Electrodialysis Deacidification of Acid Hydrolysate in Hemicellulose Saccharification Process: Membrane Fouling Identification and Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
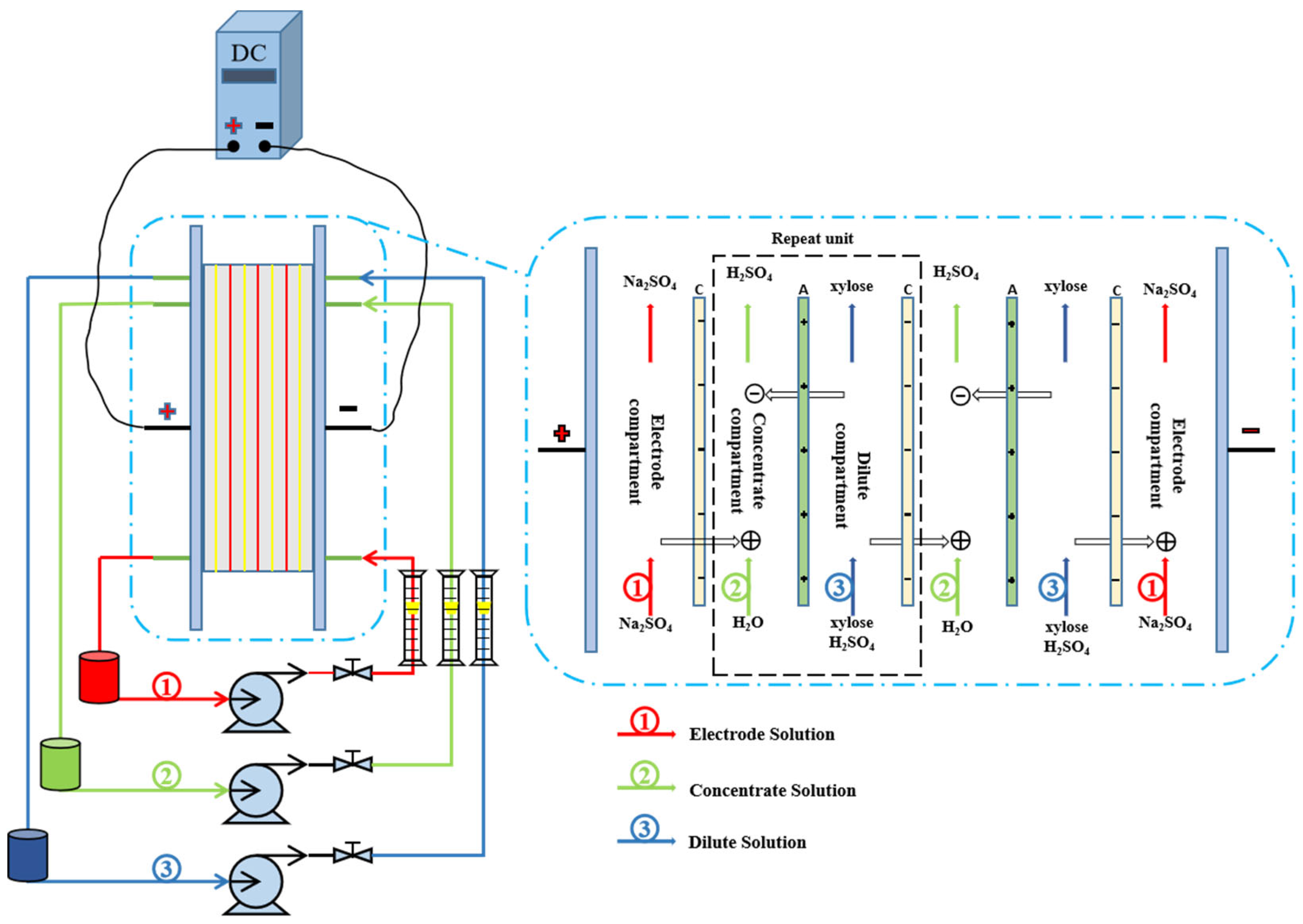
2.2. Electrochemical Deacidification Apparatus and Protocol
2.3. Process Evaluation
2.3.1. Global System Resistance
2.3.2. Deacidification Ratio
2.3.3. H+ Relative Energy Consumption
2.4. Analysis Methods
2.4.1. Quantitative Analysis of Monosaccharides and Phenolic Acids
2.4.2. pH Value and Conductivity of CAH
2.4.3. Quantitative Analysis of Protein in CAH
2.4.4. Zeta Potential of Colloidal Particles in CAH
2.4.5. Electron Microscopy and Elemental Analysis
2.4.6. Determination of Molecular Weight
2.4.7. FTIR (Fourier Transform Infrared Spectrometer)
2.4.8. HSQC (Heteronuclear Single Quantum Coherence)
3. Results and Discussion
3.1. Evaluation of ED Parameters
3.1.1. Global System Resistance
3.1.2. Deacidification Ratio
3.1.3. H+ Relative Energy Consumption
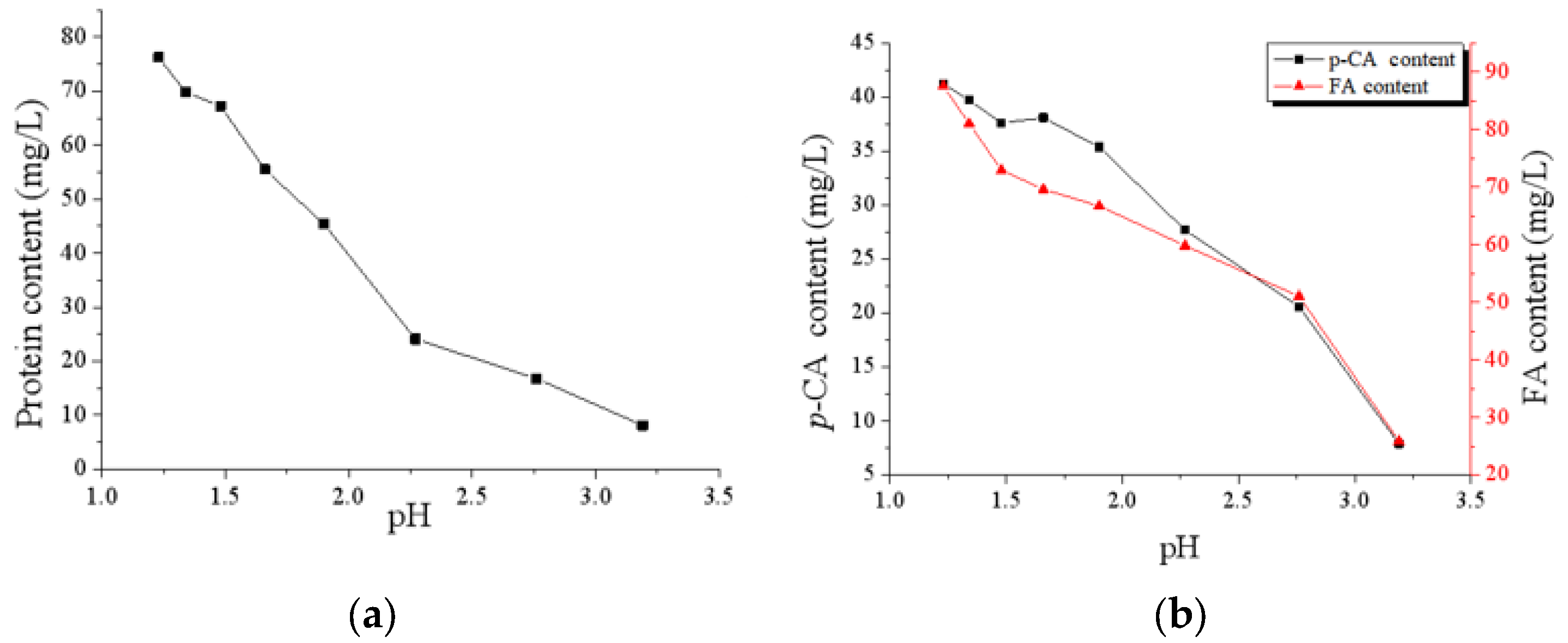
3.2. Analysis of CAH Component
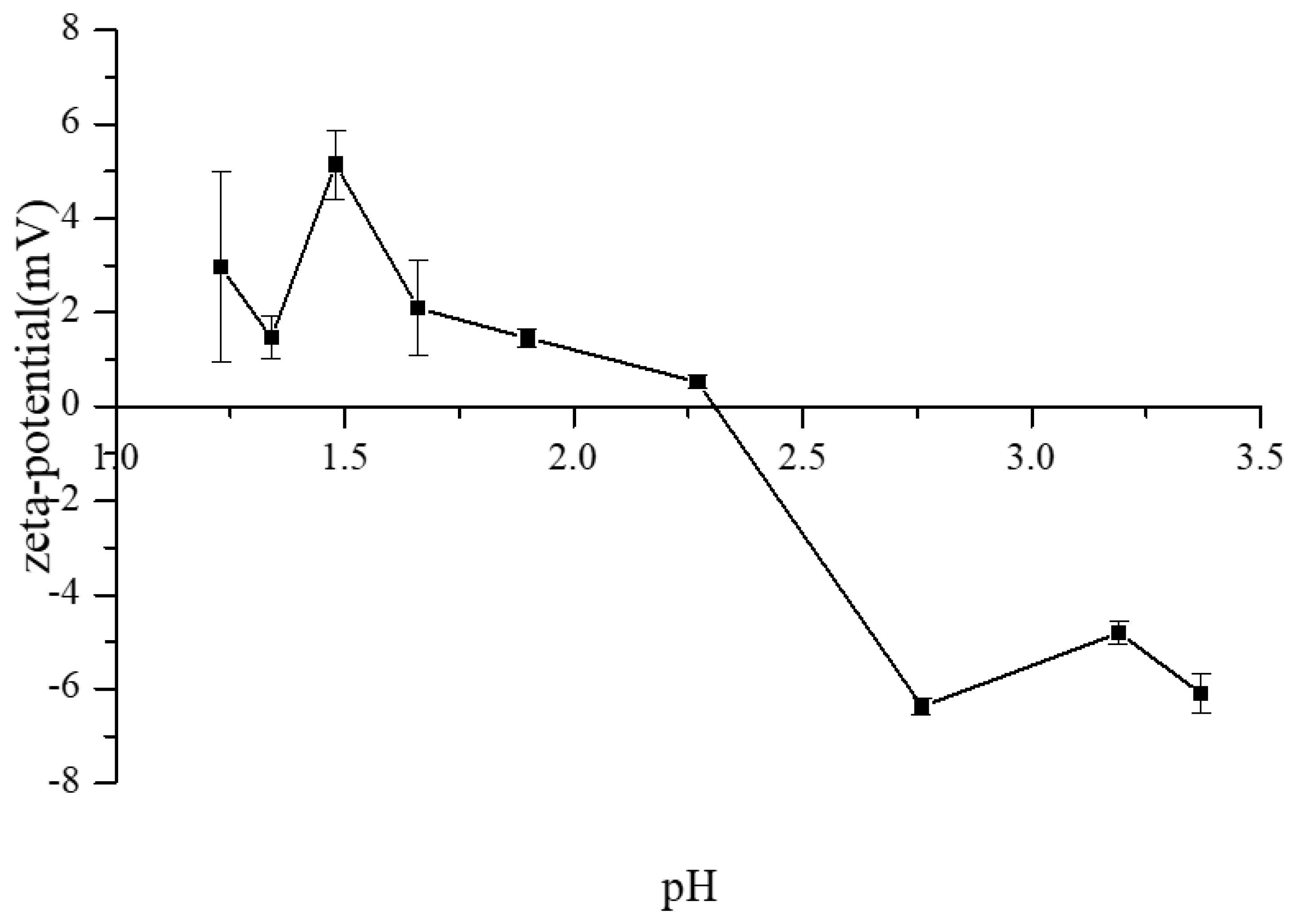
3.3. The ζ-Potential Variations of CAH
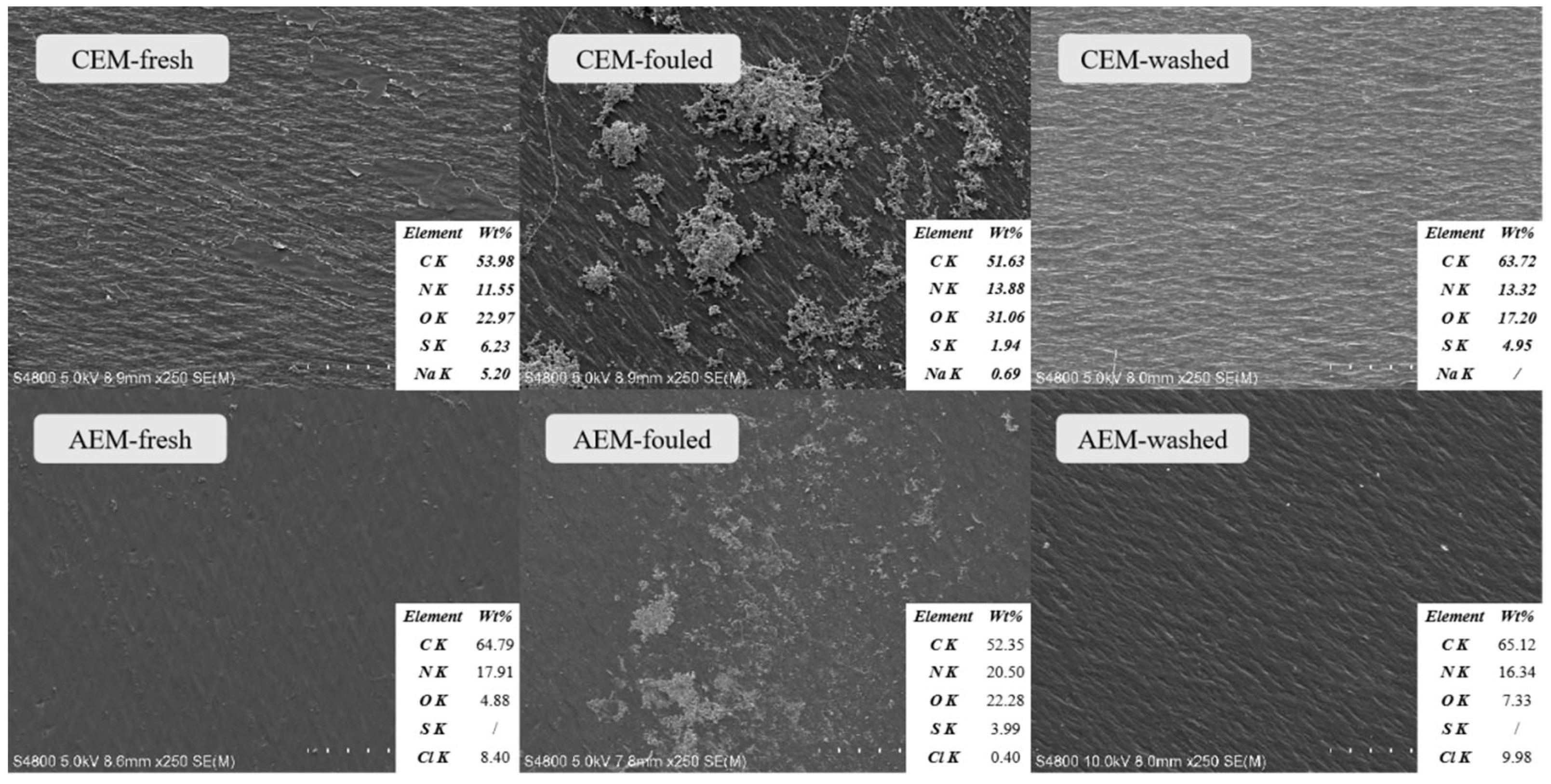
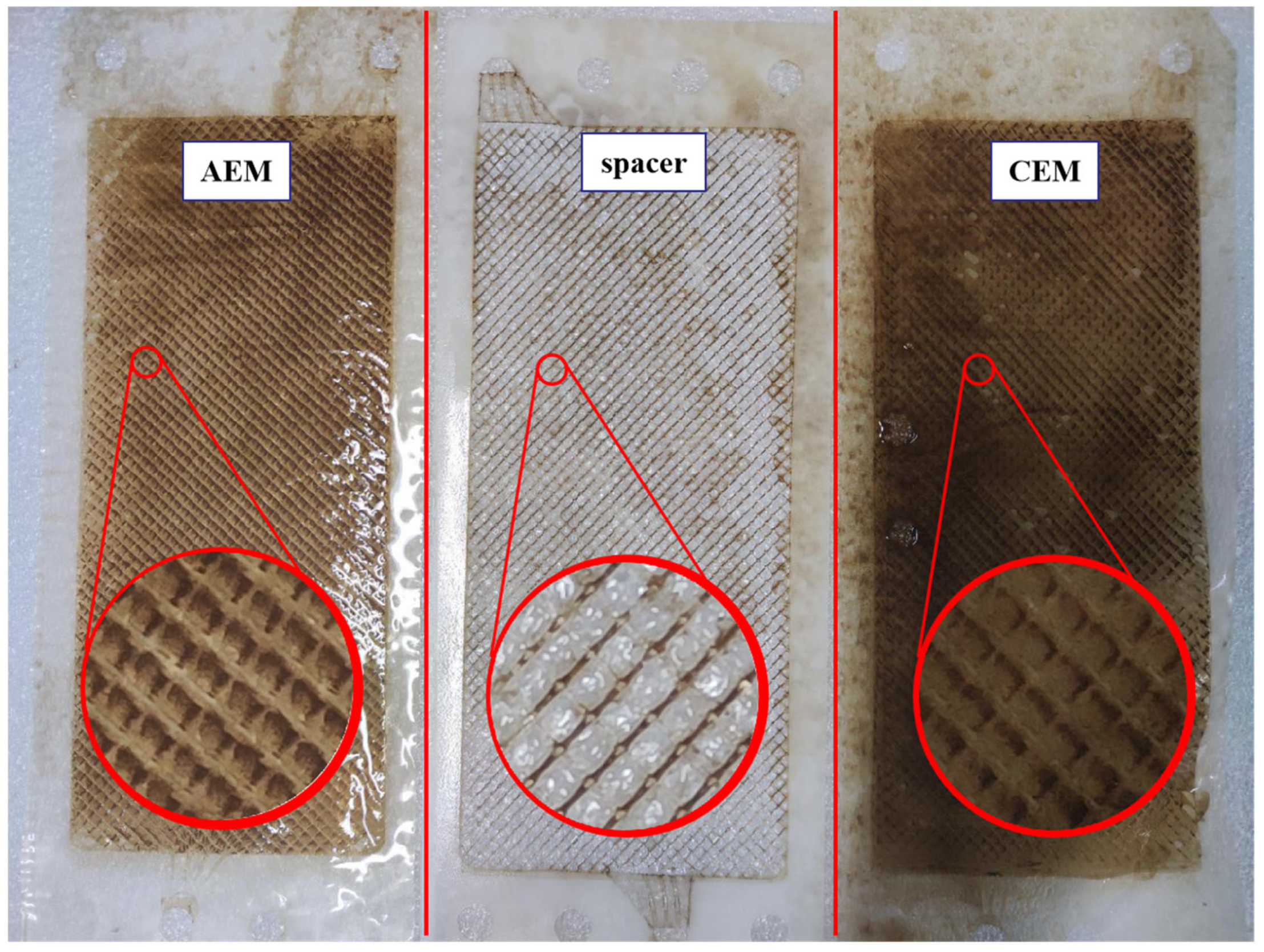
3.4. Membrane Surface Analysis for Fouling Identification
3.5. Analysis of Membrane Foulant
3.6. Proposed Fouling Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 709–719. [Google Scholar] [CrossRef]
- Shen, X.J.; Sun, R.C. Recent advances in lignocellulose prior-fractionation for biomaterials, biochemicals, and bioenergy. Carbohydr. Polym. 2021, 261, 117884. [Google Scholar] [CrossRef]
- Maki-Arvela, P.; Salmi, T.; Holmbom, B.; Willfor, S.; Murzin, D.Y. Synthesis of Sugars by Hydrolysis of Hemicelluloses—A Review. Chem. Rev. 2011, 111, 5638–5666. [Google Scholar] [CrossRef]
