Constructing a Hierarchical Hydrophilic Crosslink Network on the Surface of a Polyvinylidene Fluoride Membrane for Efficient Oil/Water Emulsion Separation
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Biocomposite Membranes
2.3. Characterisation
2.4. Separation Test of Oil/Water Emulsions
2.5. Stability and Anti-Oil Fouling Performance
3. Results and Discussion
3.1. Surface Chemistry of the Superhydrophilic Membranes
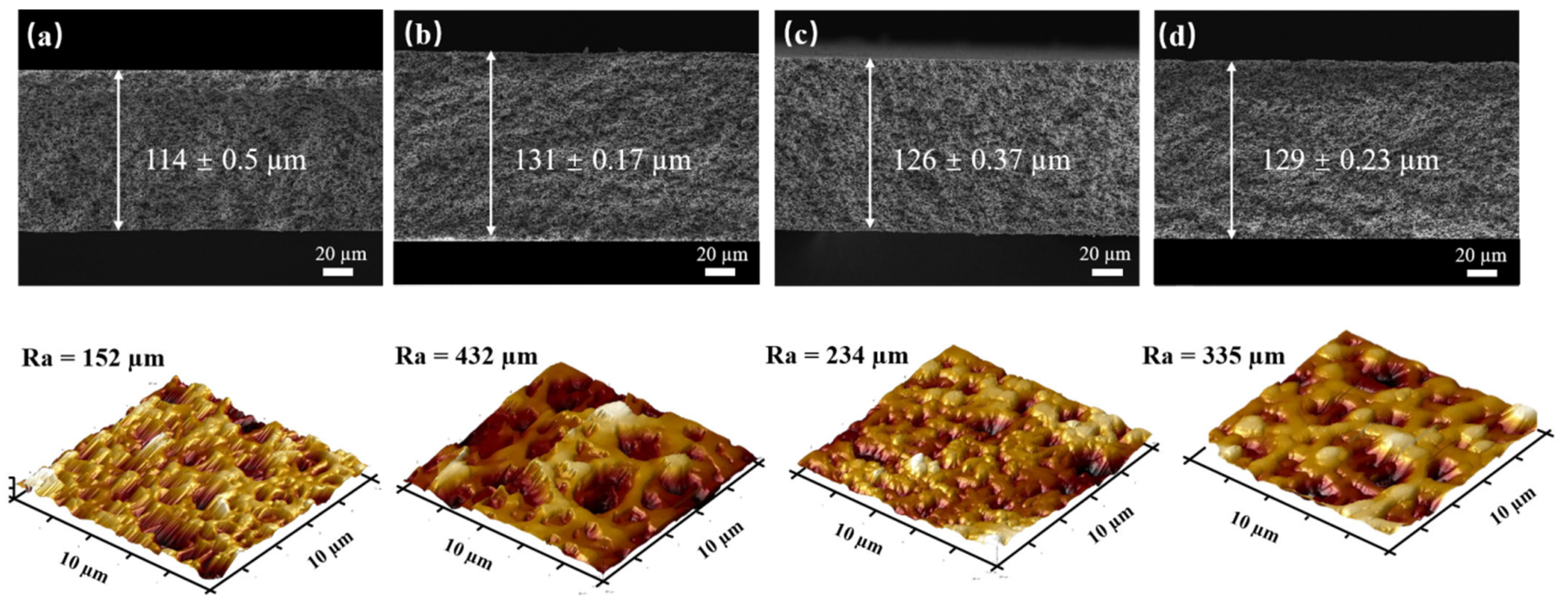
3.2. Surface Morphology
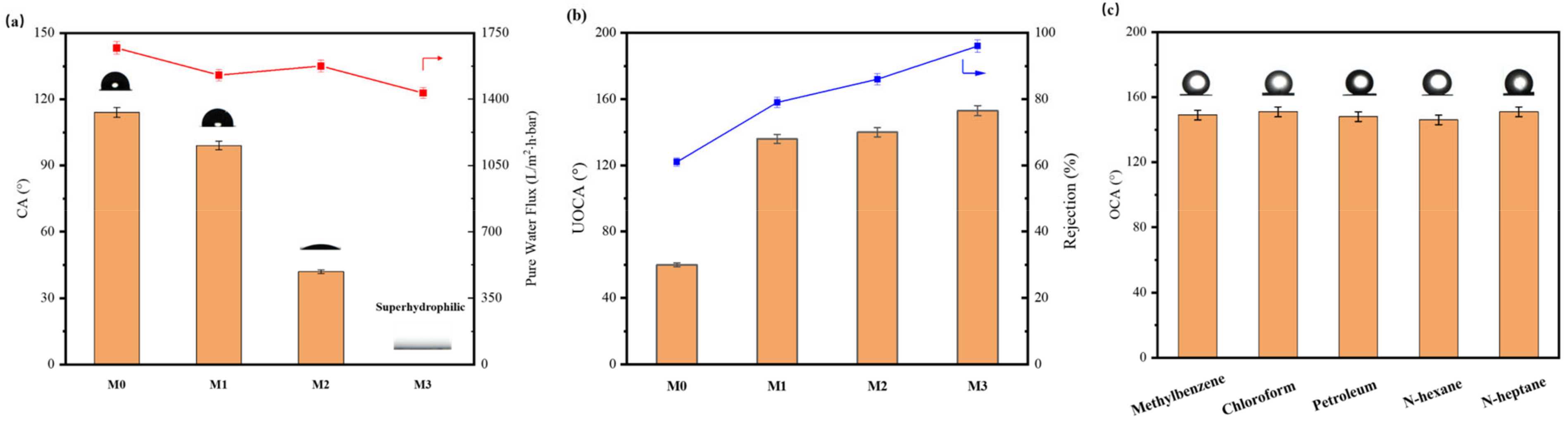
3.3. Wetting Behaviours and Water Flux
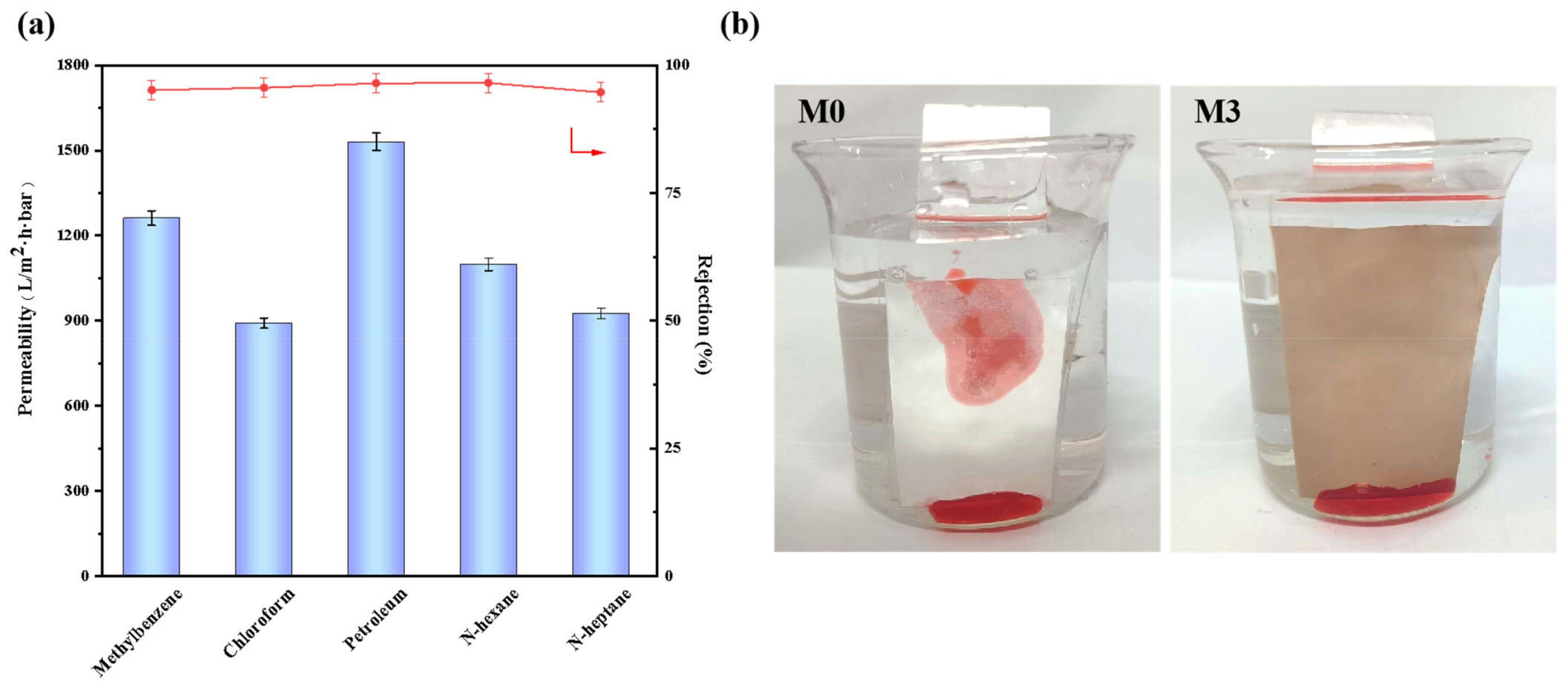
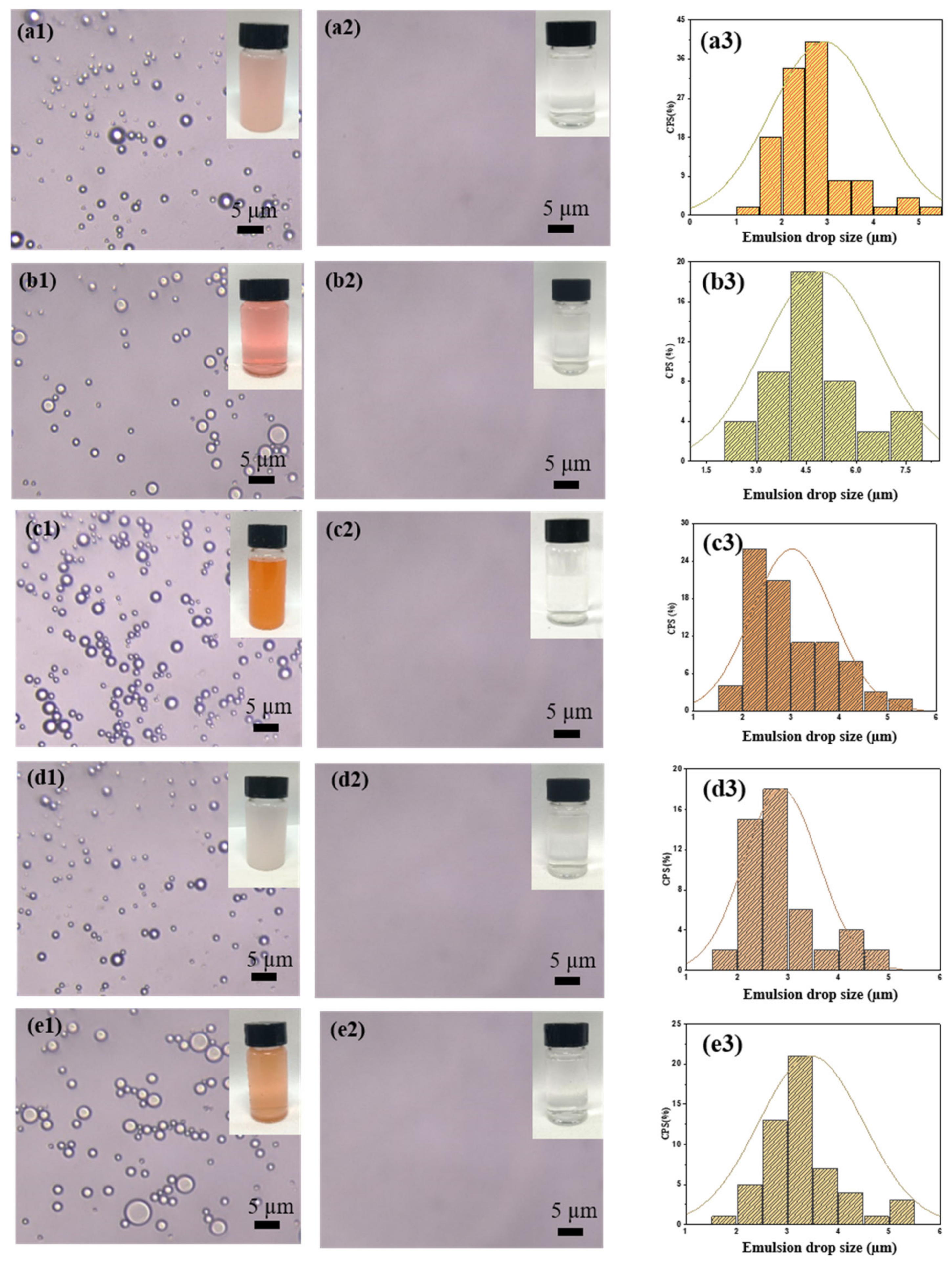
3.4. Resistance to the Oil/Water Emulsion
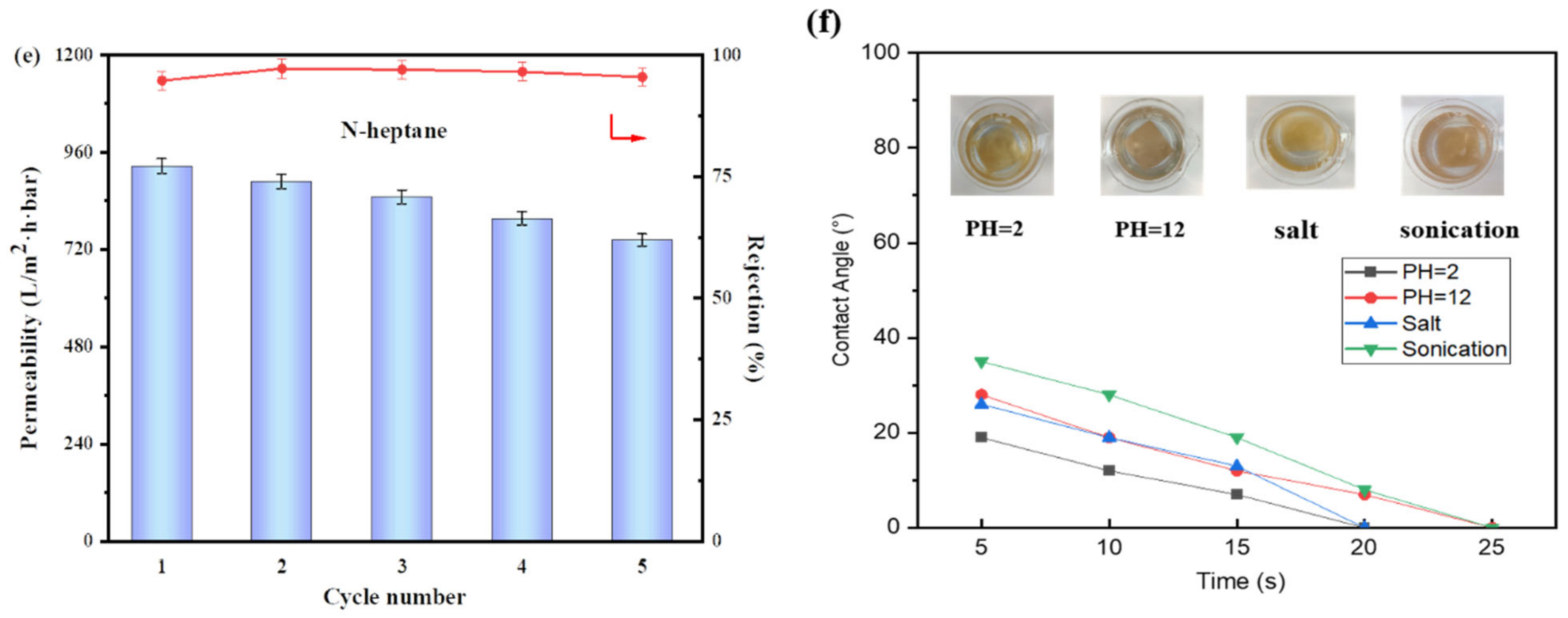
3.5. Mechanical Durability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zarghami, S.; Mohammadi, T.; Sadrzadeh, M.; Van der Bruggen, B. Superhydrophilic and underwater superoleophobic membranes—A review of synthesis methods. Prog. Polym. Sci. 2019, 98, 101166. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Li, M.-M.; Zhu, L.-J.; Wang, G.; Zeng, Z.-X. Zwitterionic polyvinylidene fluoride membranes with strong underwater superoleophobicity and oil-fouling resistance for oily water purification. J. Environ. Chem. Eng. 2022, 10, 107593. [Google Scholar] [CrossRef]
- Li, Z.-K.; Liu, Y.; Li, L.; Wei, Y.; Caro, J.; Wang, H. Ultra-thin titanium carbide (MXene) sheet membranes for high-efficient oil/water emulsions separation. J. Membr. Sci. 2019, 592, 117361. [Google Scholar] [CrossRef]
- Yao, Y.; Dang, X.; Qiao, X.; Li, R.; Chen, J.; Huang, Z.; Gong, Y.-K. Crosslinked biomimetic coating modified stainless-steel-mesh enables completely self-cleaning separation of crude oil/water mixtures. Water Res. 2022, 224, 119052. [Google Scholar] [CrossRef]
