A Strategical Improvement in the Performance of CO2/N2 Gas Permeation via Conjugation of L-Tyrosine onto Chitosan Membrane
Abstract
:1. Introduction
2. Experimental Section
2.1. Requisite Materials
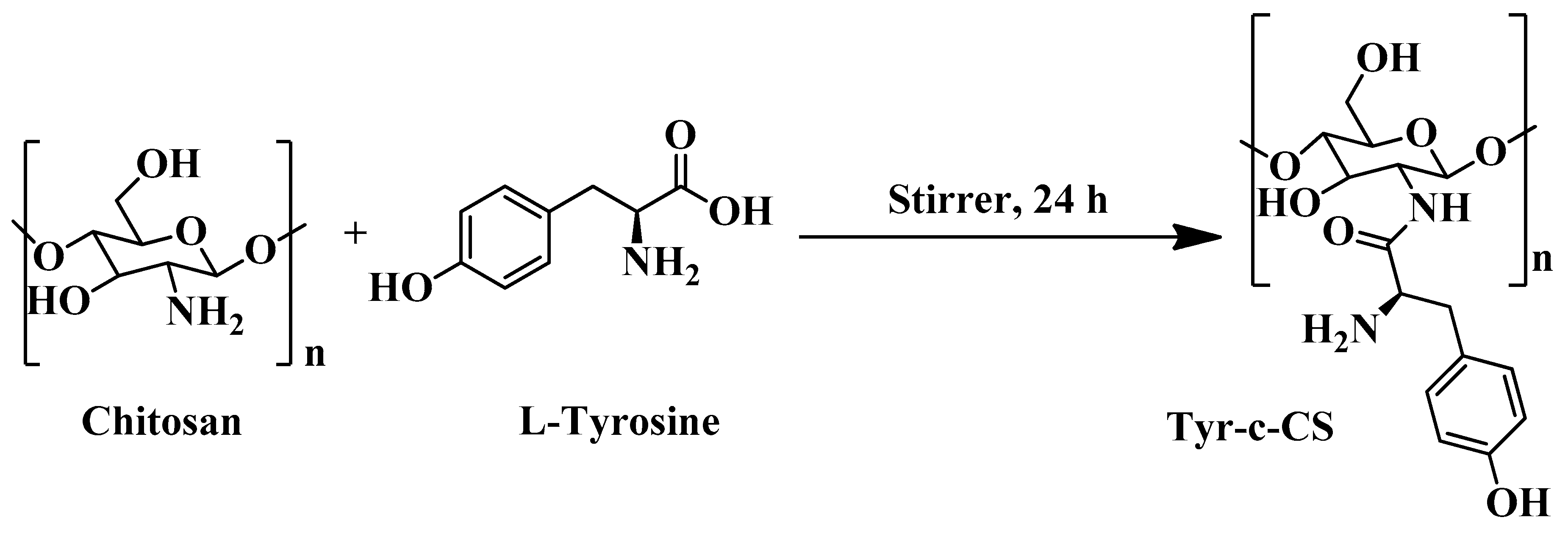
2.2. Synthesis of L-Tyrosine-Conjugated-Chitosan (Tyr-c-CS)
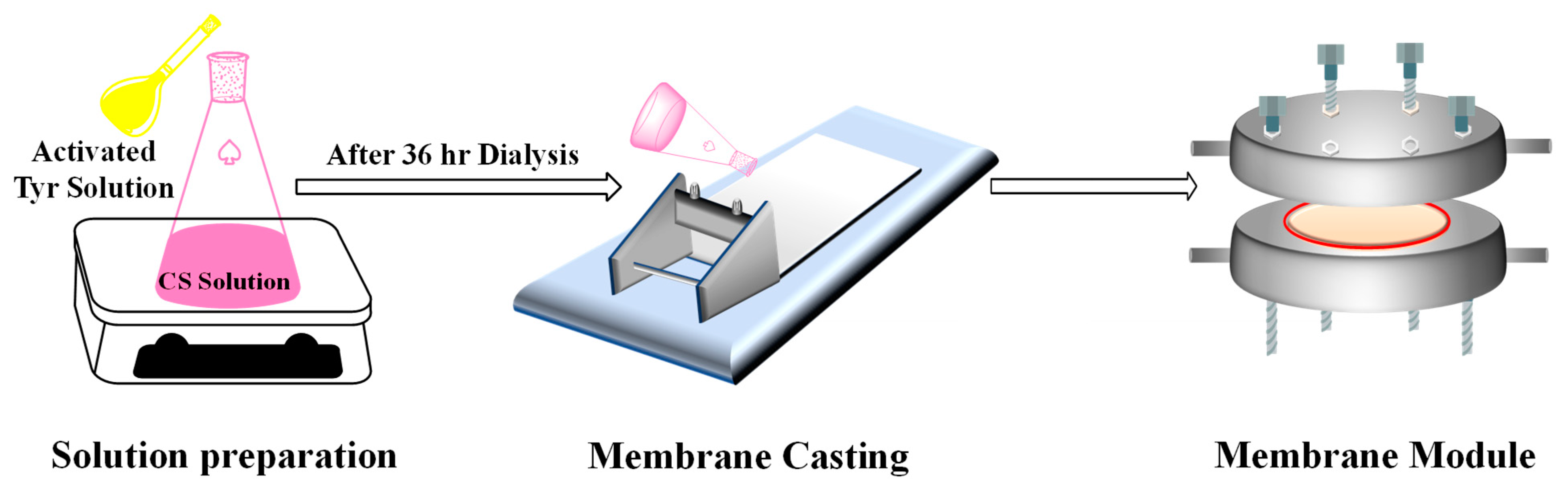
2.3. Neat CS and Tyr-c-CS Membrane Fabrication
2.4. Structural Characterization of Material and Membrane
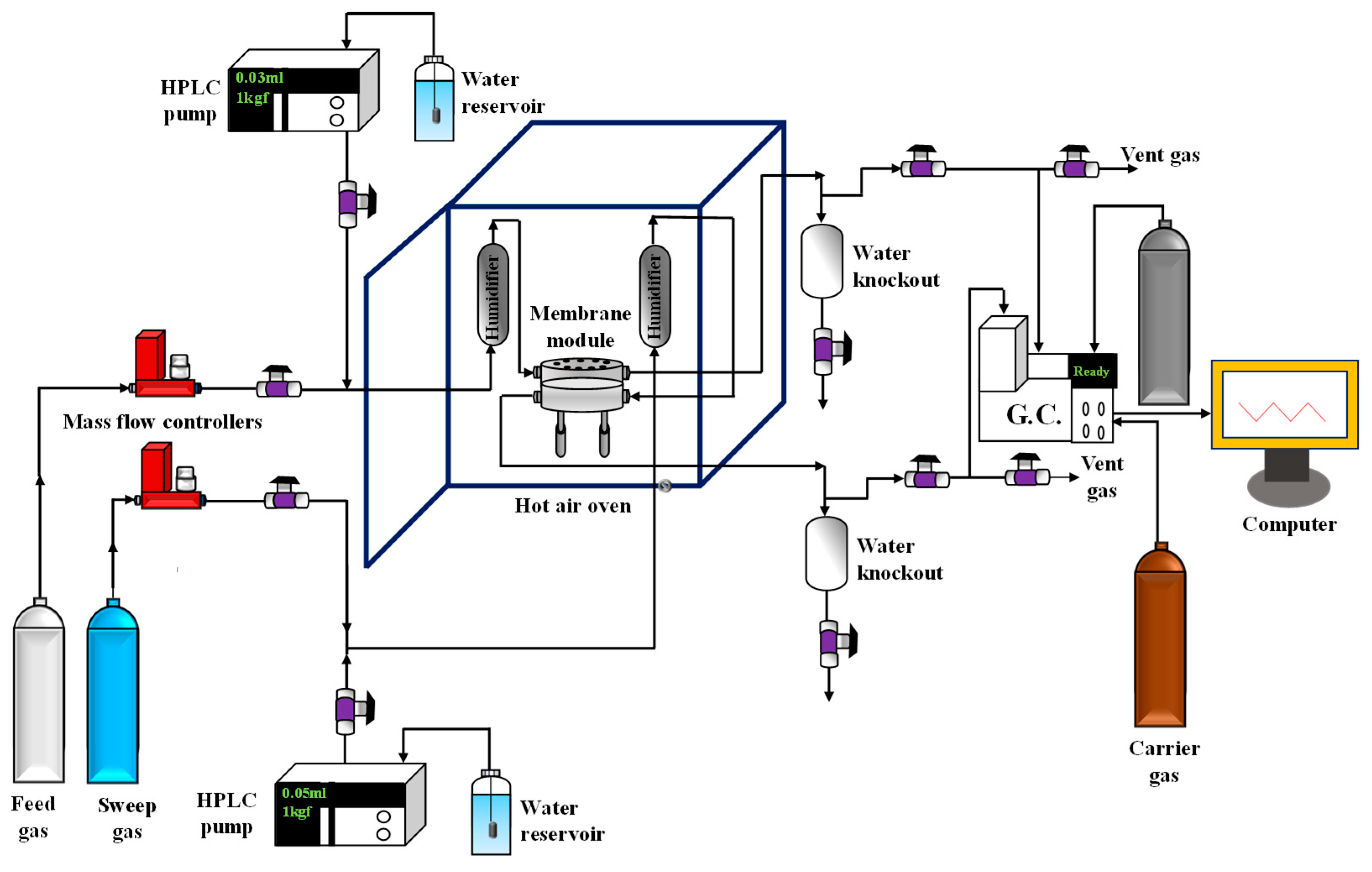
2.5. Gas Permeation Setup
3. Results and Discussions
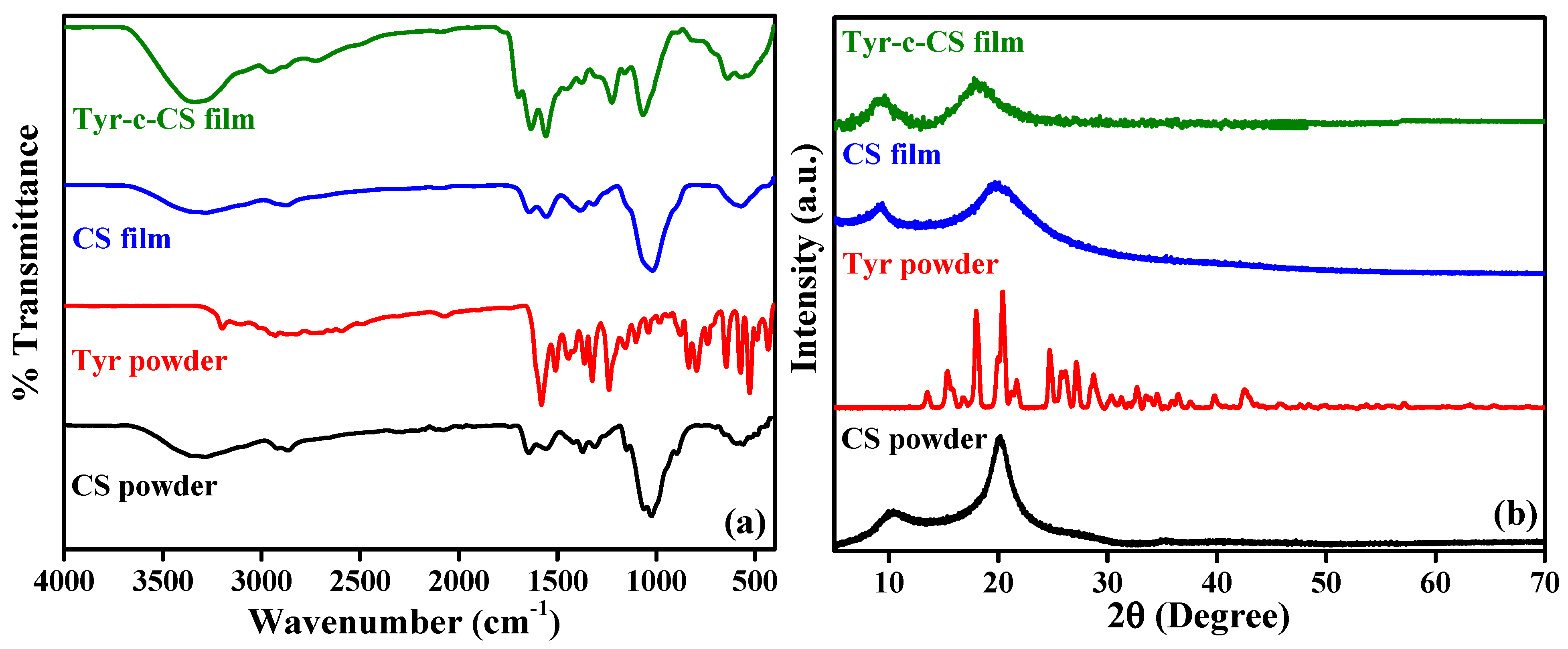
3.1. Spectroscopic Analysis
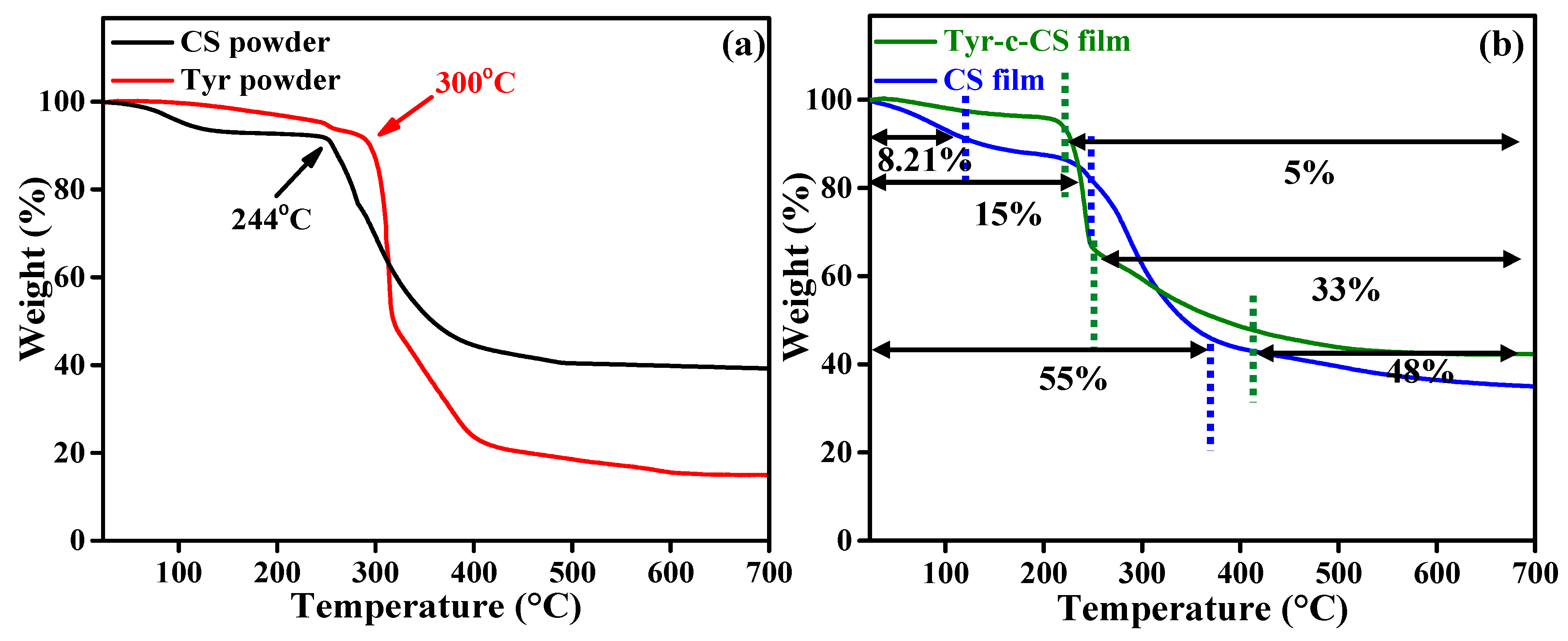
3.2. Thermal Stability Analysis
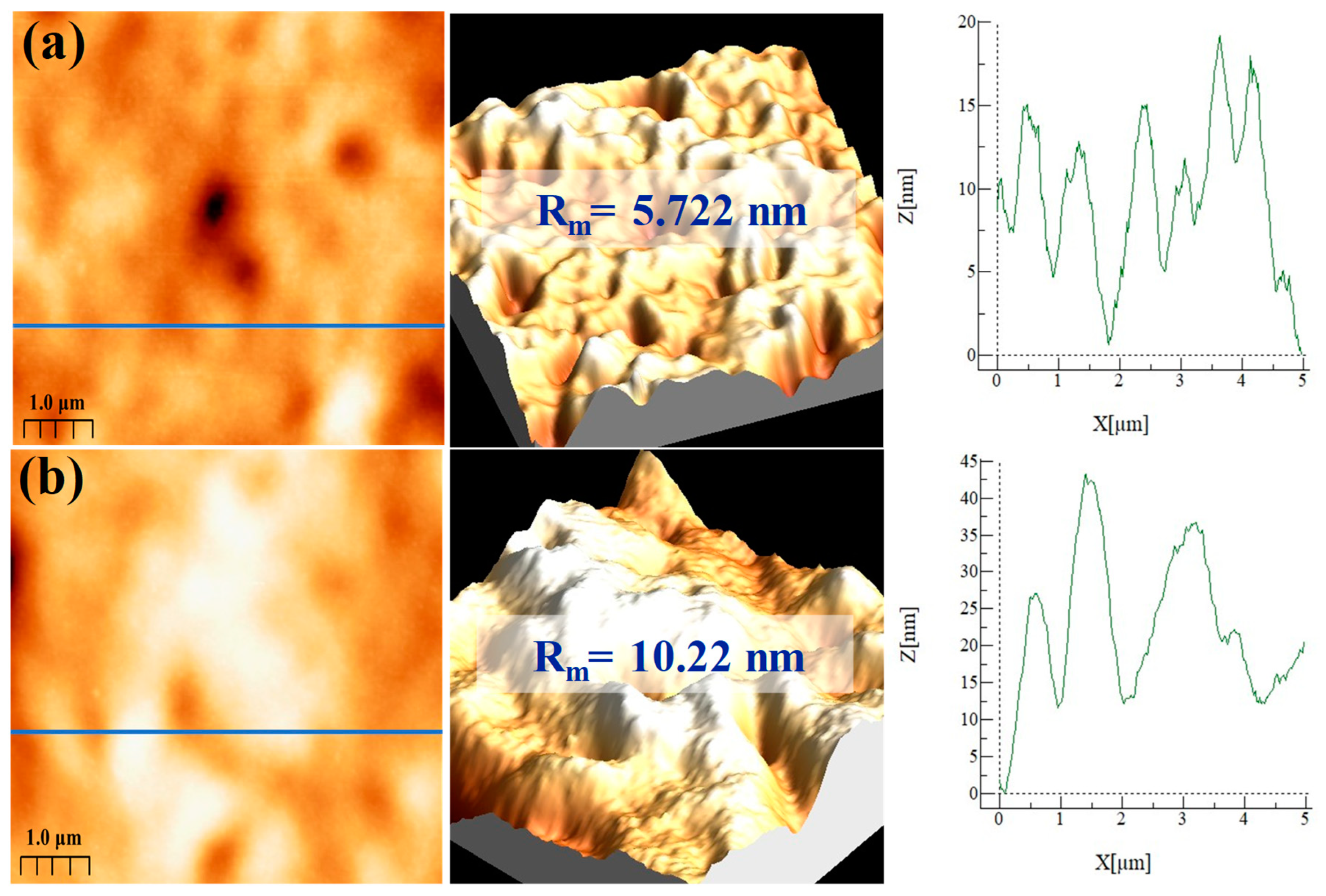
3.3. Morphological Analysis
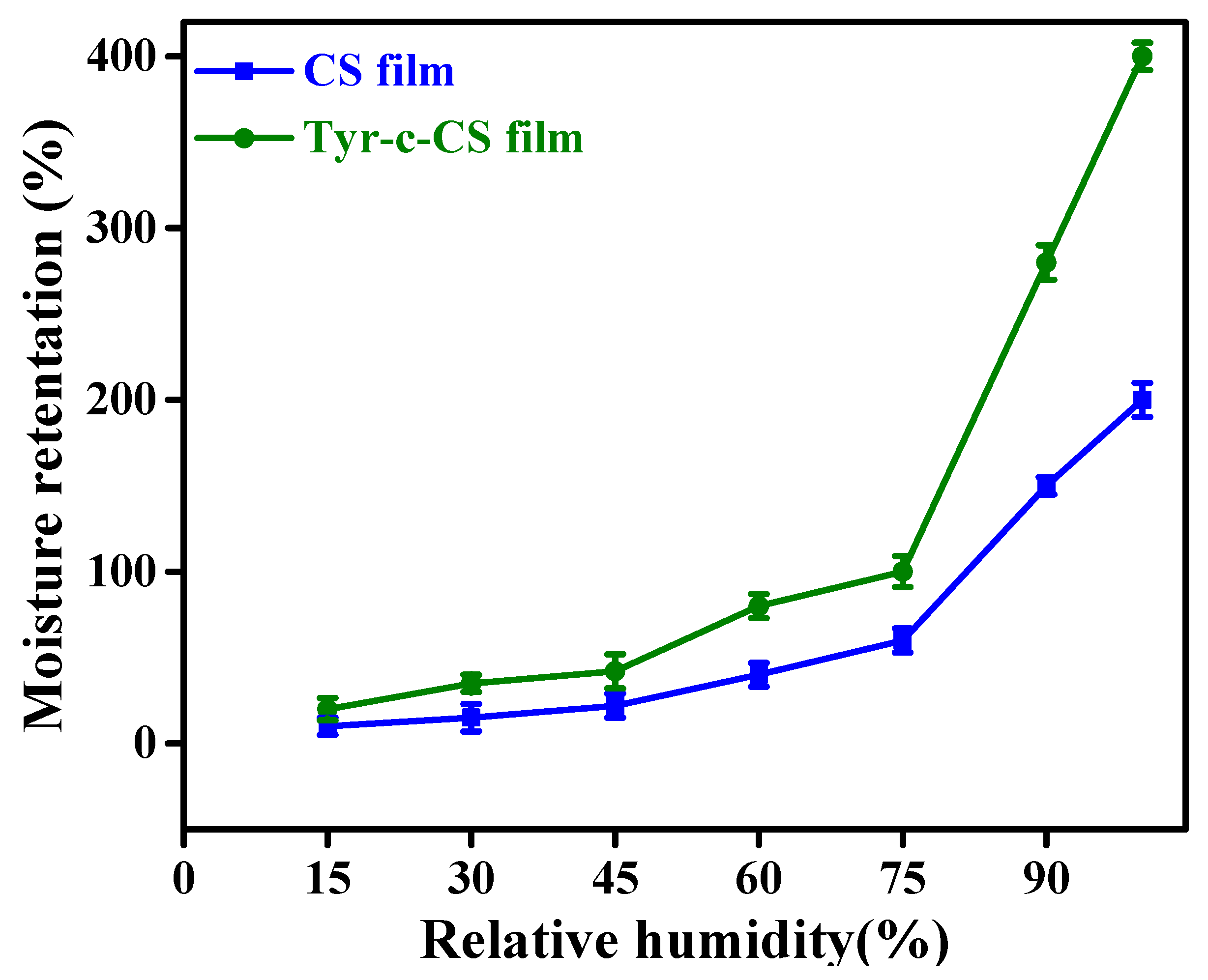
3.4. Moisture Retention Test
3.5. Gas Permeation Study
3.5.1. Effect of Temperature on the Separation Efficiency of Dry Membrane
3.5.2. Effect of Temperature on the Separation Efficiency of Swollen Membrane
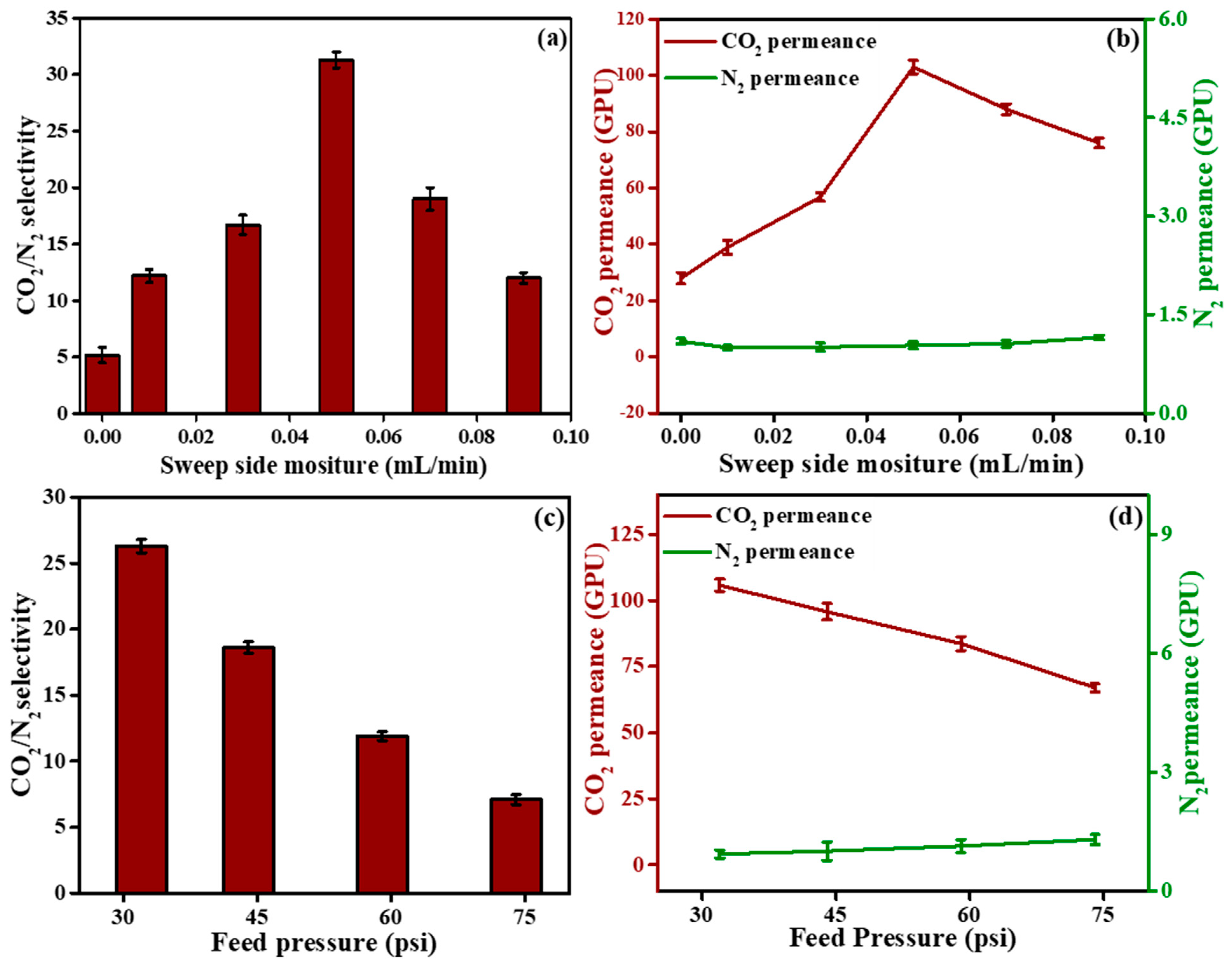
3.5.3. Effect of Sweep Side Humidity on the Separation Efficiency of Swollen Membrane
3.5.4. Effect of Feed Pressure on the Separation Efficiency of Swollen Membrane
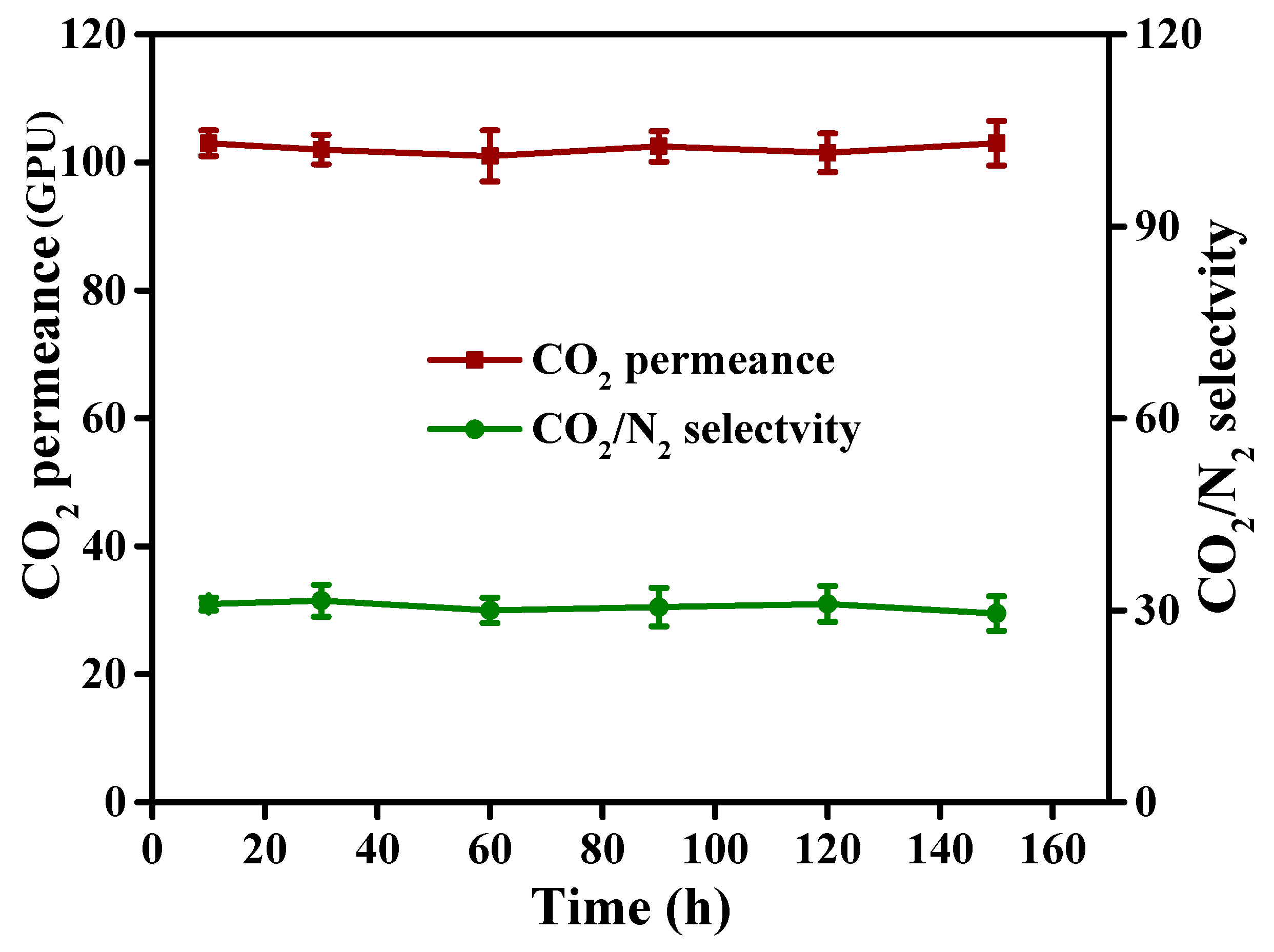
3.5.5. Prolonged CO2/N2 Gas Separation Test of Tyr-c-CS Membrane
3.5.6. Robeson Upper Bound Curve
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Li, X.; Wu, H.; Tian, Z.; Xin, Q.; He, G.; Peng, D.; Chen, S.; Yin, Y.; Jiang, Z.; et al. Advances in High Permeability Polymer-Based Membrane Materials for CO2 Separations. Energy Environ. Sci. 2016, 9, 1863–1890. [Google Scholar] [CrossRef]
- Energy Global. CO2 Status Report 2019—Analysis; IEA: Paris, France, 2019. [Google Scholar]
- Wong, K.K.; Jawad, Z.A. A Review and Future Prospect of Polymer Blend Mixed Matrix Membrane for CO2 Separation. J. Polym. Res. 2019, 26, 289. [Google Scholar] [CrossRef]
- Memon, M.Z.; Zhao, X.; Sikarwar, V.S.; Vuppaladadiyam, A.K.; Milne, S.J.; Brown, A.P.; Li, J.; Zhao, M. Alkali Metal CO2 Sorbents and the Resulting Metal Carbonates: Potential for Process Intensification of Sorption-Enhanced Steam Reforming. Environ. Sci. Technol. 