- Davison, B.H.; Drescher, S.R.; Tuskan, G.A.; Davis, M.F.; Nghiem, N.P. Variation of S/G ratio and lignin content in a Populus family influences the release of xylose by dilute acid hydrolysis. Appl. Biochem. Biotechnol. 2006, 130, 427–435. [Google Scholar] [CrossRef]
- Lavarack, B.P.; Griffin, G.J.; Rodman, D. The acid hydrolysis of sugarcane bagasse hemicellulose to produce xylose, arabinose, glucose and other products. Biomass Bioenergy 2002, 23, 367–380. [Google Scholar] [CrossRef]
- Chen, X.Q.; Yang, Q.L.; Si, C.L.; Wang, Z.J.; Huo, D.; Hong, Y.M.; Li, Z.Q. Recovery of Oligosaccharides from Prehydrolysis Liquors of Poplar by Microfiltration/Ultrafiltration Membranes and Anion Exchange Resin. ACS Sustain. Chem. Eng. 2016, 4, 937–943. [Google Scholar] [CrossRef]
- Wang, X.J.; Zhuang, J.S.; Fu, Y.J.; Tian, G.Y.; Wang, Z.J.; Qin, M.H. Separation of hemicellulose-derived saccharides from wood hydrolysate by lime and ion exchange resin. Bioresour. Technol. 2016, 206, 225–230. [Google Scholar] [CrossRef]
- Wang, X.J.; Zhuang, J.S.; Jiang, J.G.; Fu, Y.J.; Qin, M.H.; Wang, Z.J. Separation and purification of hemicellulose-derived saccharides from wood hydrolysate by combined process. Bioresour. Technol. 2015, 196, 426–430. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wang, X.J.; Fu, Y.J.; Li, Z.Q.; Zhang, F.S.; Qin, M.H. Colloidal behaviors of lignin contaminants: Destabilization and elimination for oligosaccharides separation from wood hydrolysate. Sep. Purif. Technol. 2015, 145, 1–7. [Google Scholar] [CrossRef]
- Waheed, H.; Farrukh, S.; Hussain, A.; Mukhtar, A.; Mubashir, M.; Saqib, S.; Ullah, S.; Peter, A.P.; Khoo, K.S.; Show, P.L. Green synthesized nano-cellulose polyethylene imine-based biological membrane. Food Chem. Toxicol. 2022, 160, 112773. [Google Scholar] [CrossRef]