- Lv, C.-J.; Hao, B.; Yasin, A.; Yue, X.; Ma, P.-C. H2O2-assisted preparation of superhydrophilic polyacrylonitrile fabric and its application for the separation of oil/water mixture. Colloids Surf. A Physicochem. Eng. Asp. 2022, 646, 129004. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Shen, Y.; Mu, P.; Wang, Q.; Li, J. Ultrathin 2D Ti3C2Tx MXene membrane for effective separation of oil-in-water emulsions in acidic, alkaline, and salty environment. J. Colloid Interface Sci. 2020, 561, 861–869. [Google Scholar] [CrossRef]
- Song, Y.-Z.; Kong, X.; Yin, X.; Zhang, Y.; Sun, C.-C.; Yuan, J.-J.; Zhu, B.; Zhu, L.-P. Tannin-inspired superhydrophilic and underwater superoleophobic polypropylene membrane for effective oil/water emulsions separation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 585–592. [Google Scholar] [CrossRef]
- Liu, G.; Guo, Y.; Meng, B.; Wang, Z.; Liu, G.; Jin, W. Two-dimensional MXene hollow fiber membrane for divalent ions exclusion from water. Chin. J. Chem. Eng. 2022, 41, 260–266. [Google Scholar] [CrossRef]
- Ong, C.; Shi, Y.; Chang, J.; Alduraiei, F.; Wehbe, N.; Ahmed, Z.; Wang, P. Tannin-inspired robust fabrication of superwettability membranes for highly efficient separation of oil-in-water emulsions and immiscible oil/water mixtures. Sep. Purif. Technol. 2019, 227, 115657. [Google Scholar] [CrossRef]
- Yang, J.; Lin, L.; Wang, Q.; Ma, W.; Li, X.; Liu, Z.; Yang, X.; Xu, M.; Cheng, Q.; Zhao, K.; et al. Engineering a superwetting membrane with spider-web structured carboxymethyl cellulose gel layer for efficient oil-water separation based on biomimetic concept. Int. J. Biol. Macromol. 2022, 222, 2603–2614. [Google Scholar] [CrossRef]
- Dolatkhah, F.; Mohammadi, T.; Tofighy, M.A. Polysulfone hollow fiber membrane containing charcoal-carbon nanomaterial for wastewater treatment in membrane bioreactor. J. Water Process Eng. 2022, 50, 103222. [Google Scholar] [CrossRef]
