Directly Using Ti3C2Tx MXene for a Solid-Contact Potentiometric pH Sensor toward Wearable Sweat pH Monitoring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Apparatus
2.2. Preparation of Ti3C2Tx
2.3. Fabrication of Ti3C2Tx-Based pH Electrodes
2.4. Fabrication of Flexible pH Sensor
2.5. Fabrication of Solid Ag/AgCl Reference Electrode
2.6. Electrochemical Measurement Methods
3. Results
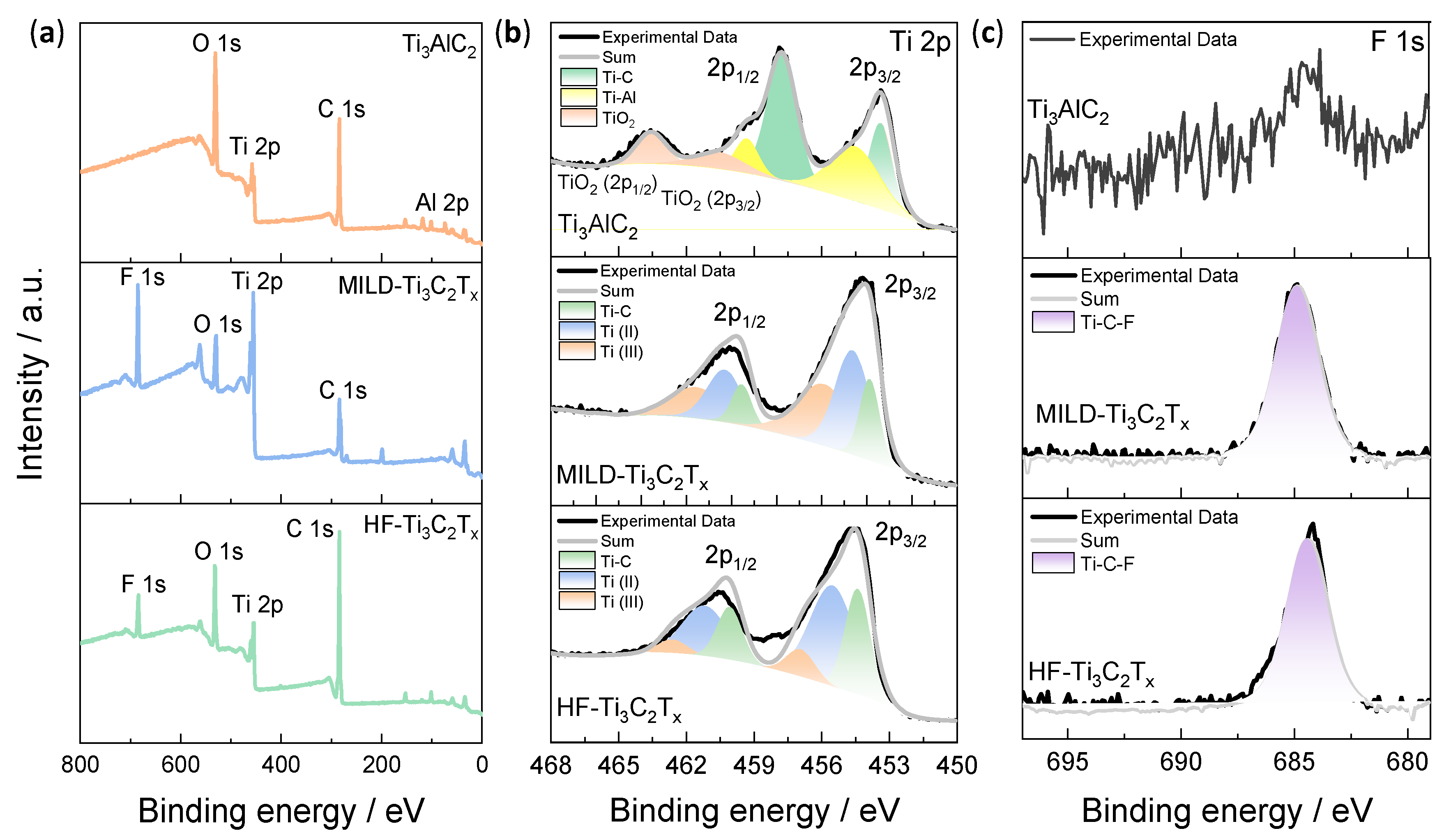
3.1. Structures and Compositions of Ti3C2Tx
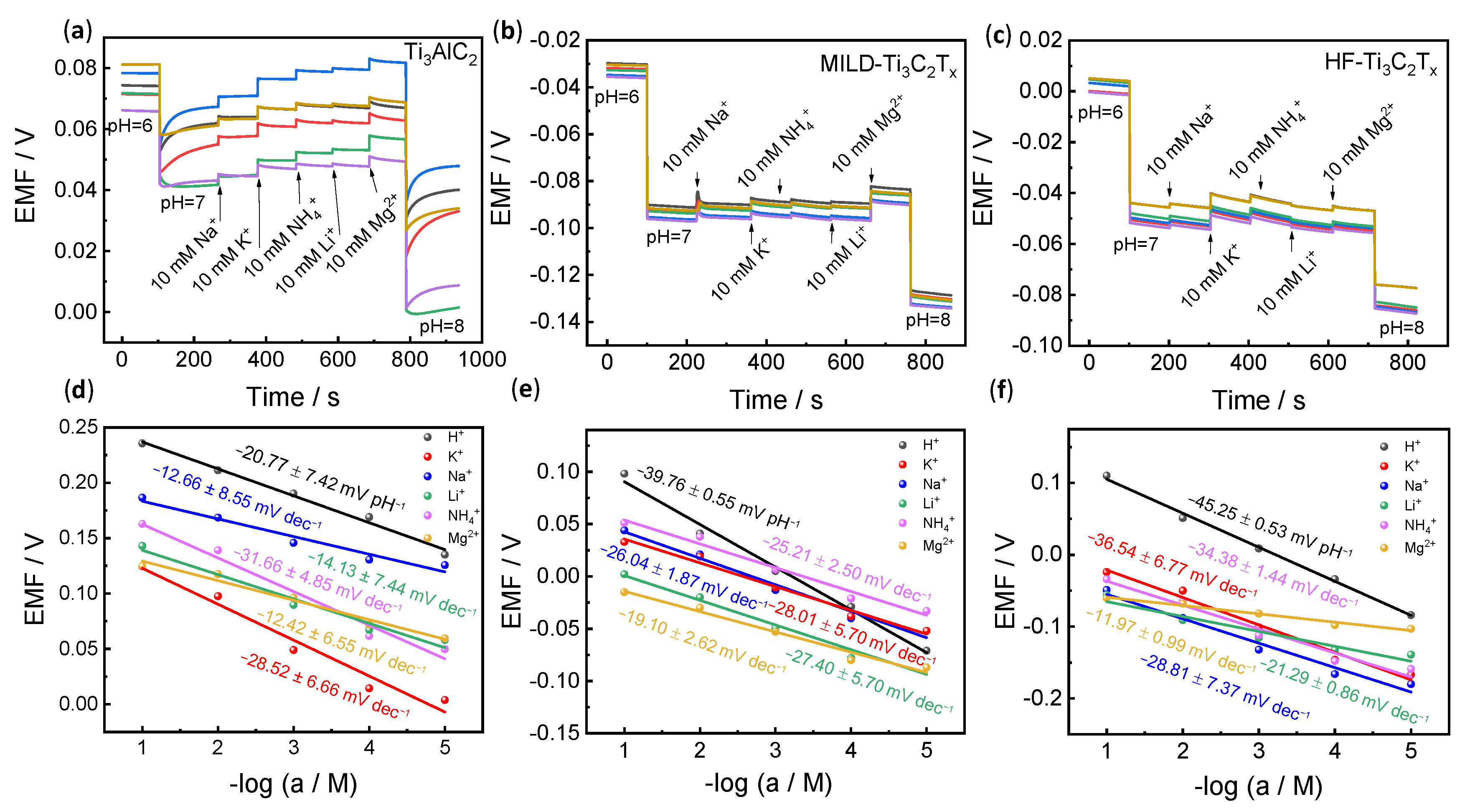
3.2. Potentiometric pH Response
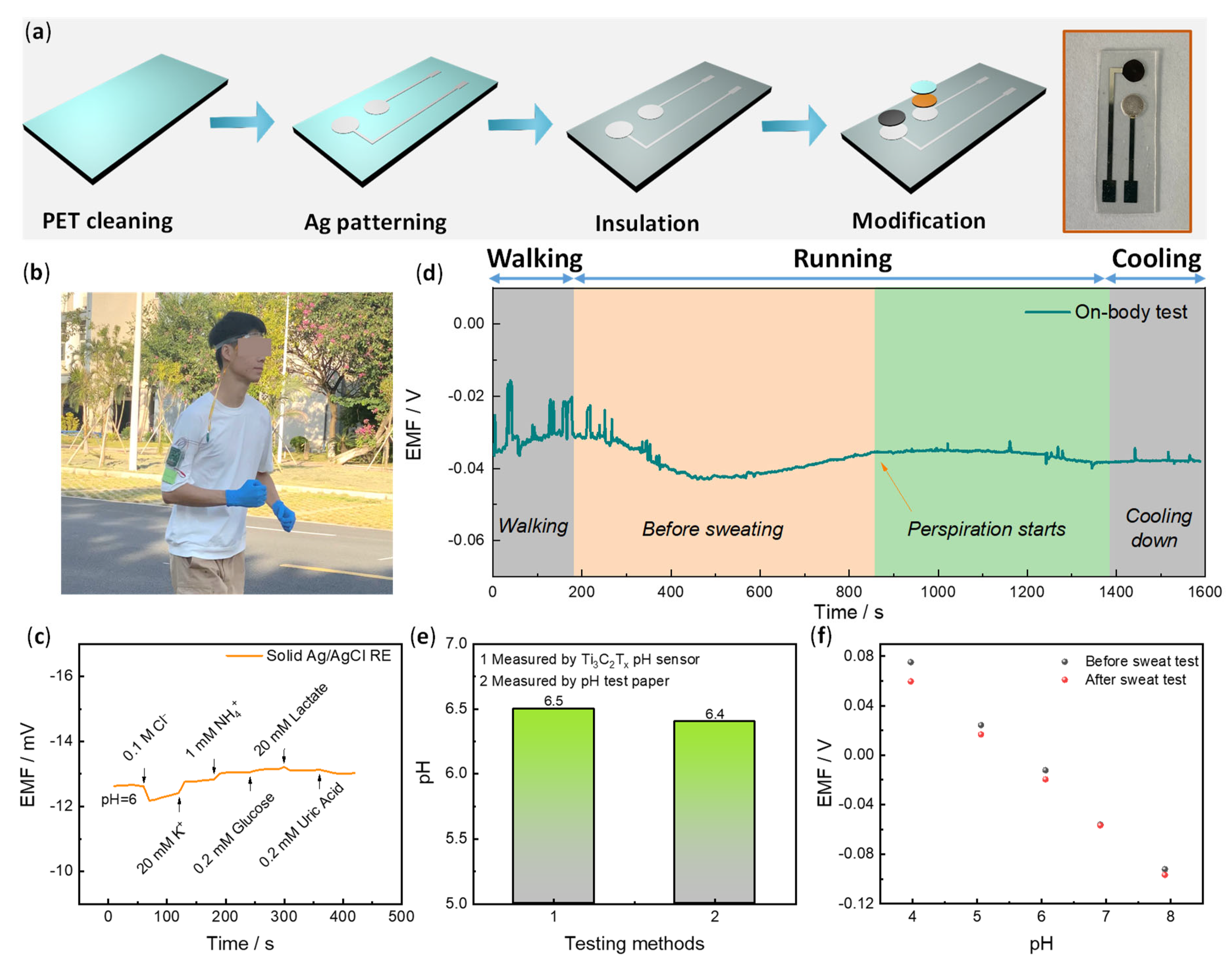
3.3. Flexible pH Sensor and On-Body Sweat Monitoring
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, A.V.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, eabf158. [Google Scholar]
- Tang, Y.; Yang, C.; Xu, X.; Kang, Y.; Henzie, J.; Que, W.; Yamauchi, Y. MXene Nanoarchitectonics: Defect-Engineered 2D MXenes towards Enhanced Electrochemical Water Splitting. Adv. Energy Mater. 2022, 12, 2103867. [Google Scholar] [CrossRef]
- You, Z.; Liao, Y.; Li, X.; Fan, J.; Xiang, Q. State-of-the-art recent progress in MXene-based photocatalysts: A comprehensive review. Nanoscale 2021, 13, 9463–9504. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, S.; Li, Y.; Fan, J.; Lv, K. MXenes as noble-metal-alternative co-catalysts in photocatalysis. Chin. J. Catal. 2021, 42, 3–14. [Google Scholar] [CrossRef]
- Li, X.; Huang, Z.; Shuck, C.E.; Liang, G.; Gogotsi, Y.; Zhi, C. MXene chemistry, electrochemistry and energy storage applications. Nat. Rev. Chem. 2022, 6, 389–404. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Ab Latif, F.E.; Numan, A.; Mubarak, N.M.; Khalid, M.; Abdullah, E.C.; Manaf, N.A.; Walvekar, R. Evolution of MXene and its 2D heterostructure in electrochemical sensor applications. Coord. Chem. Rev. 2022, 471, 214755. [Google Scholar] [CrossRef]