- Jamil, A.; Ching, O.P.; Iqbal, T.; Rafiq, S.; Zia-ul-Haq, M.; Shahid, M.Z.; Mubashir, M.; Manickam, S.; Show, P.L. Development of an extended model for the permeation of environmentally hazardous CO2 gas across asymmetric hollow fiber composite membranes. J. Hazard. Mater. 2021, 417, 126000. [Google Scholar] [CrossRef] [PubMed]
- Blanc, C.-L.; Lemaire, J.; Duval, F.; Théoleyre, M.-A.; Pareau, D. Purification of pentoses from hemicellulosic hydrolysates without neutralization for sulfuric acid recovery. Sep. Purif. Technol. 2017, 174, 513–519. [Google Scholar] [CrossRef]
- Lemaire, J.; Blanc, C.-L.; Duval, F.; Théoleyre, M.-A.; Pareau, D. Purification of pentoses from hemicellulosic hydrolysates with sulfuric acid recovery by using electrodialysis. Sep. Purif. Technol. 2016, 166, 181–186. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Bdiri, M.; Sarapulova, V.; Kozmai, A.; Fouilloux, J.; Baklouti, L.; Larchet, C.; Renard, E.; Dammak, L. A Review on Ion-Exchange Membranes Fouling during Electrodialysis Process in Food Industry, Part 2: Influence on Transport Properties and Electrochemical Characteristics, Cleaning and Its Consequences. Membranes 2021, 11, 811. [Google Scholar] [CrossRef]
- Dammak, L.; Fouilloux, J.; Bdiri, M.; Larchet, C.; Renard, E.; Baklouti, L.; Sarapulova, V.; Kozmai, A.; Pismenskaya, N. A Review on Ion-Exchange Membrane Fouling during the Electrodialysis Process in the Food Industry, Part 1: Types, Effects, Characterization Methods, Fouling Mechanisms and Interactions. Membranes 2021, 11, 789. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Bazinet, L. Fouling on ion-exchange membranes: Classification, characterization and strategies of prevention and control. Adv. Colloid. Interfac. 2016, 229, 34–56. [Google Scholar] [CrossRef]
- Haddad, M.; Bazinet, L.; Savadogo, O.; Paris, J. Electrochemical acidification of Kraft black liquor: Impacts of pulsed electric field application on bipolar membrane colloidal fouling and process. J. Membr. Sci. 2017, 524, 482–492. [Google Scholar] [CrossRef]