- Xu, J.; Cui, J.; Sun, H.; Wu, Y.; Xue, C.; Xie, A.; Li, C. Facile preparation of hydrophilic PVDF membrane via tea polyphenols modification for efficient oil-water emulsion separation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130639. [Google Scholar] [CrossRef]
- Liu, C.; Wu, L.; Zhang, C.; Chen, W.; Luo, S. Surface hydrophilic modification of PVDF membranes by trace amounts of tannin and polyethyleneimine. Appl. Surf. Sci. 2018, 457, 695–704. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, W.; Li, X.; Wang, Z. Surface engineering of filter membranes with hydrogels for oil-in-water emulsion separation. Sep. Purif. Technol. 2023, 304, 122340. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Pramanik, S.K.; Monira, S. Understanding the fragmentation of microplastics into nano-plastics and removal of nano/microplastics from wastewater using membrane, air flotation and nano-ferrofluid processes. Chemosphere 2021, 282, 131053. [Google Scholar] [CrossRef]
- Rajapakse, N.; Zargar, M.; Sen, T.; Khiadani, M. Effects of influent physicochemical characteristics on air dissolution, bubble size and rise velocity in dissolved air flotation: A review. Sep. Purif. Technol. 2022, 289, 120772. [Google Scholar] [CrossRef]
- Wu, T.; Yang, G.; Cao, J.; Xu, Z.; Jiang, X. Activation and adsorption mechanisms of methylene blue removal by porous biochar adsorbent derived from eggshell membrane. Chem. Eng. Res. Des. 2022, 188, 330–341. [Google Scholar] [CrossRef]
- Akemoto, Y.; Takahashi, S.; Inaba, Y.; Kan, M.; Sakti, S.C.W.; Tanaka, S. Collectable adsorbent based on the adsorption characteristics of sodium citrate-pretreated vermiculite for cesium ion in an aquatic environment. J. Water Process Eng. 2022, 50, 103280. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Z.; Li, Y.; Qing, Y.; Dansawad, P.; Wu, H.; Liang, J.; Li, W. Electrospun rough PVDF nanofibrous membranes via introducing fluorinated SiO2 for efficient oil-water emulsions coalescence separation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129646. [Google Scholar] [CrossRef]