2017, 51, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Luis Míguez, J.; Porteiro, J.; Pérez-Orozco, R.; Patiño, D.; Rodríguez, S. Evolution of CO2 Capture Technology between 2007 and 2017 through the Study of Patent Activity. Appl. Energy 2018, 211, 1282–1296. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Ji, N.; Deng, S.; Zhao, J.; Kitamura, Y. Advanced Cryogenic CO2 Capture Process Based on Stirling Coolers by Heat Integration. Appl. Therm. Eng. 2017, 114, 887–895. [Google Scholar] [CrossRef]
- Moftakhari Sharifzadeh, M.M.; Ebadi Amooghin, A.; Zamani Pedram, M.; Omidkhah, M. Time-Dependent Mathematical Modeling of Binary Gas Mixture in Facilitated Transport Membranes (FTMs): A Real Condition for Single-Reaction Mechanism. J. Ind. Eng. Chem. 2016, 39, 48–65. [Google Scholar] [CrossRef]
- Matteucci, S.; Yampolskii, Y.; Freeman, B.D.; Pinnau, I. Transport of Gases and Vapors in Glassy and Rubbery Polymers. In Materials Science of Membranes for Gas and Vapor Separation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 1–47. ISBN 047085345X. [Google Scholar]
- Li, T.; Pan, Y.; Peinemann, K.V.; Lai, Z. Carbon Dioxide Selective Mixed Matrix Composite Membrane Containing ZIF-7 Nano-Fillers. J. Memb. Sci. 2013, 425, 235–242. [Google Scholar] [CrossRef]
- Mubashir, M.; Yeong, Y.F.; Lau, K.K.; Chew, T.L.; Norwahyu, J. Efficient CO2/N2 and CO2/CH4 Separation Using NH2-MIL-53(Al)/Cellulose Acetate (CA) Mixed Matrix Membranes. Sep. Purif. Technol. 2018, 199, 140–151. [Google Scholar] [CrossRef]
- Lonsdale, H.K. The Evolution of Ultrathin Synthetic Membranes. J. Memb. Sci. 1987, 33, 121–136. [Google Scholar] [CrossRef]
- Kim, T.J.; Baoan, L.I.; Hägg, M.B. Novel Fixed-Site-Carrier Polyvinylamine Membrane for Carbon Dioxide Capture. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 4326–4336. [Google Scholar] [CrossRef]
- Barooah, M.; Mandal, B. Synthesis, Characterization and CO2 Separation Performance of Novel PVA/PG/ZIF-8 Mixed Matrix Membrane. J. Memb. Sci. 2019, 572, 198–209. [Google Scholar] [CrossRef]
- Mondal, A.; Barooah, M.; Mandal, B. Effect of Single and Blended Amine Carriers on CO2 Separation from CO2/N2 Mixtures Using Crosslinked Thin-Film Poly(Vinyl Alcohol) Composite Membrane. Int. J. Greenh. Gas Control 2015, 39, 27–38. [Google Scholar] [CrossRef]