- Haddad, M.; Mikhaylin, S.; Bazinet, L.; Savadogo, O.; Paris, J. Electrochemical acidification of Kraft black liquor by electrodialysis with bipolar membrane: Ion exchange membrane fouling identification and mechanisms. J. Colloid Interface Sci. 2017, 488, 39–47. [Google Scholar] [CrossRef]
- Xu, T.; Huang, C. Electrodialysis-based separation technologies: A critical review. AIChE J. 2008, 54, 3147–3159. [Google Scholar] [CrossRef]
- Casademont, C.; Farias, M.A.; Pourcelly, G.; Bazinet, L. Impact of electrodialytic parameters on cation migration kinetics and fouling nature of ion-exchange membranes during treatment of solutions with different magnesium/calcium ratios. J. Membr. Sci. 2008, 325, 570–579. [Google Scholar] [CrossRef]
- Casademont, C.; Sistat, P.; Ruiz, B.; Pourcelly, G.; Bazinet, L. Electrodialysis of model salt solution containing whey proteins: Enhancement by pulsed electric field and modified cell configuration. J. Membr. Sci. 2009, 328, 238–245. [Google Scholar] [CrossRef]
- Lee, H.J.; Moon, S.H. Fouling mitigation in the repeated batch runs of electrodialysis with humate foulant. Korean J. Chem. Eng. 2004, 21, 629–634. [Google Scholar] [CrossRef]
- Afifah, D.N.; Ariyanto, T.; Supranto, S.; Prasetyo, I. Separation of Lithium Ion from Lithium-Cobalt Mixture using Electrodialysis Monovalent Membrane. Eng. J. 2018, 22, 165–179. [Google Scholar] [CrossRef]
- Gregor, H.P.; Peterson, M.A. Electrodialytic Polarization of Ion-Exchange Membrane Systems. J. Phys. Chem. 1964, 68, 2201–2205. [Google Scholar] [CrossRef]
- Parsa, N.; Moheb, A.; Mehrabani-Zeinabad, A.; Masigol, M.A. Recovery of lithium ions from sodium-contaminated lithium bromide solution by using electrodialysis process. Chem. Eng. Res. Des. 2015, 98, 81–88. [Google Scholar] [CrossRef]