- Echols, I.J.; An, H.; Zhao, X.; Prehn, E.M.; Tan, Z.; Radovic, M.; Green, M.J.; Lutkenhaus, J.L. pH-Response of polycation/Ti3C2Tx MXene layer-by-layer assemblies for use as resistive sensors. Mol. Syst. Des. Eng. 2020, 5, 366–375. [Google Scholar] [CrossRef]
- Ghoneim, M.T.; Nguyen, A.; Dereje, N.; Huang, J.; Moore, G.C.; Murzynowski, P.J.; Dagdeviren, C. Recent Progress in Electrochemical pH-Sensing Materials and Configurations for Biomedical Applications. Chem. Rev. 2019, 119, 5248–5297. [Google Scholar] [CrossRef]
- Yin, L.; Cao, M.; Kim, K.N.; Lin, M.; Moon, J.-M.; Sempionatto, J.R.; Yu, J.; Liu, R.; Wicker, C.; Trifonov, A.; et al. A stretchable epidermal sweat sensing platform with an integrated printed battery and electrochromic display. Nat. Electron. 2022, 5, 694–705. [Google Scholar] [CrossRef]
- Ghaffari, R.; Yang, D.S.; Kim, J.; Mansour, A.; Wright, J.A., Jr.; Model, J.B.; Wright, D.E.; Rogers, J.A.; Ray, T.R. State of Sweat: Emerging Wearable Systems for Real-Time, Noninvasive Sweat Sensing and Analytics. ACS Sens. 2021, 6, 2787–2801. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.M.V.; Rajendran, V.; Mishra, R.K.; Jayaraman, M. Recent advances and perspectives in sweat based wearable electrochemical sensors. TrAC-Trends Anal. Chem. 2020, 131, 116024. [Google Scholar] [CrossRef]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Zdrachek, E.; Bakker, E. Potentiometric Sensing. Anal. Chem. 2021, 93, 72–102. [Google Scholar] [CrossRef]
- Shao, Y.; Ying, Y.; Ping, J. Recent advances in solid-contact ion-selective electrodes: Functional materials, transduction mechanisms, and development trends. Chem. Soc. Rev. 2020, 49, 4405–4465. [Google Scholar] [CrossRef]