- Mondal, A.; Mandal, B. CO2 Separation Using Thermally Stable Crosslinked Poly(Vinyl Alcohol) Membrane Blended with Polyvinylpyrrolidone/Polyethyleneimine/Tetraethylenepentamine. J. Memb. Sci. 2014, 460, 126–138. [Google Scholar] [CrossRef]
- Prasad, B.; Mandal, B. Preparation and Characterization of CO2-Selective Facilitated Transport Membrane Composed of Chitosan and Poly(Allylamine) Blend for CO2/N2 Separation. J. Ind. Eng. Chem. 2018, 66, 419–429. [Google Scholar] [CrossRef]
- Prasad, B.; Mandal, B. Moisture Responsive and CO2 Selective Biopolymer Membrane Containing Silk Fibroin as a Green Carrier for Facilitated Transport of CO2. J. Memb. Sci. 2018, 550, 416–426. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Kopel, P. The Effect of Nanofillers on the Functional Properties of Biopolymer-Based Films: A Review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef]
- Aitken, C.L.; Koros, W.J.; Paul, D.R. Effect of Structural Symmetry on Gas Transport Properties of Polysulfones. Macromolecules 1992, 25, 3424–3434. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, S.R. Gas Permeation Characteristics by Chitosan/Pebax Composite Membranes. Membr. J. 2017, 27, 319–327. [Google Scholar] [CrossRef]
- Duan, K.; Wang, J.; Zhang, Y.; Liu, J. Covalent Organic Frameworks (COFs) Functionalized Mixed Matrix Membrane for Effective CO2/N2 Separation. J. Memb. Sci. 2019, 572, 588–595. [Google Scholar] [CrossRef]
- Ahmad, J.; Rehman, W.U.; Deshmukh, K.; Basha, S.K.; Ahamed, B.; Chidambaram, K. Recent Advances in Poly (Amide-B-Ethylene) Based Membranes for Carbon Dioxide (CO2) Capture: A Review. Polym. Technol. Mater. 2019, 58, 366–383. [Google Scholar] [CrossRef]
- Scofield, J.M.P.; Gurr, P.A.; Kim, J.; Fu, Q.; Halim, A.; Kentish, S.E.; Qiao, G.G. High-Performance Thin Film Composite Membranes with Well-Defined Poly(dimethylsiloxane)-b-poly(ethylene glycol) Copolymer Additives for CO2 Separation. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1500–1511. [Google Scholar] [CrossRef]