- Suwal, S.; Doyen, A.; Bazinet, L. Characterization of protein, peptide and amino acid fouling on ion-exchange and filtration membranes: Review of current and recently developed methods. J. Membr. Sci. 2015, 496, 267–283. [Google Scholar] [CrossRef]
- Lee, H.-J.; Hong, M.-K.; Han, S.-D.; Cho, S.-H.; Moon, S.-H. Fouling of an anion exchange membrane in the electrodialysis desalination process in the presence of organic foulants. Desalination 2009, 238, 60–69. [Google Scholar] [CrossRef]
- Norgren, M.; Edlund, H.; Wågberg, L.; Lindström, B.; Annergren, G. Aggregation of kraft lignin derivatives under conditions relevant to the process, part I: Phase behaviour. Colloids Surf. A 2001, 194, 85–96. [Google Scholar] [CrossRef]
- Derkacheva, O.; Sukhov, D. Investigation of Lignins by FTIR Spectroscopy. Macromol. Symp. 2008, 265, 61–68. [Google Scholar] [CrossRef]
- Moghaddam, L.; Rencoret, J.; Maliger, V.R.; Rackemann, D.W.; Harrison, M.D.; Gutiérrez, A.; del Río, J.C.; Doherty, W.O.S. Structural Characteristics of Bagasse Furfural Residue and Its Lignin Component. An NMR, Py-GC/MS, and FTIR Study. ACS Sustain. Chem. Eng. 2017, 5, 4846–4855. [Google Scholar] [CrossRef]
- De Ninno, A.; Castellano, A.C. Deprotonation of Glutamic Acid Induced by Weak Magnetic Field: An FTIR-ATR Study. Bioelectromagnetics 2011, 32, 218–225. [Google Scholar] [CrossRef]
- Mnich, E.; Bjarnholt, N.; Eudes, A.; Harholt, J.; Holland, C.; Jørgensen, B.; Larsen, F.H.; Liu, M.; Manat, R.; Meyer, A.S.; et al. Phenolic cross-links: Building and de-constructing the plant cell wall. Nat. Prod. Rep. 2020, 37, 919–961. [Google Scholar] [CrossRef] [PubMed]
- Hood, C.; Laredo, T.; Marangoni, A.G.; Pensini, E. Water-repellent films from corn protein and tomato cutin. J. Appl. Polym. Sci. 2021, 138, 50831. [Google Scholar] [CrossRef]
- Al-Rudainy, B.; Galbe, M.; Wallberg, O. Influence of prefiltration on membrane performance during isolation of lignin-carbohydrate complexes from spent sulfite liquor. Sep. Purif. Technol. 2017, 187, 380–388. [Google Scholar] [CrossRef]