- Shijie, F.; Jiefeng, Z.; Pengyu, Z.; Yunling, G.A.O.; Junxian, Y. Superhydrophilic/underwater superoleophobic oil-in-water emulsion separation membrane modified by the co-deposition of polydopamine and chitosan-tripolyphosphate nanoparticles. J. Environ. Chem. Eng. 2022, 10, 107407. [Google Scholar] [CrossRef]
- Nie, J.; Huang, H.; Rao, P.; Chen, H.; Du, X.; Wang, Z.; Zhang, W.; Liang, H. Composite functional particle enhanced gravity driven ceramic membrane bioreactor for simultaneous removal of nitrogen and phosphorus from groundwater. Chem. Eng. J. 2023, 452, 139134. [Google Scholar] [CrossRef]
- Chen, M.; Nan, J.; Ji, X.; Wu, F.; Ye, X.; Ge, Z. Effect of adsorption and coagulation pretreatment sequence on ultrafiltration membrane fouling: Process study and targeted prediction. Desalination 2022, 540, 115967. [Google Scholar] [CrossRef]
- Mansha, M.; Salhi, B.; Ali, S.; Khan, S.A.; Baig, N. Novel procaine-based gemini zwitterion incorporated PVDF membranes for efficient treatment of oily wastewater. J. Environ. Chem. Eng. 2022, 10, 107935. [Google Scholar] [CrossRef]
- Xiao, F.; Zhang, H.; Wu, T.; Liu, J.; Liu, J.; Zhang, J.; Liu, W.; Liang, T.; Hu, J. Superhydrophobic/superlipophilic interface layer for oil-water separation. Process Saf. Environ. Prot. 2022, 161, 13–21. [Google Scholar] [CrossRef]
- Samal, S.; Kosjakova, O. Surface feature of PMMA films on NiTi alloy substrate by the spin coating method. Ceram. Int. 2022. [Google Scholar] [CrossRef]
- Song, D.; Liu, K.; Zuo, T.; Jia, J.; Wang, N.; Che, Q. Constructing foldable and stretchable proton exchange membranes through spin coating technology. J. Mol. Liq. 2022, 360, 119421. [Google Scholar] [CrossRef]