- Shan, M.; Seoane, B.; Pustovarenko, A.; Wang, X.; Liu, X.; Yarulina, I.; Abou-Hamad, E.; Kapteijn, F.; Gascon, J. Benzimidazole Linked Polymers (BILPs) in Mixed-Matrix Membranes: Influence of Filler Porosity on the CO2/N2 Separation Performance. J. Memb. Sci. 2018, 566, 213–222. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, H.; Liu, J.; Zhang, Y. Enhanced Performance of a Novel Polyvinyl Amine/Chitosan/Graphene Oxide Mixed Matrix Membrane for CO2 Capture. ACS Sustain. Chem. Eng. 2015, 3, 1819–1829. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Zhang, X.; Li, J.; Liu, C.; Li, N.; Xie, Z. Polyvinylamine/Graphene Oxide/PANI@CNTs Mixed Matrix Composite Membranes with Enhanced CO2/N2 Separation Performance. J. Memb. Sci. 2019, 589, 117246. [Google Scholar] [CrossRef]
- Chen, Y.; Ho, W.S.W. High-Molecular-Weight Polyvinylamine/Piperazine Glycinate Membranes for CO2 Capture from Flue Gas. J. Memb. Sci. 2016, 514, 376–384. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, Z.; Qiao, Z.; Wang, M.; Wang, J.; Wang, S. Improvement of CO2/N2 Separation Characteristics of Polyvinylamine by Modifying with Ethylenediamine. J. Memb. Sci. 2011, 378, 425–437. [Google Scholar] [CrossRef]
- Gonzales, R.R.; Park, M.J.; Bae, T.H.; Yang, Y.; Abdel-Wahab, A.; Phuntsho, S.; Shon, H.K. Melamine-Based Covalent Organic Framework-Incorporated Thin Film Nanocomposite Membrane for Enhanced Osmotic Power Generation. Desalination 2019, 459, 10–19. [Google Scholar] [CrossRef]
- Prasad, B.; Mandal, B. Graphene-Incorporated Biopolymeric Mixed-Matrix Membrane for Enhanced CO2 Separation by Regulating the Support Pore Filling. ACS Appl. Mater. Interfaces 2018, 10, 27810–27820. [Google Scholar] [CrossRef]
- Wu, S.Y.; Hsiao, I.C.; Liu, C.M.; Mt Yusuf, N.Y.; Wan Isahak, W.N.R.; Masdar, M.S. A Novel Bio-Cellulose Membrane and Modified Adsorption Approach in CO2/H2 Separation Technique for PEM Fuel Cell Applications. Int. J. Hydrogen Energy 2017, 42, 27630–27640. [Google Scholar] [CrossRef]
- Demontigny, D.; Tontiwachwuthikul, P.; Chakma, A. Using Polypropylene and Polytetrafluoroethylene Membranes in a Membrane Contactor for CO2 Absorption. J. Memb. Sci. 2006, 277, 99–107. [Google Scholar] [CrossRef]
- Hwang, H.Y.; Nam, S.Y.; Koh, H.C.; Ha, S.Y.; Barbieri, G.; Drioli, E. The Effect of Operating Conditions on the Performance of Hollow Fiber Membrane Modules for CO2/N2 Separation. J. Ind. Eng. Chem. 2012, 18, 205–211. [Google Scholar] [CrossRef]