- Yuan, T.-Q.; Sun, S.-N.; Xu, F.; Sun, R.-C. Characterization of Lignin Structures and Lignin–Carbohydrate Complex (LCC) Linkages by Quantitative 13C and 2D HSQC NMR Spectroscopy. J. Agric. Food. Chem. 2011, 59, 10604–10614. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.-L.; Xue, B.-L.; Xu, F.; Sun, R.-C.; Pinkert, A. Unmasking the structural features and property of lignin from bamboo. Ind. Crops Prod. 2013, 42, 332–343. [Google Scholar] [CrossRef]
- Balakshin, M.; Capanema, E.; Gracz, H.; Chang, H.-M.; Jameel, H. Quantification of lignin–carbohydrate linkages with high-resolution NMR spectroscopy. Planta 2011, 233, 1097–1110. [Google Scholar] [CrossRef]
- Norgren, M.; Edlund, H.; Wågberg, L. Aggregation of Lignin Derivatives under Alkaline Conditions. Kinetics and Aggregate Structure. Langmuir 2002, 18, 2859–2865. [Google Scholar] [CrossRef]



| Item | Value | Unit |
|---|---|---|
| Brix | 7.8 | % |
| pH | 1.1 | – |
| Conductivity | 17.24 | mS/cm |
| OD420 | 2.2 | – |
| Glucose | 3.57 | g/L |
| Xylose | 47.53 | |
| Arabinose | 4.26 | |
| Sodium | 355.0 | mg/L |
| Potassium | 473.8 | |
| Magnesium | 39.4 | |
| Calcium | 98.5 | |
| Chlorure | 159.4 | |
| Sulfate | 3935.7 |
| Membrane Type | AEM | CEM |
|---|---|---|
| Membrane code | TWEDA1 | TWEDC1 |
| Thickness/wet (µm) | 40–50 | 40–50 |
| IEC a (ion exchange capacity, mmol/g) | 0.90–1.10 | 0.90–1.10 |
| Area resistance b (Ω·cm2) | ≤2.5 | ≤3.3 |
| Water uptake c (%) | 15–20 | 15–20 |
| Transport number d | ≥0.98 | ≥0.97 |
| Operational Conditions | Data |
|---|---|
| Number of operating units | 4 |
| Re-circulation flow rate | 0.7 L/min |
| Applied current density | 64.33 A/m2 |
| Effective membrane surface area | 0.0187 m2 |
| Initial volume of each solution | 2 L |
| Operational temperature | 25 °C |
| Initial Na2SO4 concentration | 0.1 mol/L |