- Kim, K.J.; Chae, Y.; An, S.J.; Jo, J.H.; Park, S.; Chi, W.S. Microphase-separated morphology controlled polyimide graft copolymer membranes for CO2 separation. Sep. Purif. Technol. 2023, 304, 122315. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Meng, M.; Lv, P.; Yan, M.; Wei, X.; Li, H.; Yan, Y.; Li, C. Bio-inspired adhesion: Fabrication of molecularly imprinted nanocomposite membranes by developing a hybrid organic–inorganic nanoparticles composite structure. J. Membr. Sci. 2015, 490, 169–178. [Google Scholar] [CrossRef]
- Ronte, A.; Dangwal, S.; Lin, H.; Wagle, P.; Echeverria, E.; Lee, J.S.; Zhu, J.; McIlroy, D.N.; Kim, S.-J. Modification of ZIF-8 membranes by atomic layer deposition for high propylene/propane selectivity. Microporous Mesoporous Mater. 2022, 343, 112173. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, B.; Fu, Z.; Li, J.; Yu, M.; Li, L.; Li, J. Poly (vinyl alcohol) modification of poly(vinylidene fluoride) microfiltration membranes for oil/water emulsion separation via an unconventional radiation method. J. Membr. Sci. 2021, 619, 118792. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, Y.; Tong, Y.; Ding, W.; Li, W. Plant-inspired biomimetic hybrid PVDF membrane co-deposited by tea polyphenols and 3-amino-propyl-triethoxysilane for high-efficiency oil-in-water emulsion separation. Chin. J. Chem. Eng. 2022, 53, 170–180. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q. Oxidant-induced plant phenol surface chemistry for multifunctional coatings: Mechanism and potential applications. J. Membr. Sci. 2019, 570–571, 176–183. [Google Scholar] [CrossRef]
- Li, H.; Wu, Y.; Huang, H.; Zhang, B.; Liang, Y.; Chen, Y.; Wang, T. Surface synthesis of a polyethylene glutaraldehyde coating for improving the oil removal from wastewater of microfiltration carbon membranes. J. Water Process Eng. 2022, 47, 102724. [Google Scholar] [CrossRef]
- Dong, Y.; Li, J.; Pedersen-Bjergaard, S.; Huang, C. Unidirectional solute transfer using a Janus membrane. J. Membr. Sci. 2020, 596, 117723. [Google Scholar] [CrossRef]