- Gomez-Coma, L.; Garea, A.; Irabien, A. Carbon Dioxide Capture by [Emim][Ac] Ionic Liquid in a Polysulfone Hollow Fiber Membrane Contactor. Int. J. Greenh. Gas Control 2016, 52, 401–409. [Google Scholar] [CrossRef]
- Houde, A.Y.; Krishnakumar, B.; Charati, S.G.; Stern, S.A. Permeability of Dense (Homogeneous) Cellulose Acetate Membranes to Methane, Carbon Dioxide, and Their Mixtures at Elevated Pressures. J. Appl. Polym. Sci. 1996, 62, 2181–2192. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The Solution-Diffusion Model: A Review. J. Memb. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of Separation Factor versus Permeability for Polymeric Membranes. J. Memb. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Reijerkerk, S.R.; Wessling, M.; Nijmeijer, K. Pushing the Limits of Block Copolymer Membranes for CO2 Separation. J. Memb. Sci. 2011, 378, 479–484. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, R.; Hou, J.; Wei, Z.; Li, X. Mixed-Matrix Membranes Containing Carbon Nanotubes Composite with Hydrogel for Efficient CO2 Separation. ACS Appl. Mater. Interfaces 2016, 8, 29044–29051. [Google Scholar] [CrossRef] [PubMed]
- Borgohain, R.; Jain, N.; Prasad, B.; Mandal, B.; Su, B. Carboxymethyl Chitosan/Carbon Nanotubes Mixed Matrix Membranes for CO2 Separation. React. Funct. Polym. 2019, 143, 104331. [Google Scholar] [CrossRef]
- Xia, J.; Liu, S.; Chung, T.S. Effect of End Groups and Grafting on the CO2 Separation Performance of Poly(Ethylene Glycol) Based Membranes. Macromolecules 2011, 44, 7727–7736. [Google Scholar] [CrossRef]
- Caplow, M. Kinetics of Carbamate Formation and Breakdown. J. Am. Chem. Soc. 1968, 90, 6795–6803. [Google Scholar] [CrossRef]
- Tong, Z.; Ho, W.S.W. Facilitated Transport Membranes for CO2 Separation and Capture. Sep. Sci. Technol. 2017, 52, 156–167. [Google Scholar] [CrossRef]
- Prasad, B.; Mandal, B. CO2 Separation Performance by Chitosan/Tetraethylenepentamine/Poly(Ether Sulfone) Composite Membrane. J. Appl. Polym. Sci. 2017, 134, 45206. [Google Scholar] [CrossRef]
- Borgohain, R.; Mandal, B. PH Responsive Carboxymethyl Chitosan/Poly(Amidoamine) Molecular Gate Membrane for CO2/N2 Separation. ACS Appl. Mater. Interfaces 2019, 11, 42616–42628. [Google Scholar] [CrossRef]