| Initial pure water | ≥18.2 MΩ·cm |
| Characteristics | Before ED | After ED |
|---|---|---|
| pH | 1.24 ± 0.01 | 3.29 ± 0.06 |
| Conductivity(mS/cm) | 18.04 ± 0.46 | 1.05 ± 0.04 |
| Protein (mg/L) * | 76.26 | 8.09 |
| FA (mg/L) * | 87.52 | 25.92 |
| p-CA (mg/L) * | 41.24 | 7.91 |
| Maxima at cm−1, Absorbance | Chemical Group | Band Assignment |
|---|---|---|
| 835 | S units | C–H out of plane at positions 2 and 6 of in etherified syringyl units (S units) |
| 1032 | Glucosidic bonds | C–O–C stretching |
| 1170 | C=O in ester groups of lignin units | S ring+G ring condensed (G ring substituted at position 5) C–C, C–O, C=O stretching; G condensed>G etherfied; aromatic C–H in-plane deformation; typical for G units; primary OH |
| 1227 | C-C, C-O, and C=O bonds | S ring + G ring condensed (G ring substituted at position 5) C–C, C–O, C=O stretch; G condensed>G etherfied; aromatic C–H in-plane deformation; typical for G units; primary OH |
| 1270 | C=O bond | C=O stretching |
| 1365 | Aliphatic C–H in CH3 not in OCH3; phenolic OH | aliphatic C–H stretching in CH3 not in OCH3; phenolic O–H stretching |
| 1430 | Aromatic skeletal | aromatic skeletal vibrations combined with C–H in-plane deformation |
| 1463 | Aromatic skeletal | C–H deformation |
| 1515 | Aromatic skeletal | aromatic skeletal vibrations G>S |
| 1604 | Aromatic skeletal | vibration of aromatic skeletal; C6-point double bond O stretch; S>G, G condensed>G etherified |
| 1655 | Amide I peak | α-helix |
| 1701 | Ester group | C=O stretching |
| 2840 | Methylene group | C–H stretching |
| 2938 | Methyl group |
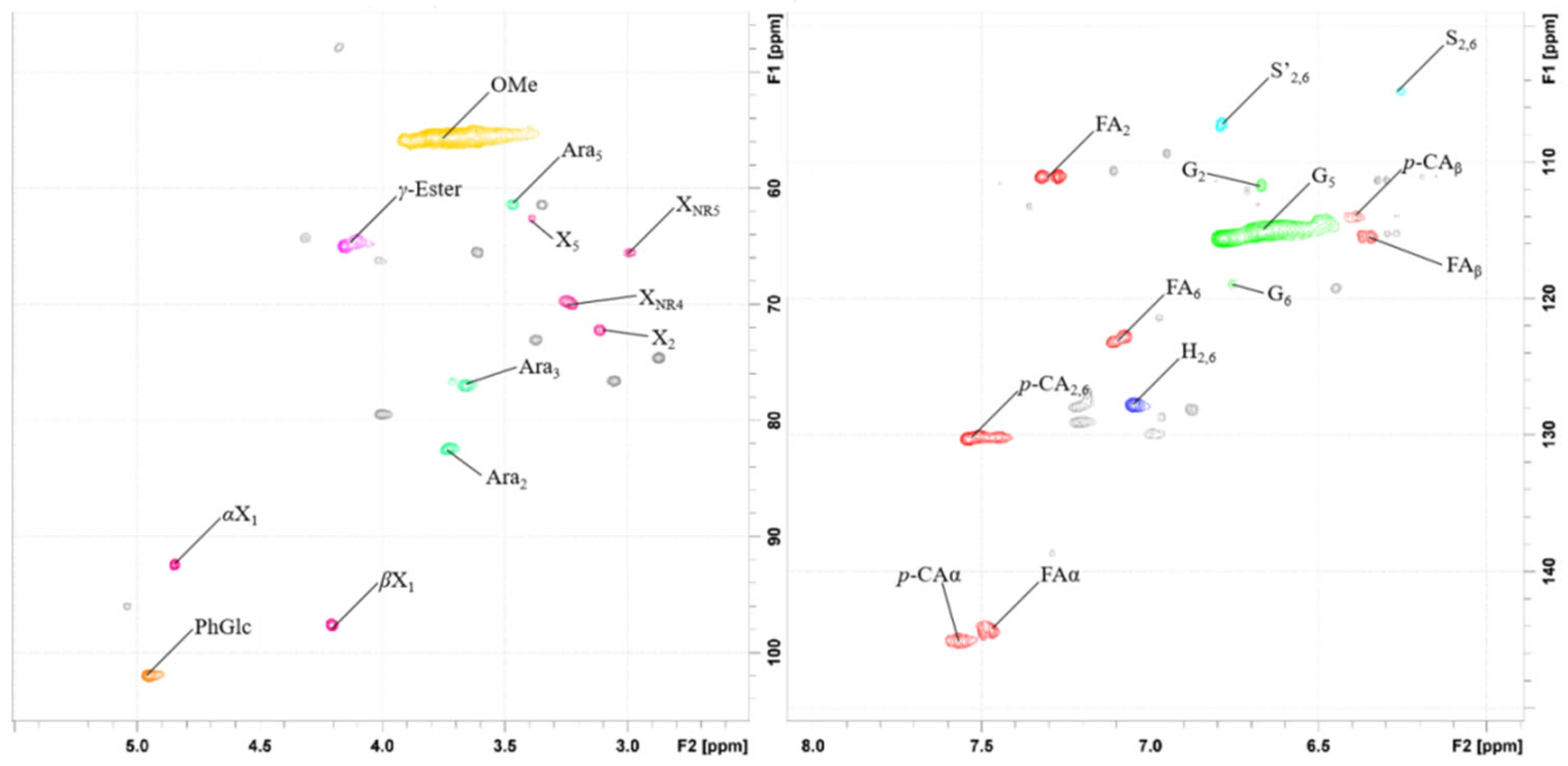
| Labels | δC/δH | Assignment |
|---|---|---|
| -OCH3 | 55.7/3.74 | C–H in methoxyls |
| γ-Ester | 65.1/4.16 | γ-Ester linkages in LCC |
| Ara3 | 77.1/3.66 | C3–H3 in α-(1→ 3)-L-arabinofuranoside |
| Dβ | 79.6/4.01 | Cβ’-Hβ’ in spirodienone substructures(D) |