- Khayet, M.; García-Payo, C.; Matsuura, T. Superhydrophobic nanofibers electrospun by surface segregating fluorinated amphiphilic additive for membrane distillation. J. Membr. Sci. 2019, 588, 117215. [Google Scholar] [CrossRef]
- Fang, S.; Zhang, Z.; Yang, H.; Wang, G.; Gu, L.; Xia, L.; Zeng, Z.; Zhu, L. Mussel-inspired hydrophilic modification of polypropylene membrane for oil-in-water emulsion separation. Surf. Coat. Technol. 2020, 403, 126375. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, Y.; Wu, L.; Deng, X.; Zhao, Z.; Zhang, C. Preparation of refreshable membrane by partially sacrificial hydrophilic coating. Polym. Biopolym. 2021, 56, 10676–10690. [Google Scholar] [CrossRef]
- Xu, Q.; Ji, X.; Tian, J.; Jin, X.; Wu, L. Inner Surface Hydrophilic Modification of PVDF Membrane with Tea Polyphenols/Silica Composite Coating. Polymers 2021, 13, 4186. [Google Scholar] [CrossRef]
- Qiu, W.-Z.; Yang, H.-C.; Wan, L.-S.; Xu, Z.-K. Co-deposition of catechol/polyethyleneimine on porous membranes for efficient decolorization of dye water. J. Mater. Chem. A 2015, 3, 14438–14444. [Google Scholar] [CrossRef]
- Zeng, X.; Lin, J.; Cai, W.; Lu, Q.; Fu, S.; Li, J.; Yan, X.; Wen, X.; Zhou, C.; Zhang, M. Fabrication of superhydrophilic PVDF membranes by one-step modification with eco-friendly phytic acid and polyethyleneimine complex for oil-in-water emulsions separation. Chemosphere 2021, 264, 128395. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, S.; He, F.; Cao, M.; Peng, S.; Li, Y. One-step transformation of highly hydrophobic membranes into superhydrophilic and underwater superoleophobic ones for high-efficiency separation of oil-in-water emulsions. J. Mater. Chem. A 2018, 6, 3391–3396. [Google Scholar] [CrossRef]