- Dai, Z.; Deng, J.; Peng, K.J.; Liu, Y.L.; Deng, L. Pebax/PEG Grafted CNT Hybrid Membranes for Enhanced CO2/N2 Separation. Ind. Eng. Chem. Res. 2019, 58, 12226–12234. [Google Scholar] [CrossRef]
- Chen, X.Y.; Nik, O.G.; Rodrigue, D.; Kaliaguine, S. Mixed Matrix Membranes of Aminosilanes Grafted FAU/EMT Zeolite and Cross-Linked Polyimide for CO2/CH4 Separation. Polymer 2012, 53, 3269–3280. [Google Scholar] [CrossRef]
- Jaiswal, S.; Dutta, P.K.; Kumar, S.; Koh, J.; Pandey, S. Methyl Methacrylate Modified Chitosan: Synthesis, Characterization and Application in Drug and Gene Delivery. Carbohydr. Polym. 2019, 211, 109–117. [Google Scholar] [CrossRef]
- Kim, E.; Xiong, Y.; Cheng, Y.; Wu, H.C.; Liu, Y.; Morrow, B.H.; Ben-Yoav, H.; Ghodssi, R.; Rubloff, G.W.; Shen, J.; et al. Chitosan to Connect Biology to Electronics: Fabricating the Bio-Device Interface and Communicating across This Interface. Polymers 2015, 7, 1–46. [Google Scholar] [CrossRef]
- Hu, Y.; Zhan, C.; Zhou, A.; Zhang, S.; Chen, J.; Huang, X. Synthesis and Characterization of L-Tyrosine-Conjugated Quaternary Ammonium Salt Chitosan and Their Cytocompatibility as a Potential Tissue Engineering Scaffold. J. Biomater. Sci. Polym. Ed. 2020, 31, 833–848. [Google Scholar] [CrossRef]
- Forney, C.F.; Brandl, D.G. Control of humidity in small controlled-environment chambers using glycerol-water solutions. HortTechnology 1992, 2, 52–54. [Google Scholar] [CrossRef]
- Purwanto, M.; Atmaja, L.; Mohamed, M.A.; Salleh, M.T.; Jaafar, J.; Ismail, A.F.; Santoso, M.; Widiastuti, N. Biopolymer-Based Electrolyte Membranes from Chitosan Incorporated with Montmorillonite-Crosslinked GPTMS for Direct Methanol Fuel Cells. RSC Adv. 2016, 6, 2314–2322. [Google Scholar] [CrossRef]
- Yoksan, R.; Akashi, M. Low Molecular Weight Chitosan-g-l-Phenylalanine: Preparation, Characterization, and Complex Formation with DNA. Carbohydr. Polym. 2009, 75, 95–103. [Google Scholar] [CrossRef]
- Takara, E.A.; Vega-Hissi, E.G.; Garro-Martinez, J.C.; Marchese, J.; Ochoa, N.A. About Endothermic Sorption of Tyrosine on Chitosan Films. Carbohydr. Polym. 2019, 206, 57–64. [Google Scholar] [CrossRef]