- Ding, J.; Qin, W. Recent advances in potentiometric biosensors. TrAC-Trends Anal. Chem. 2020, 124, 115803. [Google Scholar] [CrossRef]
- Zdrachek, E.; Bakker, E. Potentiometric Sensing. Anal. Chem. 2019, 91, 2–26. [Google Scholar] [CrossRef] [Green Version]
- Bobacka, J. Conducting polymer-based solid-state ion-selective electrodes. Electroanalysis 2006, 18, 7–18. [Google Scholar] [CrossRef]
- Lyu, Y.; Gan, S.; Bao, Y.; Zhong, L.; Xu, J.; Wang, W.; Liu, Z.; Ma, Y.; Yang, G.; Niu, L. Solid-Contact Ion-Selective Electrodes: Response Mechanisms, Transducer Materials and Wearable Sensors. Membranes 2020, 10, 128. [Google Scholar] [CrossRef]
- Parrilla, M.; Cuartero, M.; Crespo, G.A. Wearable potentiometric ion sensors. TrAC-Trends Anal. Chem. 2019, 110, 303–320. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.; Wang, C.; Wang, H.; Jian, M.; Lu, W.; Liang, X.; Zhang, X.; Yang, F.; Zhang, Y. Integrated textile sensor patch for real-time and multiplex sweat analysis. Sci. Adv. 2019, 5, eaax0649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Zhong, L.; Wang, W.; He, Y.; Han, T.; Xu, L.; Mo, X.; Liu, Z.; Ma, Y.; Bao, Y.; et al. Recent Advances in Wearable Potentiometric pH Sensors. Membranes 2022, 12, 504. [Google Scholar] [CrossRef]
- An, Q.; Gan, S.; Xu, J.; Bao, Y.; Wu, T.; Kong, H.; Zhong, L.; Ma, Y.; Song, Z.; Niu, L. A multichannel electrochemical all-solid-state wearable potentiometric sensor for real-time sweat ion monitoring. Electrochem. Commun. 2019, 107, 106553. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Z.; Gan, S.; Gao, H.; Kong, H.; Song, Z.; Ge, X.; Bao, Y.; Niu, L. Highly Stretchable Fiber-Based Potentiometric Ion Sensors for Multichannel Real-Time Analysis of Human Sweat. ACS Sens. 2020, 5, 2834–2842. [Google Scholar] [CrossRef]
