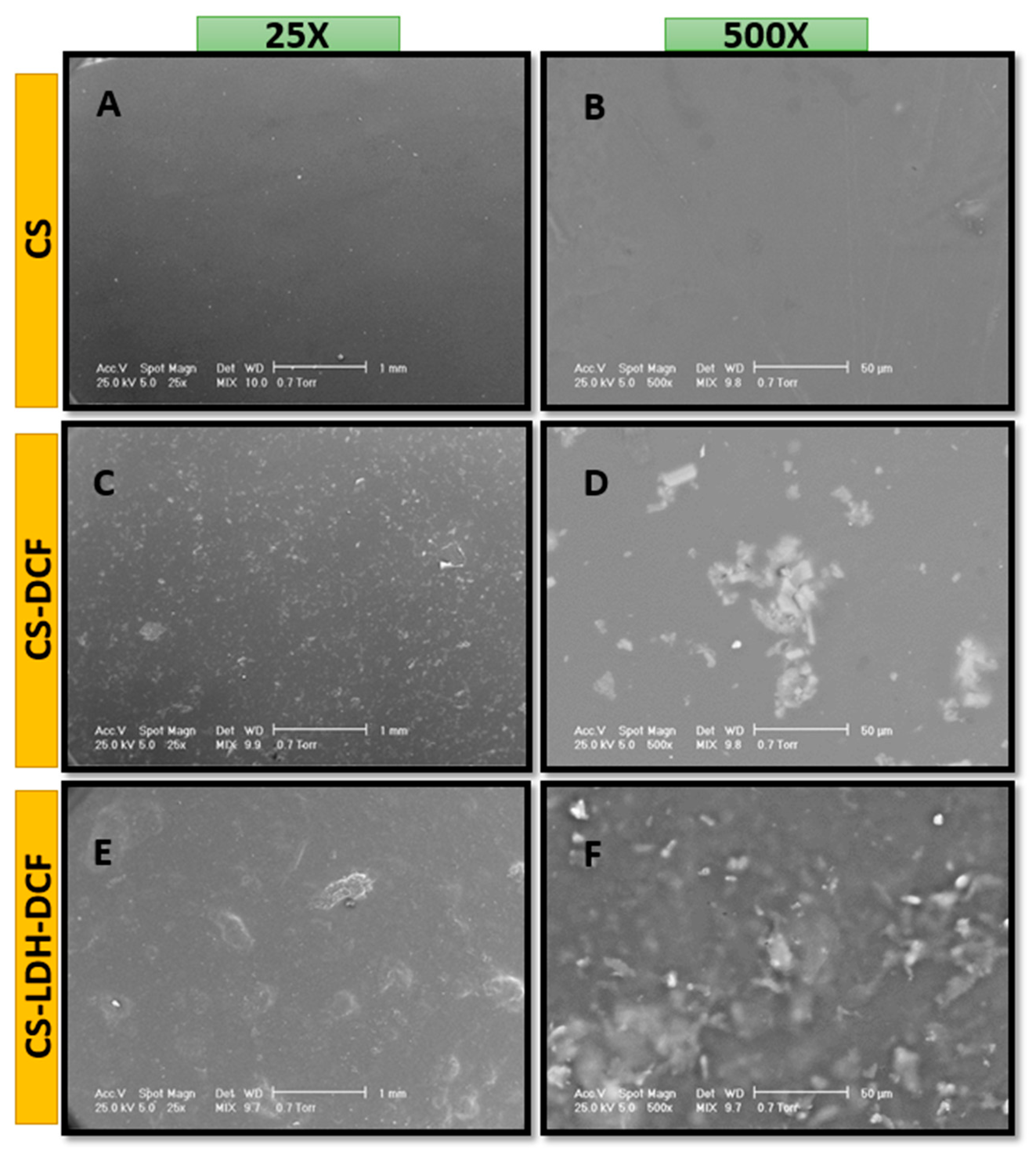
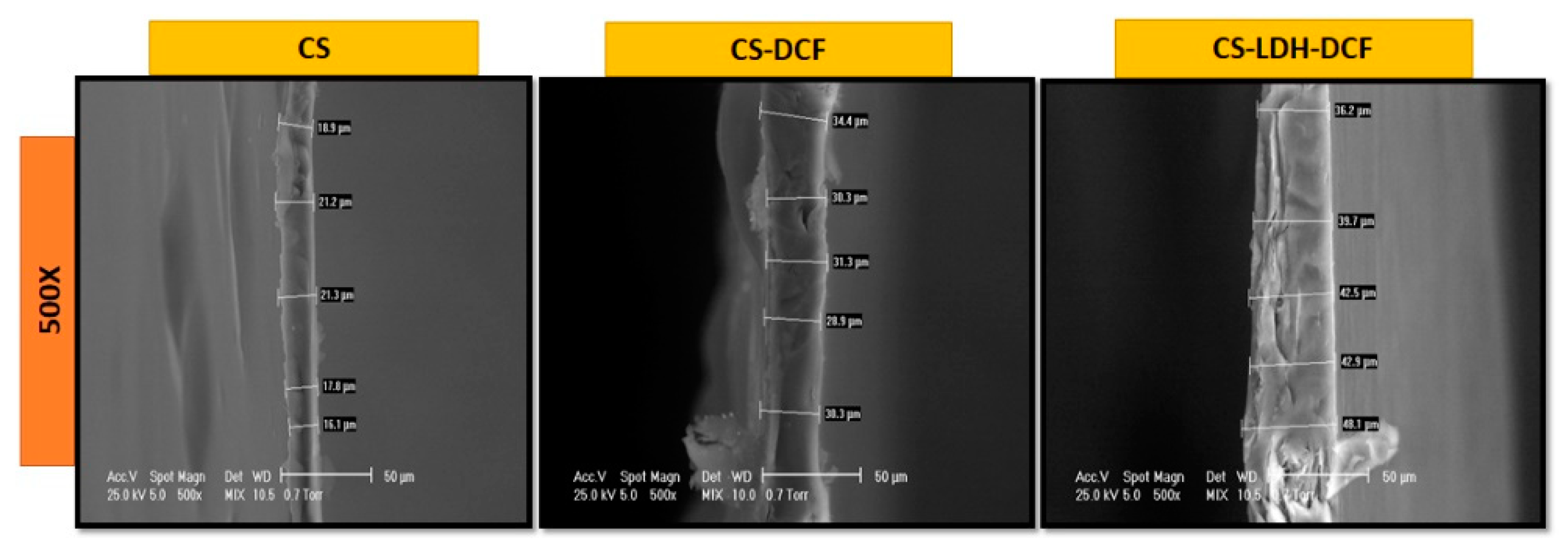
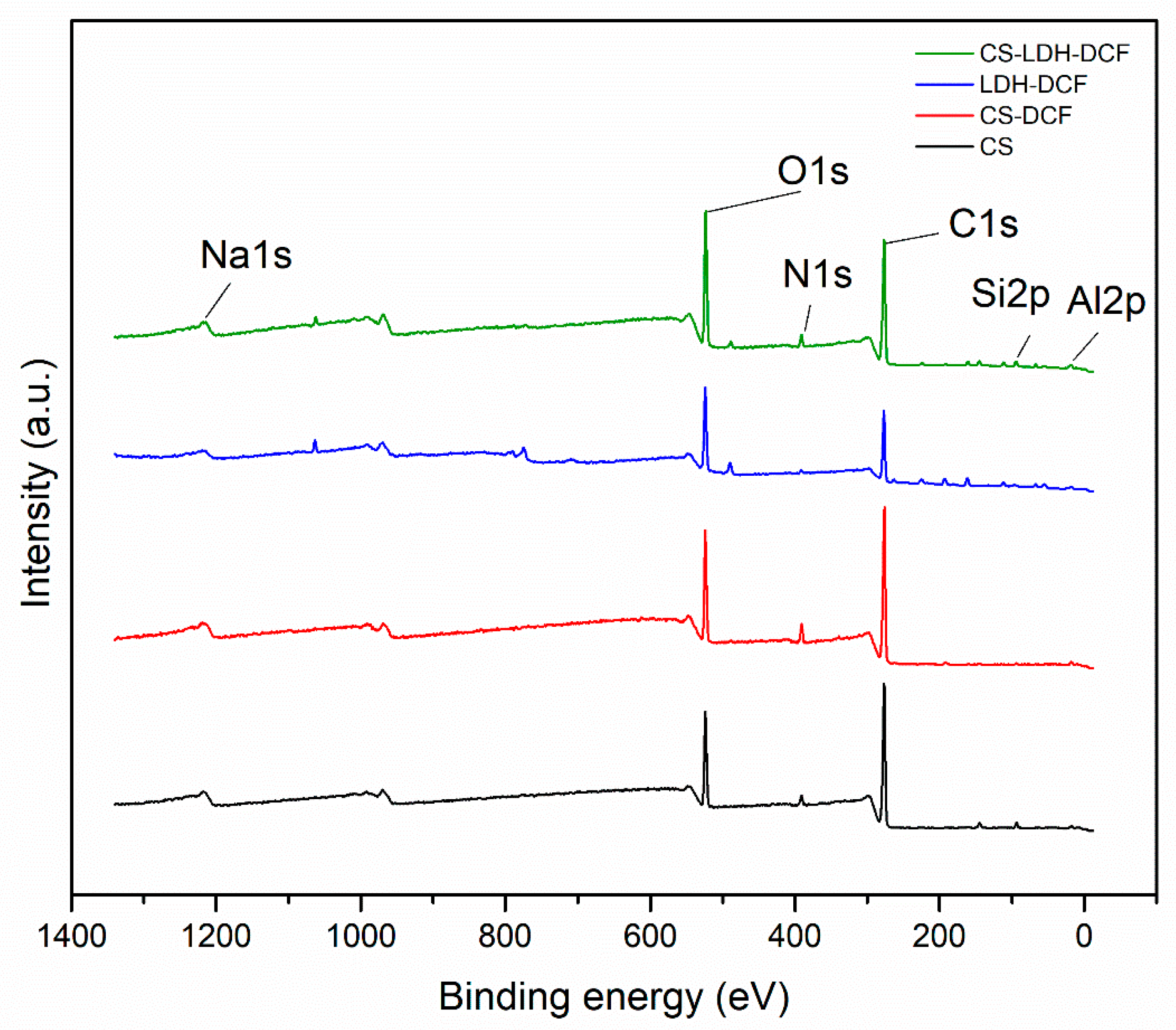
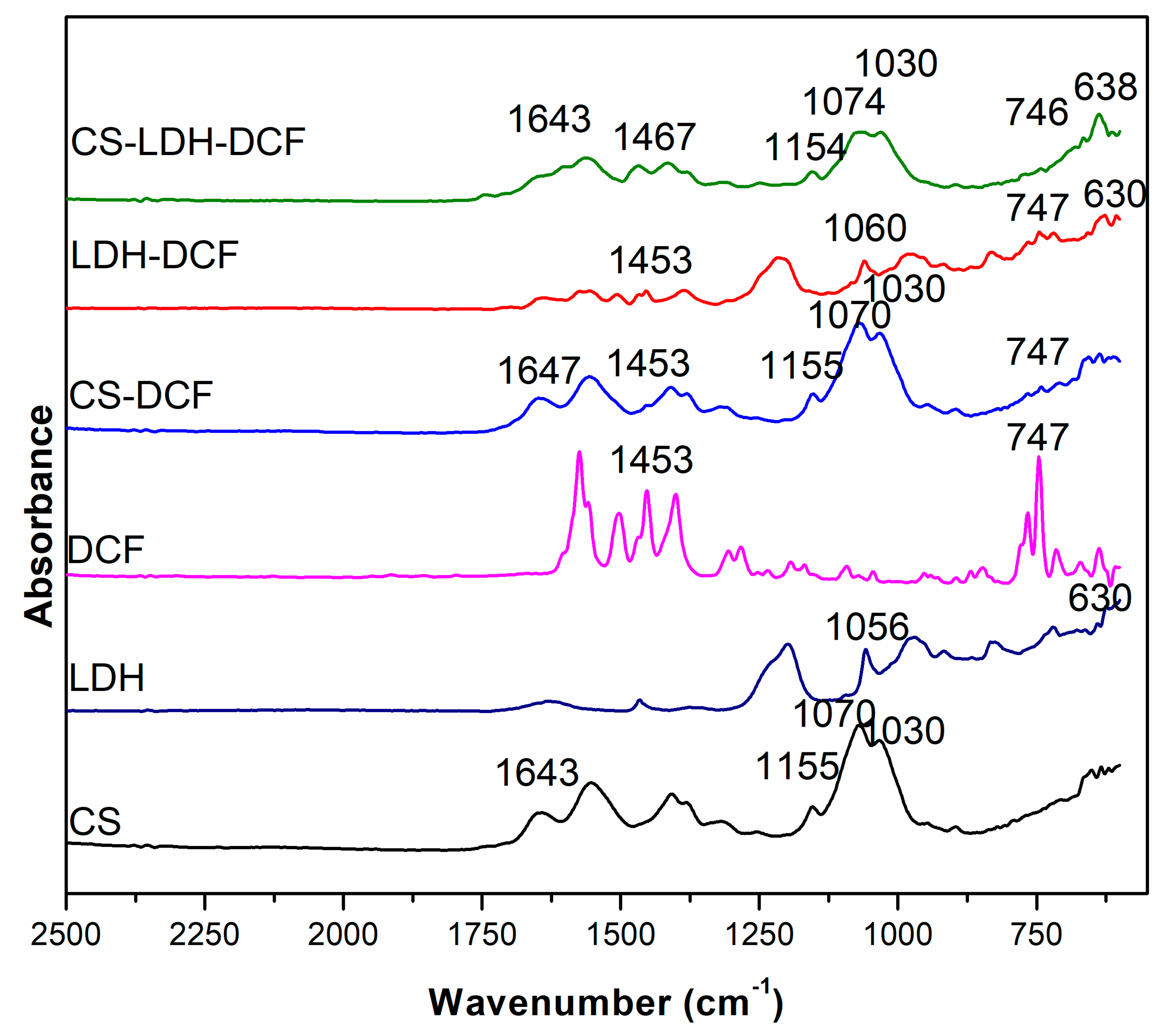
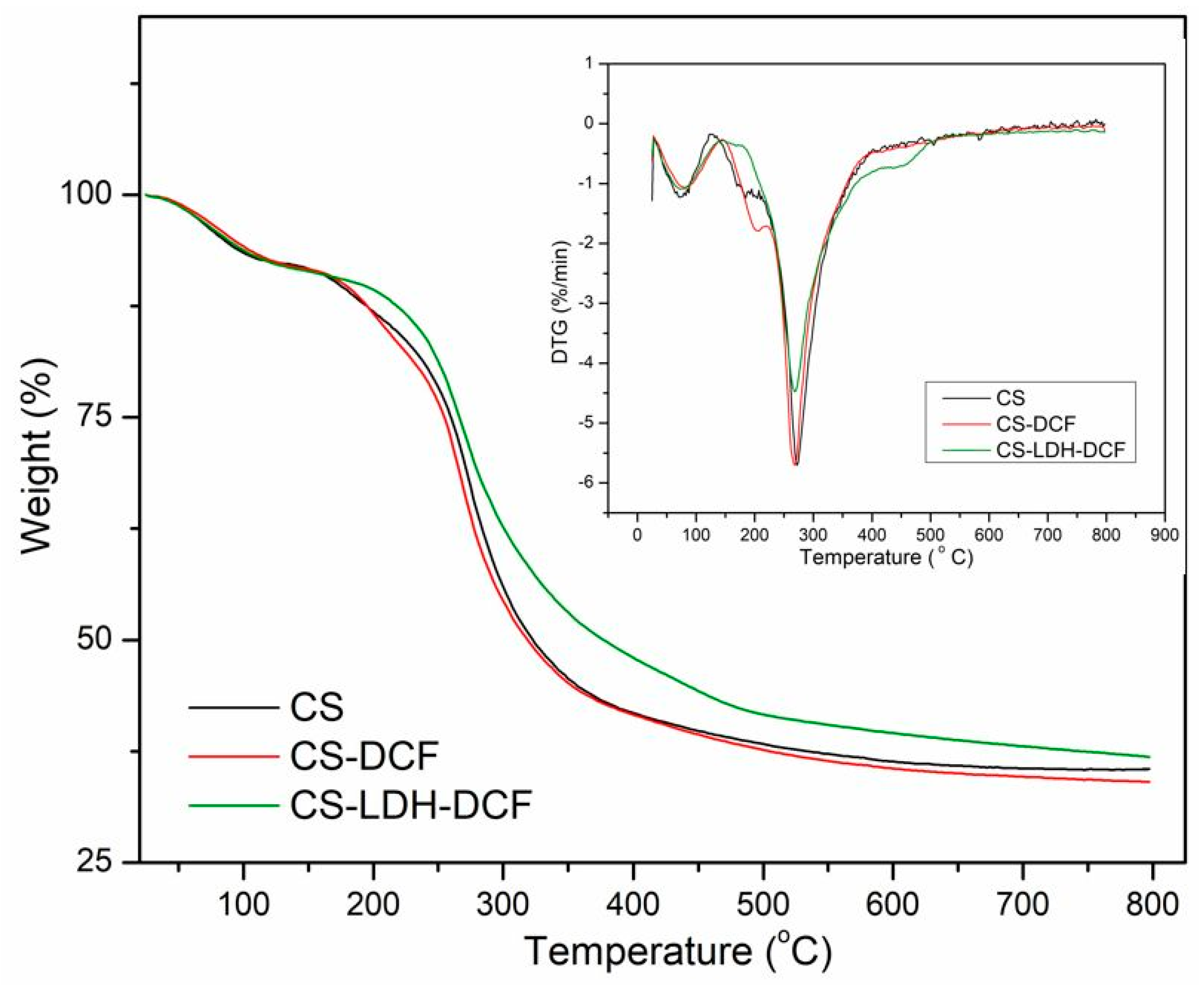
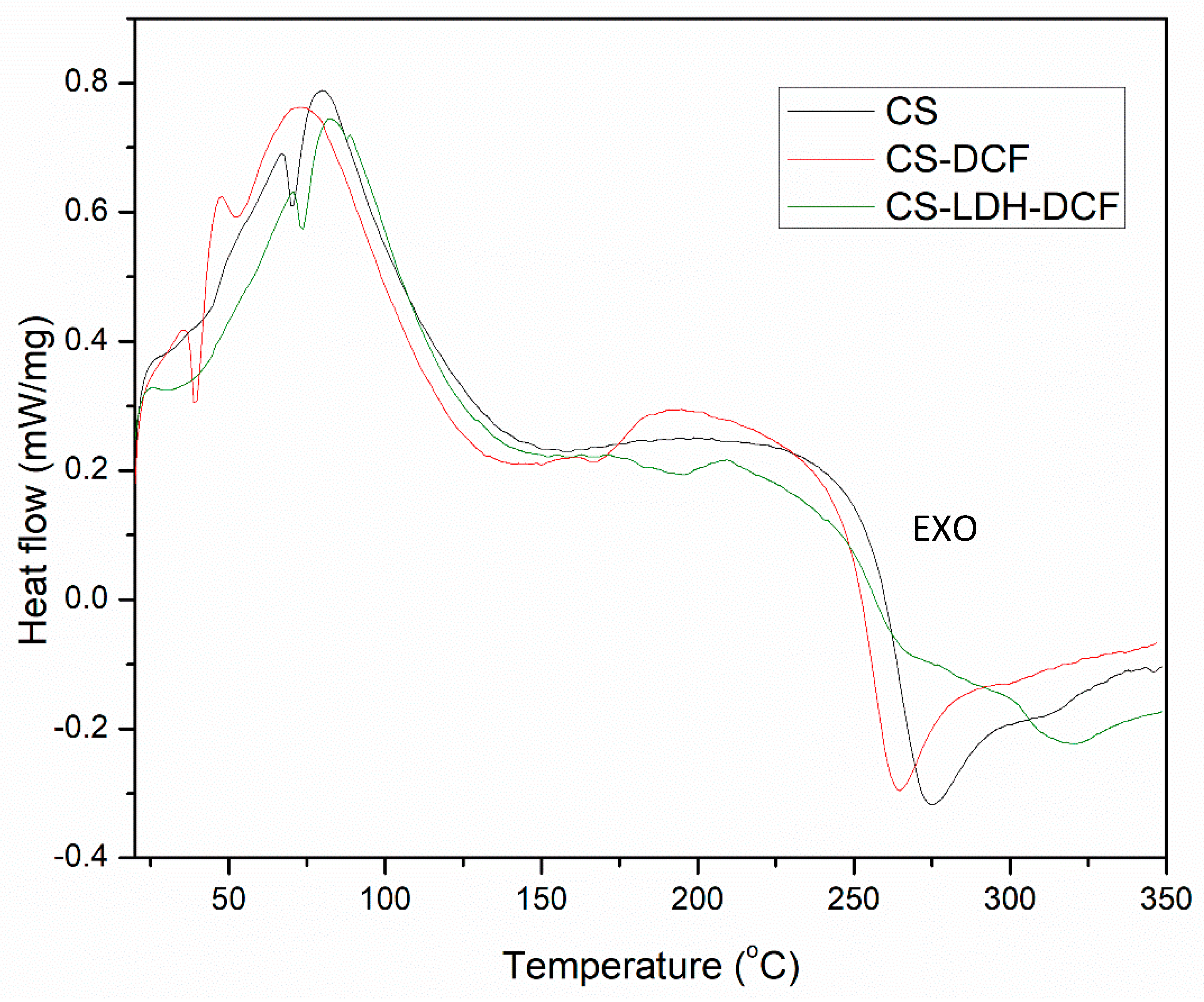

| Sample | Atomic Percent (%) | ||||||