- Cassano, R.; Trapani, A.; Luisa, M.; Gioia, D.; Mandracchia, D.; Pellitteri, R.; Tripodo, G.; Trombino, S.; Di, S.; Conese, M. Synthesis and Characterization of Novel Chitosan-Dopamine or Chitosan- Tyrosine Conjugates for Potential Nose-to-Brain Delivery. Int. J. Pharm. 2020, 589, 119829. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Dutta, P.K.; Mehrotra, G.K. Lignin Incorporated Antimicrobial Chitosan Film for Food Packaging Application. J. Polym. Mater. 2017, 34, 171–183. [Google Scholar]
- Liu, B.; Ye, H.; Liang, Q.; Jiang, L.; Chen, M.; Yang, S. Development and Characterization of Pectin and Chitosan Films Incorporated with a New Cross-Linking Agent. J. Sci. Food Agric. 2023, 103, 1964–1973. [Google Scholar] [CrossRef]
- Mehta, R.; Kumari, R.; Das, P.; Bhowmick, A.K. Synthesis and Characterization of a Biocompatible Monotyrosine-Based Polymer and Its Interaction with DNA. J. Mater. Chem. B 2014, 2, 6236–6248. [Google Scholar] [CrossRef]
- Shruthi, S.B.; Roy, P.; Sailaja, R.R.N.; Sengupta, C. Encapsulation And Release Characteristics Of Marigold Oleoresin In Chitosan Grafted Sodium Acrylate-Acrylate-Co-Acrylamide. Adv. Mater. Lett. 2016, 7, 795–801. [Google Scholar] [CrossRef]
- Shen, J.N.; Yu, C.C.; Zeng, G.N.; van der Bruggen, B. Preparation of a Facilitated Transport Membrane Composed of Carboxymethyl Chitosan and Polyethylenimine for CO2/N2 Separation. Int. J. Mol. Sci. 2013, 14, 3621–3638. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.; Thakur, R.M.; Mandal, B.; Su, B. Enhanced CO2 Separation Membrane Prepared from Waste By-Product of Silk Fibroin. J. Memb. Sci. 2019, 587, 117170. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Ahmadi, R.; Ebadi Amooghin, A.; Ghanbari, D. A Novel Ternary Mixed Matrix Membrane Containing Glycerol-Modified Poly(Ether-Block-Amide) (Pebax 1657)/Copper Nanoparticles for CO2 Separation. J. Memb. Sci. 2019, 573, 234–246. [Google Scholar] [CrossRef]
- Xiao, Y.; Low, B.T.; Hosseini, S.S.; Chung, T.S.; Paul, D.R. The Strategies of Molecular Architecture and Modification of Polyimide-Based Membranes for CO2 Removal from Natural Gas-A Review. Prog. Polym. Sci. 2009, 34, 561–580. [Google Scholar] [CrossRef]
- Gay, J.S. Effects of Relative Humidity and Temperature on Gas Transport Properties in MFC and MFC + Lupamin Films. 2015. Available online: https://zaguan.unizar.es/record/48047/files/TAZ-TFG-2015-314.pdf (accessed on 20 September 2022).