| Ara2 | 82.4/3.73 | C2–H2 in α-(1 → 3)-L-arabinofuranoside |
| PhGlc | 102.1/4.96 | Phenyl glycoside linkages in LCC |
| S2, 6 | 104.9/6.26 | C2, 6-H2,6 in etherified syringyl units (S) |
| S’2, 6 | 107.2/6.79 | C2, 6-H2,6 in etherified syringyl units (S’) |
| FA2 | 111.2/7.33 | C2–H2 in ferulate (FA) |
| G2 | 111.9/6.68 | C2–H2 in guaiacyl units (G) |
| p-CAβ | 113.8/6.29 | Cβ–Hβ in p-coumarate (p-CA) |
| G5 | 115.1/6.67 | C5–H5 in guaiacyl units (G) |
| G6 | 119.0/6.68 | C6–H6 in guaiacyl units (G) |
| FA6 | 123.2/7.12 | C6–H6 in ferulate (FA) |
| H2,6 | 127.9/7.05 | C2, 6-H2,6 in p-hydroxyphenyl units (H) |
| p-CA2, 6 | 130.3/7.54 | C2, 6-H2,6 in p-hydroxyphenyl units (H) |
| p-CAa, FAa | 145.1/7.57 | Ca-Ha in p-coumarate (p-CA) and ferulate (FA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Sun, L.; Shou, Q.; Liang, X.; Liu, H. Electrodialysis Deacidification of Acid Hydrolysate in Hemicellulose Saccharification Process: Membrane Fouling Identification and Mechanisms. Membranes 2023, 13, 256. https://doi.org/10.3390/membranes13030256
Luo X, Sun L, Shou Q, Liang X, Liu H. Electrodialysis Deacidification of Acid Hydrolysate in Hemicellulose Saccharification Process: Membrane Fouling Identification and Mechanisms. Membranes. 2023; 13(3):256. https://doi.org/10.3390/membranes13030256
Chicago/Turabian StyleLuo, Xitao, Lingling Sun, Qinghui Shou, Xiangfeng Liang, and Huizhou Liu. 2023. "Electrodialysis Deacidification of Acid Hydrolysate in Hemicellulose Saccharification Process: Membrane Fouling Identification and Mechanisms" Membranes 13, no. 3: 256. https://doi.org/10.3390/membranes13030256
APA StyleLuo, X., Sun, L., Shou, Q., Liang, X., & Liu, H. (2023). Electrodialysis Deacidification of Acid Hydrolysate in Hemicellulose Saccharification Process: Membrane Fouling Identification and Mechanisms. Membranes, 13(3), 256. https://doi.org/10.3390/membranes13030256