- Guinovart, T.; Valdés-Ramírez, G.; Windmiller, J.R.; Andrade, F.J.; Wang, J. Bandage-Based Wearable Potentiometric Sensor for Monitoring Wound pH. Electroanalysis 2014, 26, 1345–1353. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Gao, W.; Shahpar, Z.; Emaminejad, S.; Challa, S.; Chen, K.; Fahad, H.M.; Tai, L.-C.; Ota, H.; Davis, R.W.; et al. A Wearable Electrochemical Platform for Noninvasive Simultaneous Monitoring of Ca2+ and pH. ACS Nano 2016, 10, 7216–7224. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Q.; Yap, L.W.; Wang, R.; Gong, S.; Guo, Z.; Liu, Y.; Lyu, Q.; Wang, J.; Simon, G.P.; Cheng, W. Vertically Aligned Gold Nanowires as Stretchable and Wearable Epidermal Ion-Selective Electrode for Noninvasive Multiplexed Sweat Analysis. Anal. Chem. 2020, 92, 4647–4655. [Google Scholar] [CrossRef]
- Chen, L.; Chen, F.; Liu, G.; Lin, H.; Bao, Y.; Han, D.; Wang, W.; Ma, Y.; Zhang, B.; Niu, L. Superhydrophobic Functionalized Ti3C2Tx MXene-Based Skin-Attachable and Wearable Electrochemical pH Sensor for Real-Time Sweat Detection. Anal. Chem. 2022, 94, 7319–7328. [Google Scholar] [CrossRef]
- Ibarra, L.E.; Tarres, L.; Bongiovanni, S.; Barbero, C.A.; Kogan, M.J.; Rivarola, V.A.; Bertuzzi, M.L.; Yslas, E.I. Assessment of polyaniline nanoparticles toxicity and teratogenicity in aquatic environment using Rhinella arenarum model. Ecotoxicol. Environ. Saf. 2015, 114, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Manjakkal, L.; Szwagierczak, D.; Dahiya, R. Metal oxides based electrochemical pH sensors: Current progress and future perspectives. Prog. Mater. Sci. 2020, 109, 100635. [Google Scholar] [CrossRef]