- Borgohain, R.; Mandal, B. Thermally Stable and Moisture Responsive Carboxymethyl Chitosan/Dendrimer/Hydrotalcite Membrane for CO2 Separation. J. Memb. Sci. 2020, 608, 118214. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Gurkan, B. Graphene Oxide Reinforced Facilitated Transport Membrane with Poly(Ionic Liquid) and Ionic Liquid Carriers for CO2/N2 Separation. J. Memb. Sci. 2021, 638, 119652. [Google Scholar] [CrossRef]
- Han, Y.; Wu, D.; Ho, W.S.W. Simultaneous Effects of Temperature and Vacuum and Feed Pressures on Facilitated Transport Membrane for CO2/N2 Separation. J. Memb. Sci. 2019, 573, 476–484. [Google Scholar] [CrossRef]
- Tong, Z.; Ho, W.S.W. New Sterically Hindered Polyvinylamine Membranes for CO2 Separation and Capture. J. Memb. Sci. 2017, 543, 202–211. [Google Scholar] [CrossRef]
- Lv, B.; Guo, B.; Zhou, Z.; Jing, G. Mechanisms of CO2 Capture into Monoethanolamine Solution with Different CO2 Loading during the Absorption/Desorption Processes. Environ. Sci. Technol. 2015, 49, 10728–10735. [Google Scholar] [CrossRef]
- Balzani, V.; Armaroli, N. Energy for a Sustainable World: From the Oil Age to a Sun-Powered Future; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Borgohain, R.; Prasad, B.; Mandal, B. Synthesis and Characterization of Water-Soluble Chitosan Membrane Blended with a Mobile Carrier for CO2 Separation. Sep. Purif. Technol. 2019, 222, 177–187. [Google Scholar] [CrossRef]
- Duan, S.; Kouketsu, T.; Kazama, S.; Yamada, K. Development of PAMAM Dendrimer Composite Membranes for CO2 Separation. J. Memb. Sci. 2006, 283, 2–6. [Google Scholar] [CrossRef]
- Lilleby Helberg, R.M.; Dai, Z.; Ansaloni, L.; Deng, L. PVA/PVP Blend Polymer Matrix for Hosting Carriers in Facilitated Transport Membranes: Synergistic Enhancement of CO2 Separation Performance. Green Energy Environ. 2019, 5, 59–68. [Google Scholar] [CrossRef]
- Kim, S.J. Gas Permeation through Water-Swollen Sericin/PVA Membranes. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2007. [Google Scholar]
- Zhang, Z.; Rao, S.; Han, Y.; Pang, R.; Ho, W.S.W. CO2-Selective Membranes Containing Amino Acid Salts for CO2/N2 Separation. J. Memb. Sci. 2021, 638, 119696. [Google Scholar] [CrossRef]
- Yoshida, W.; Cohen, Y. Topological AFM Characterization of Graft Polymerized Silica Membranes. J. Memb. Sci. 2003, 215, 249–264. [Google Scholar] [CrossRef]
- Fernández, L.; Sánchez, M.; Carmona, F.J.; Palacio, L.; Calvo, J.I.; Hernández, A.; Prádanos, P. Analysis of the Grafting Process of PVP on a Silicon Surface by AFM and Contact Angle. Langmuir 2011, 27, 11636–11649. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Zhang, J.; Wang, Z.; Wang, J.; Zhao, P.; Cao, X.; Zhang, Y. Interfacial Property Modulation of PIM-1 through Polydopamine-Derived Submicrospheres for Enhanced CO2 /N2 Separation Performance. ACS Appl. Mater. Interfaces 2019, 11, 19613–19622. [Google Scholar] [CrossRef] [PubMed]



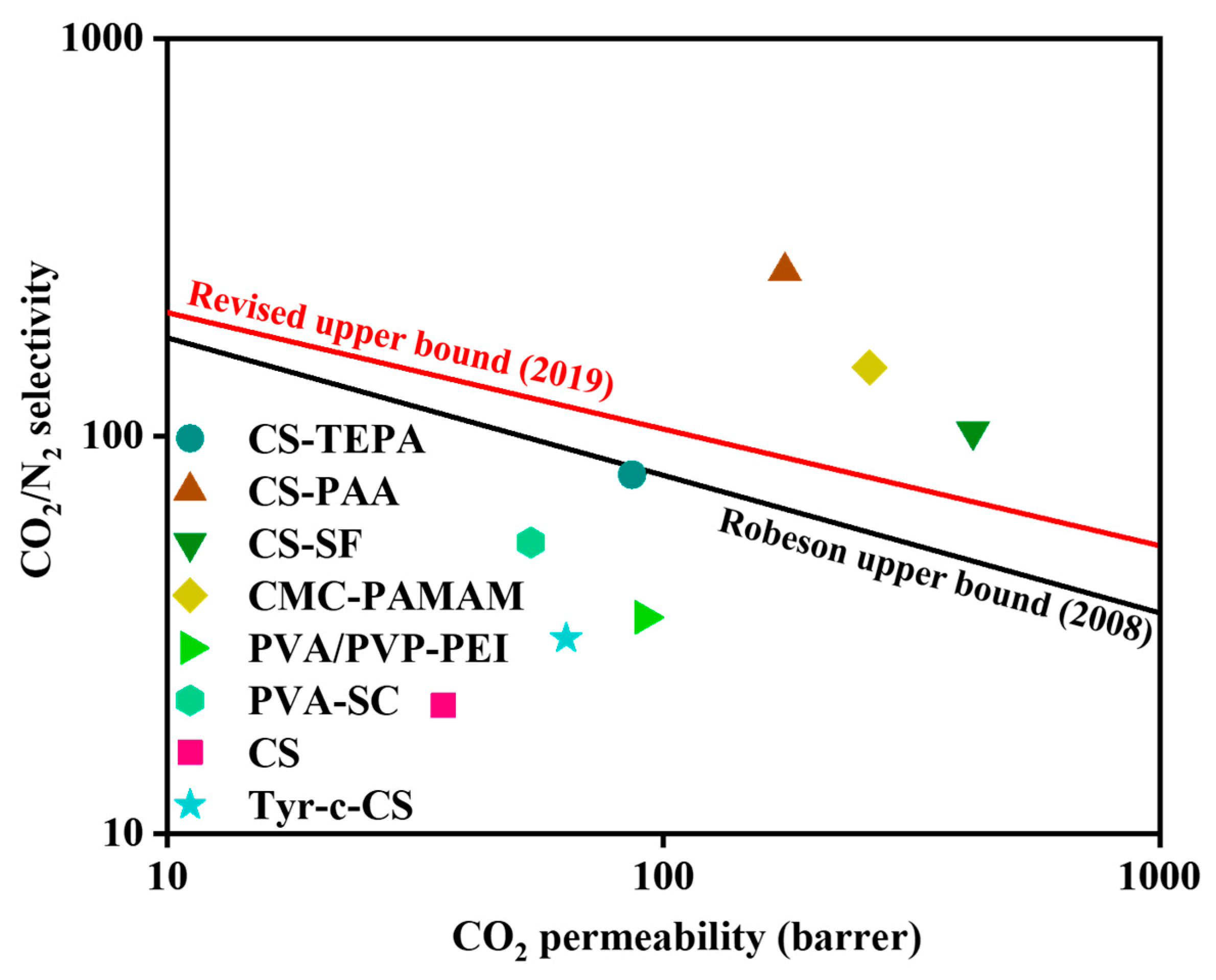
| Polymer | Carrier | Top (°C), P (psi) | Thickness (μm) | CO2 Permeance (GPU) | References | |
|---|---|---|---|---|---|---|
| CS | - | 90, 32 | 3.5 | 12.5 | 54 a | [44] |
| CS | TEPA | 90, 32 | 3.5 | 24.7 | 80 a | [44] |
| CS | PAA | 90, 32 | 4.5 | 39 | 260 a | [16] |
| CS | SF | 90, 32 | 3 | 140 | 103 a | [17] |
| CMC | PAMAM | 90, 32 | 2.6 | 100 | 149 a | [45] |
| CS | PAMAM | 40, 14 | 0.1 | 61 | 230 a | [72] |
| PVA/PVP | PEI | 25, 32 | 0.5 | 183 | 35 b | [73] |
| PVA | SC | 25, 32 | 25 | 2.16 | 54 c | [74] |
| CS | - | 85, 32 | 0.6 | 60 | 21 a | This work |
| CS | L-Tyr | 85, 32 | 0.6 | 103 | 31 a | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katare, A.; Borgohain, R.; Prasad, B.; Mandal, B. A Strategical Improvement in the Performance of CO2/N2 Gas Permeation via Conjugation of L-Tyrosine onto Chitosan Membrane. Membranes 2023, 13, 487. https://doi.org/10.3390/membranes13050487
Katare A, Borgohain R, Prasad B, Mandal B. A Strategical Improvement in the Performance of CO2/N2 Gas Permeation via Conjugation of L-Tyrosine onto Chitosan Membrane. Membranes. 2023; 13(5):487. https://doi.org/10.3390/membranes13050487
Chicago/Turabian StyleKatare, Aviti, Rajashree Borgohain, Babul Prasad, and Bishnupada Mandal. 2023. "A Strategical Improvement in the Performance of CO2/N2 Gas Permeation via Conjugation of L-Tyrosine onto Chitosan Membrane" Membranes 13, no. 5: 487. https://doi.org/10.3390/membranes13050487