- Sun, H.; Yang, X.; Zhang, Y.; Cheng, X.; Xu, Y.; Bai, Y.; Shao, L. Segregation-induced in situ hydrophilic modification of poly (vinylidene fluoride) ultrafiltration membranes via sticky poly (ethylene glycol) blending. J. Membr. Sci. 2018, 563, 22–30. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, G.; Wang, K.; Wan, Q.; Tao, L.; Zhang, X.; Wei, Y. Recent developments in polydopamine: An emerging soft matter for surface modification and biomedical applications. Nanoscale 2016, 8, 16819–16840. [Google Scholar] [CrossRef]
- Balkenende, D.W.R.; Winkler, S.M.; Messersmith, P.B. Marine-inspired polymers in medical adhesion. Eur. Polym. J. 2019, 116, 134–143. [Google Scholar] [CrossRef]
- Wang, Z.; Han, M.; Zhang, J.; He, F.; Peng, S.; Li, Y. Investigating and significantly improving the stability of tannic acid (TA)-aminopropyltriethoxysilane (APTES) coating for enhanced oil-water separation. J. Membr. Sci. 2020, 593, 134–143. [Google Scholar] [CrossRef]
- Luo, C.; Liu, Q. Oxidant-Induced High-Efficient Mussel-Inspired Modification on PVDF Membrane with Superhydrophilicity and Underwater Superoleophobicity Characteristics for Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 8297–8307. [Google Scholar] [CrossRef]
- Qiu, W.Z.; Wu, G.P.; Xu, Z.K. Robust Coatings via Catechol-Amine Codeposition: Mechanism, Kinetics, and Application. ACS Appl Mater Interfaces 2018, 10, 5902–5908. [Google Scholar] [CrossRef]
- Chen, C.; Weng, D.; Mahmood, A.; Chen, S.; Wang, J. Separation Mechanism and Construction of Surfaces with Special Wettability for Oil/Water Separation. ACS Appl. Mater. Interfaces 2019, 304, 122340. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Chen, Z.; Yang, H.; Hou, K.; Zeng, X.; Zheng, Y.; Cheng, J. Nature-Inspired Strategy toward Superhydrophobic Fabrics for Versatile Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 9184–9194. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Z.; Li, T.; Ye, J.; Shen, L.; She, Z.; Liu, F. Catalytic PVDF membrane for continuous reduction and separation of p-nitrophenol and methylene blue in emulsified oil solution. Chem. Eng. J. 2018, 334, 579–586. [Google Scholar] [CrossRef]