- Liao, Y.-H.; Chou, J.-C. Preparation and characteristics of ruthenium dioxide for pH array sensors with real-time measurement system. Sens. Actuators B Chem. 2008, 128, 603–612. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, W.-D. Modification of vertically aligned carbon nanotubes with RuO2 for a solid-state pH sensor. Electrochim. Acta 2010, 55, 2859–2864. [Google Scholar] [CrossRef]
- Marzouk, S.A.M.; Ufer, S.; Buck, R.P.; Johnson, T.A.; Dunlap, L.A.; Cascio, W.E. Electrodeposited Iridium Oxide pH Electrode for Measurement of Extracellular Myocardial Acidosis during Acute Ischemia. Anal. Chem. 1998, 70, 5054–5061. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-D.; Cao, H.; Deb, S.; Chiao, M.; Chiao, J.C. A flexible pH sensor based on the iridium oxide sensing film. Sens. Actuators A Phys. 2011, 169, 1–11. [Google Scholar] [CrossRef]
- Prats-Alfonso, E.; Abad, L.; Casañ-Pastor, N.; Gonzalo-Ruiz, J.; Baldrich, E. Iridium oxide pH sensor for biomedical applications. Case urea–urease in real urine samples. Biosens. Bioelectron. 2013, 39, 163–169. [Google Scholar] [CrossRef]
- Zamora, M.L.; Dominguez, J.M.; Trujillo, R.M.; Goy, C.B.; Sánchez, M.A.; Madrid, R.E. Potentiometric textile-based pH sensor. Sens. Actuators B Chem. 2018, 260, 601–608. [Google Scholar] [CrossRef] [Green Version]
- Chiang, J.L.; Jan, S.S.; Chou, J.C.; Chen, Y.C. Study on the temperature effect, hysteresis and drift of pH-ISFET devices based on amorphous tungsten oxide. Sens. Actuators B Chem. 2001, 76, 624–628. [Google Scholar] [CrossRef]
- Zhang, W.-D.; Xu, B. A solid-state pH sensor based on WO3-modified vertically aligned multiwalled carbon nanotubes. Electrochem. Commun. 2009, 11, 1038–1041. [Google Scholar] [CrossRef]