|---|---|---|---|---|---|---|---|
| O1s | C1s | N1s | Na1s | S2p | Si2p | Al2p | |
| CS | 17.46 | 75.92 | 3.58 | - | - | 2.79 | - |
| CS-DCF | 18.32 | 75.95 | 0.65 | 5.08 | - | - | - |
| LDH-DCF | 25.69 | 56.71 | 1.79 | 2.33 | 4.09 | 1.87 | 3.1 |
| CS-LDH-DCF | 24.35 | 65.78 | 3.16 | 0.96 | 0.93 | 2.95 | 1.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radu, E.-R.; Pandele, A.M.; Tuncel, C.; Miculescu, F.; Voicu, S.I. Preparation and Characterization of Chitosan/LDH Composite Membranes for Drug Delivery Application. Membranes 2023, 13, 179. https://doi.org/10.3390/membranes13020179
Radu E-R, Pandele AM, Tuncel C, Miculescu F, Voicu SI. Preparation and Characterization of Chitosan/LDH Composite Membranes for Drug Delivery Application. Membranes. 2023; 13(2):179. https://doi.org/10.3390/membranes13020179
Chicago/Turabian StyleRadu, Elena-Ruxandra, Andreea Madalina Pandele, Cristina Tuncel, Florin Miculescu, and Stefan Ioan Voicu. 2023. "Preparation and Characterization of Chitosan/LDH Composite Membranes for Drug Delivery Application" Membranes 13, no. 2: 179. https://doi.org/10.3390/membranes13020179
APA StyleRadu, E.-R., Pandele, A. M., Tuncel, C., Miculescu, F., & Voicu, S. I. (2023). Preparation and Characterization of Chitosan/LDH Composite Membranes for Drug Delivery Application. Membranes, 13(2), 179. https://doi.org/10.3390/membranes13020179