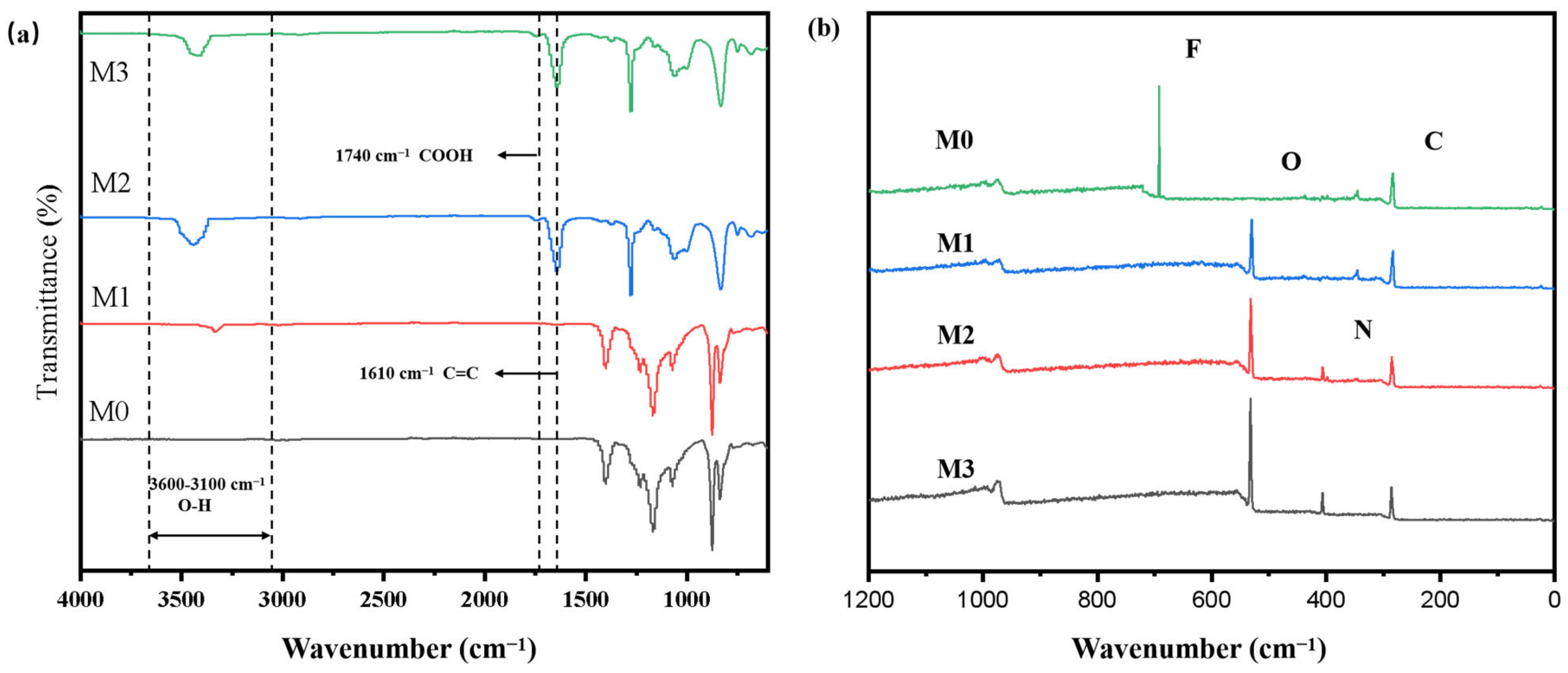
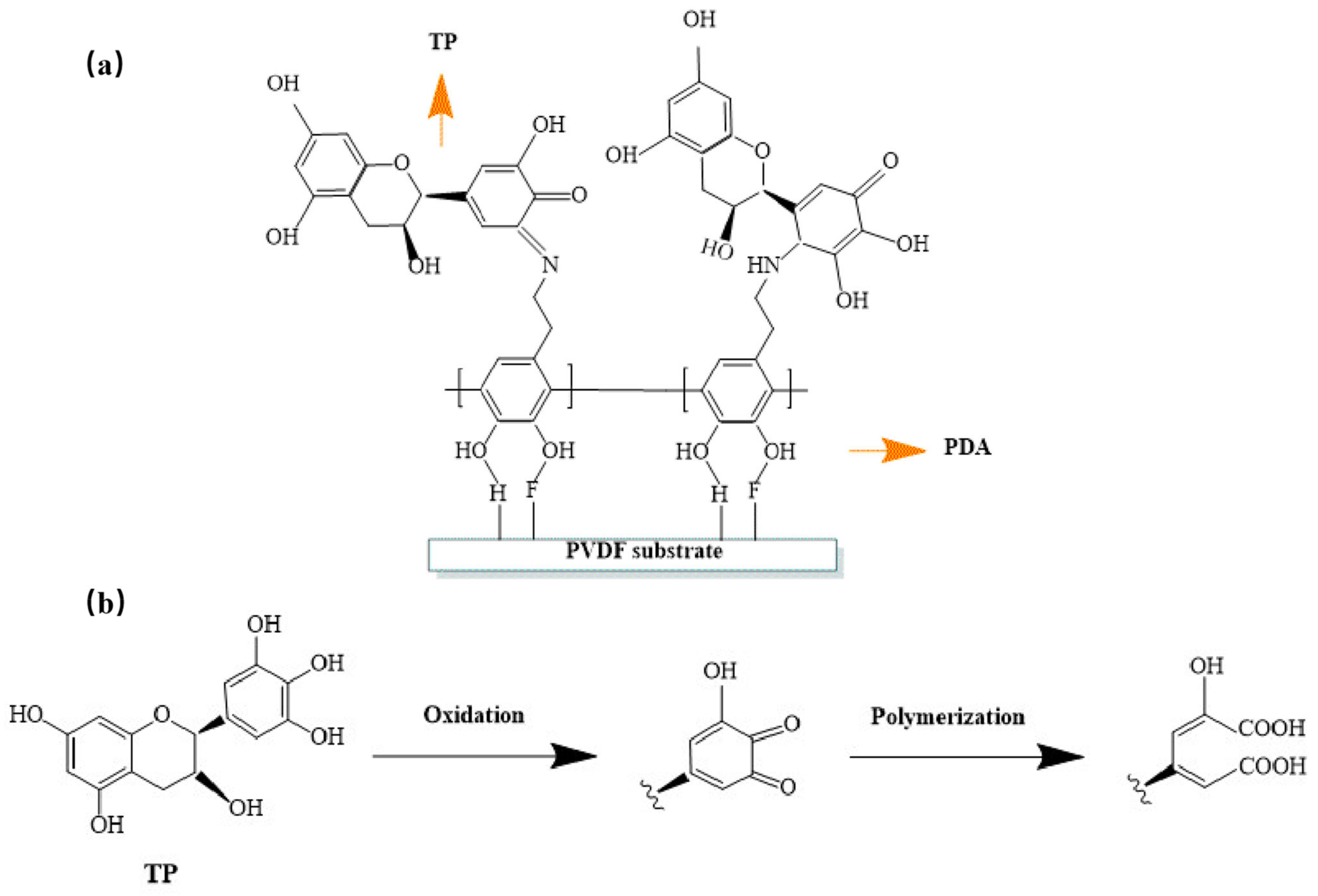
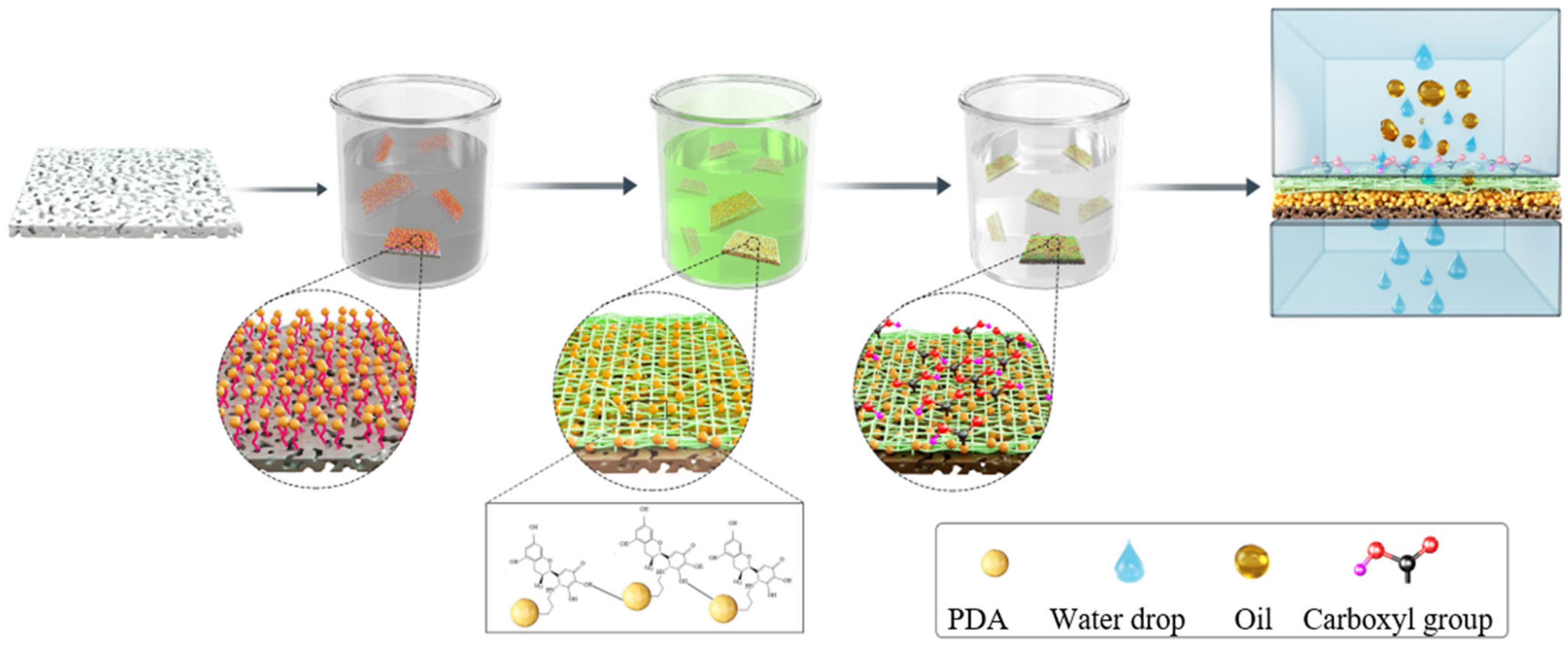
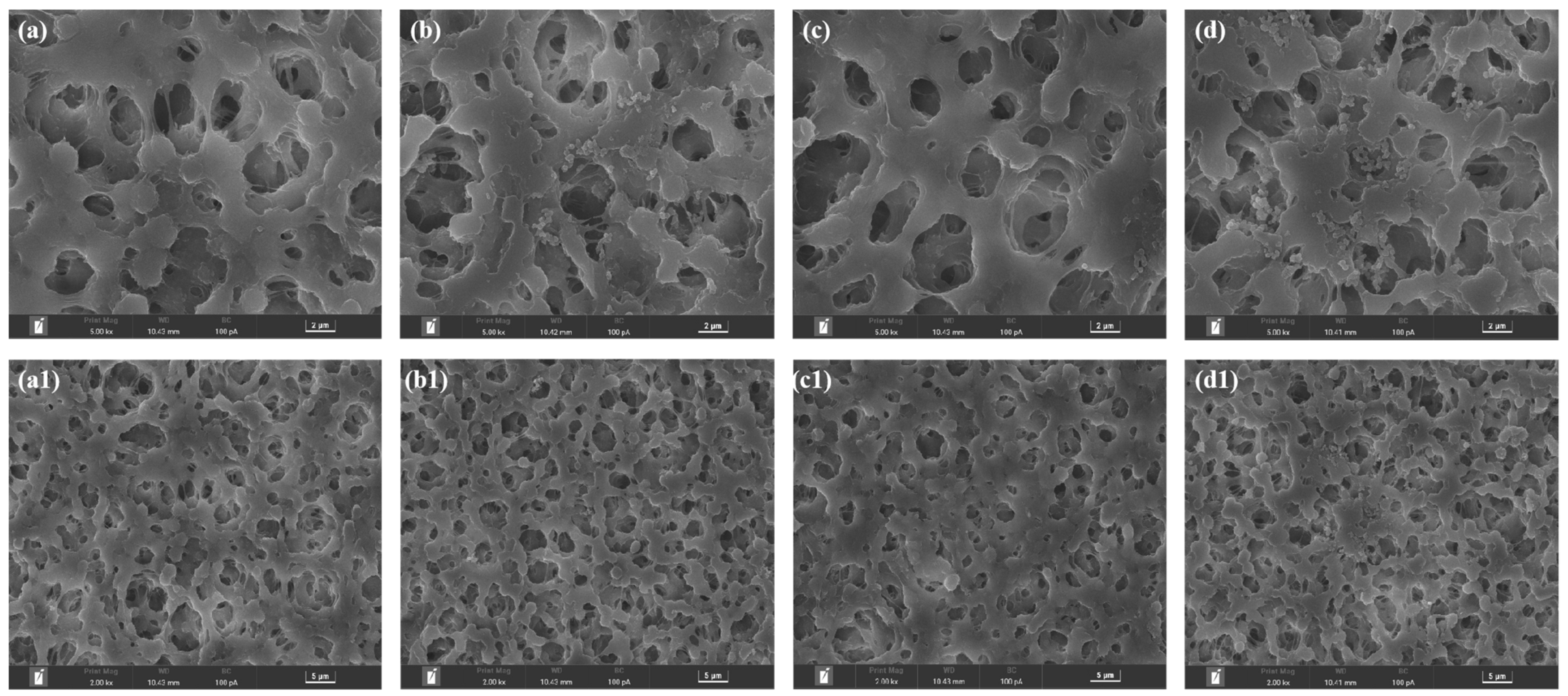


| Sample | Modification Method | Transmembrane Pressure | Reaction Time | Literature |
|---|---|---|---|---|
| TA/APTES | Dip coating | 0.1 MPa | 24 h | [45] |
| CA/SP | Dip coating | 0.075 MPa | 2 h | [32] |
| TP/APTES | Dip coating | 0.01 MPa | 12 h | [31] |
| PDA/SP | Dip coating | 0.038 MPa | 2 h | [46] |
| CA/MPD | Dip coating | 0.6 MPa | 3 h | [47] |
| PDA/TP/NaIO4 | Dip coating | 0.01 MPa | 0.25 h | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Mo, Y.; Gao, Y.; Zhou, Z.; Hou, X.; Ren, X.; Wang, J.; Chu, X.; Lu, Y. Constructing a Hierarchical Hydrophilic Crosslink Network on the Surface of a Polyvinylidene Fluoride Membrane for Efficient Oil/Water Emulsion Separation. Membranes 2023, 13, 255. https://doi.org/10.3390/membranes13030255
Zhang R, Mo Y, Gao Y, Zhou Z, Hou X, Ren X, Wang J, Chu X, Lu Y. Constructing a Hierarchical Hydrophilic Crosslink Network on the Surface of a Polyvinylidene Fluoride Membrane for Efficient Oil/Water Emulsion Separation. Membranes. 2023; 13(3):255. https://doi.org/10.3390/membranes13030255
Chicago/Turabian StyleZhang, Ruixian, Yuanbin Mo, Yanfei Gao, Zeguang Zhou, Xueyi Hou, Xiuxiu Ren, Junzhong Wang, Xiaokun Chu, and Yanyue Lu. 2023. "Constructing a Hierarchical Hydrophilic Crosslink Network on the Surface of a Polyvinylidene Fluoride Membrane for Efficient Oil/Water Emulsion Separation" Membranes 13, no. 3: 255. https://doi.org/10.3390/membranes13030255
APA StyleZhang, R., Mo, Y., Gao, Y., Zhou, Z., Hou, X., Ren, X., Wang, J., Chu, X., & Lu, Y. (2023). Constructing a Hierarchical Hydrophilic Crosslink Network on the Surface of a Polyvinylidene Fluoride Membrane for Efficient Oil/Water Emulsion Separation. Membranes, 13(3), 255. https://doi.org/10.3390/membranes13030255