- Choi, S.-J.; Savagatrup, S.; Kim, Y.; Lang, J.H.; Swager, T.M. Precision pH Sensor Based on WO3 Nanofiber-Polymer Composites and Differential Amplification. ACS Sens. 2019, 4, 2593–2598. [Google Scholar] [CrossRef]
- Tang, Y.; Gan, S.; Zhong, L.; Sun, Z.; Xu, L.; Liao, C.; Lin, K.; Cui, X.; He, D.; Ma, Y.; et al. Lattice Proton Intercalation to Regulate WO3-Based Solid-Contact Wearable pH Sensor for Sweat Analysis. Adv. Funct. Mater. 2022, 32, 2107653. [Google Scholar] [CrossRef]
- Shahzad, F.; Iqbal, A.; Kim, H.; Koo, C.M. 2D Transition Metal Carbides (MXenes): Applications as an Electrically Conducting Material. Adv Mater. 2020, 32, 2002159. [Google Scholar] [CrossRef] [PubMed]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2TX MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Li, J.; Qin, R.; Yan, L.; Chi, Z.; Yu, Z.; Li, N.; Hu, M.; Chen, H.; Shan, G. Plasmonic Light Illumination Creates a Channel to Achieve Fast Degradation of Ti3C2Tx Nanosheets. Inorg. Chem. 2019, 58, 7285–7294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, S.J.; Lai, L.T.; Tang, W.L. Improving the Performance of pH Sensors with One-Dimensional ZnO Nanostructures. IEEE Sens. J. 2019, 19, 10972–10976. [Google Scholar] [CrossRef]
- Sharifuzzaman, M.; Chhetry, A.; Zahed, M.A.; Yoon, S.H.; Park, C.I.; Zhang, S.; Chandra Barman, S.; Sharma, S.; Yoon, H.; Park, J.Y. Smart bandage with integrated multifunctional sensors based on MXene-functionalized porous graphene scaffold for chronic wound care management. Biosens. Bioelectron. 2020, 169, 112637. [Google Scholar] [CrossRef]
- Choi, M.-Y.; Lee, M.; Kim, J.-H.; Kim, S.; Choi, J.; So, J.-H.; Koo, H.-J. A fully textile-based skin pH sensor. J. Ind. Text. 2022, 51, 441S–457S. [Google Scholar] [CrossRef]


| Materials | Linear pH Range | Sensitivity (mV pH-1) | References |
|---|---|---|---|
| PANI/MXene | 1–11 | −41.91 | [30] |
| RuO2 | 1–13 | −49.8 to −59.1 | [33] |
| RuO2 | 2–12 | −55 | [34] |
| IrOx | 2–12 | −51.1 | [36] |
| IrO2 | 4–8 | −47.54 | [38] |
| WO3 | 1–7 | −44.85 | [39] |
| WO3/MWCNT | 2–12 | −41.0 | [40] |
| WO3 nanofiber | 3–11 | −38.5 | [41] |
| ZnO nanorods | 4–10 | −44.56 | [46] |
| PANI/LGG-MXene | 4–9 | −57.03 | [47] |
| PANI/CNT | 5–9 | −45.9 | [48] |
| MILD-Ti3C2Tx | 1–11 | −37.91 ± 0.63 | This work |
| HF-Ti3C2Tx | 1–11 | −43.51 ± 0.53 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, R.; Zhong, L.; Zhang, Y.; Tang, Y.; Lai, M.; Han, T.; Wang, W.; Bao, Y.; Ma, Y.; Gan, S.; et al. Directly Using Ti3C2Tx MXene for a Solid-Contact Potentiometric pH Sensor toward Wearable Sweat pH Monitoring. Membranes 2023, 13, 376. https://doi.org/10.3390/membranes13040376
Liang R, Zhong L, Zhang Y, Tang Y, Lai M, Han T, Wang W, Bao Y, Ma Y, Gan S, et al. Directly Using Ti3C2Tx MXene for a Solid-Contact Potentiometric pH Sensor toward Wearable Sweat pH Monitoring. Membranes. 2023; 13(4):376. https://doi.org/10.3390/membranes13040376
Chicago/Turabian StyleLiang, Rongfeng, Lijie Zhong, Yirong Zhang, Yitian Tang, Meixue Lai, Tingting Han, Wei Wang, Yu Bao, Yingming Ma, Shiyu Gan, and et al. 2023. "Directly Using Ti3C2Tx MXene for a Solid-Contact Potentiometric pH Sensor toward Wearable Sweat pH Monitoring" Membranes 13, no. 4: 376. https://doi.org/10.3390/membranes13040376
APA StyleLiang, R., Zhong, L., Zhang, Y., Tang, Y., Lai, M., Han, T., Wang, W., Bao, Y., Ma, Y., Gan, S., & Niu, L. (2023). Directly Using Ti3C2Tx MXene for a Solid-Contact Potentiometric pH Sensor toward Wearable Sweat pH Monitoring. Membranes, 13(4), 376. https://doi.org/10.3390/membranes13040376







