Measuring Physical Properties of Electrospun Nanofiber Mats for Different Biomedical Applications
Abstract
:1. Introduction
2. Porosity
3. Pore Size Distribution
4. Specific Surface Area
5. Nanofiber Diameter
6. Nanofiber Orientation
7. Surface Roughness
8. Nanofiber Mat Thickness
9. Hydrophobicity/Hydrophilicity
10. Water Uptake
11. Mechanical Properties
12. Electrical Conductivity
13. Water Vapor Permeability
14. Air Permeability
15. Thermal Properties
16. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Fujihara, K.; Teo, W.E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: Solving global issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Grothe, T.; Wehlage, D.; Böhm, T.; Remche, A.; Ehrmann, A. Needleless electrospinning of PAN nanofibre mats. Tekstilec 2017, 60, 290–295. [Google Scholar] [CrossRef]
- Klinkhammer, K.; Seiler, N.; Grafahrend, D.; Gerardo-Nava, J.; Mey, J.; Brook, G.A.; Möller, M.; Dalton, P.D.; Klee, D. Deposition of electrospun fibers on reactive substrates for In Vitro investigations. Tissue Eng. Part C 2009, 15, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Mamun, A. Review of possible applications of nanofibrous mats for wound dressings. Tekstilec 2019, 62, 89–100. [Google Scholar] [CrossRef]
- Gao, S.T.; Tang, G.S.; Hua, D.W.; Xiong, R.H.; Han, J.Q.; Jiang, S.H.; Zhang, Q.L.; Huang, C.B. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B 2019, 7, 709–729. [Google Scholar] [CrossRef]
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for biomedical and healthcare applications. Macromol. Biosci. 2019, 19, 1800256. [Google Scholar] [CrossRef]
- Wehlage, D.; Blattner, H.; Mamun, A.; Kutzli, I.; Diestelhorst, E.; Rattenholl, A.; Gudermann, F.; Lütkemeyer, D.; Ehrmann, A. Cell growth on electrospun nanofiber mats from polyacrylonitrile (PAN) blends. AIMS Bioeng. 2020, 7, 43–54. [Google Scholar] [CrossRef]
- Tanzli, E.; Ehrmann, A. Electrospun nanofibrous membranes for tissue engineering and cell growth. Appl. Sci. 2021, 11, 6929. [Google Scholar] [CrossRef]
- Zahedi, P.; Khatibi, A.; Fallah-Darrehchi, M. ANtimicrobial electrospun membranes. In Electrospun and Nanofibrous Membranes; Elsevier: Amsterdam, The Netherlands, 2023; pp. 501–519. [Google Scholar]
- Jafari, S.; Hosseini Salekdeh, S.S.; Solouk, A.; Yousefzadeh, M. Electrospun polyethylene terephthalate (PET) nanofibrous conduit for biomedical application. Polym. Adv. Technol. 2020, 31, 284–296. [Google Scholar] [CrossRef]
- More, N.; Ranglani, D.; Kharche, S.; Kapusetti, G. Electrospun mat of thermal-treatment-induced nanocomposite hydrogel of polyvinyl alcohol and cerium oxide for biomedical applications. J. Appl. Polym. Sci. 2020, 137, 49426. [Google Scholar] [CrossRef]
- Ramos, C.; Lanno, G.-M.; Laidmäe, I.; Meos, A.; Härmas, R.; Kogermann, K. High humidity electrospinning of porous fibers for tuning the release of drug delivery systems. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 880–892. [Google Scholar] [CrossRef]
- Chen, S.X.; John, J.V.; McCarthy, A.; Xie, J.W. New forms of electrospun nanofiber materials for biomedical applications. J. Mater. Chem. B 2020, 8, 3733–3746. [Google Scholar] [CrossRef]
- Zou, S.Z.; Wang, X.R.; Fan, S.N.; Yao, X.; Zhang, Y.P.; Shao, H.L. Electrospun regenerated Antheraea pernyi silk fibroin scaffolds with improved pore size, mechanical properties and cytocompatibility using mesh collectors. J. Mater. Chem. B 2021, 9, 5514–5527. [Google Scholar] [CrossRef]
- Selvaras, T.; Alshamrani, S.A.; Gopal, R.; Jaganathan, S.K.; Sivalingam, S.; Kadiman, S.; Saidin, S. Biodegradable and antithrombogenic chitosan/elastin blended polyurethane electrospun membrane for vascular tissue integration. J. Biomed. Mater. Res. B 2023, 111, 1171–1181. [Google Scholar] [CrossRef]
- Entekhabi, E.; Nazarpak, M.H.; Shafieian, M.; Mohammadi, H.; Firouzi, M.; Hassannejad, Z. Fabrication and in vitro evaluation of 3D composite scaffold based on collagen/hyaluronic acid sponge and electrospun polycaprolactone nanofibers for peripheral nerve regeneration. J. Biomed. Mater. Res. A 2021, 109, 300–312. [Google Scholar] [CrossRef]
- Yardimci, A.I. Comparative Study of the Structural, Mechanical and Electrochemical Properties of Polyacrylonitrile (PAN)-Based Polypyrrole (PPy) and Polyvinylidene Fluoride (PVDF) Electrospun Nanofibers. J. Macromol. Sci. B 2022, 61, 1103–1115. [Google Scholar] [CrossRef]
- Zhang, H.N.; Zhang, T.T.; Qiu, Q.H.; Qin, X.H. Quaternary ammonium salt–modified polyacrylonitrile/polycaprolactone electrospun nanofibers with enhanced antibacterial properties. Text. Res. J. 2021, 91, 2194–2203. [Google Scholar] [CrossRef]
- Jirofti, N.; Golandi, M.; Movaffagh, J.; Ahmadi, F.S.; Kalalinia, F. Improvement of the Wound-Healing Process by Curcumin-Loaded Chitosan/Collagen Blend Electrospun Nanofibers: In Vitro and In Vivo Studies. ACS Biomater. Sci. Eng. 2021, 7, 3886–3897. [Google Scholar] [CrossRef]
- Akbarzadeh, M.; Pezeshki-Modaress, M.; Zandi, M. Biphasic, tough composite core/shell PCL/PVA-GEL nanofibers for biomedical application. J. Appl. Polym. Sci. 2020, 137, 48713. [Google Scholar] [CrossRef]
- Islam, M.A.; Begum, H.A.; Shahid, M.A.; Ali, Y. Antibacterial electrospun nanofibers from poly (vinyl alcohol) and Mikania micrantha with augmented moisture properties: Formation and evaluation. J. Text. Inst. 2021, 112, 1602–1610. [Google Scholar] [CrossRef]
- Stella, S.M.; Vijayalakshi, U. Influence of chemically modified Luffa on the preparation of nanofiber and its biological evaluation for biomedical applications. J. Biomed. Mater. Res. A 2019, 107, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.W.; Jia, S.W.; Xiong, P.; Cai, Z.J. Preparation of protein nanoparticle-coated poly(hydroxybutyrate) electrospun nanofiber based scaffold for biomedical applications. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 677–691. [Google Scholar] [CrossRef]
- Malik, S.; Hussain, T.; Nazir, A.; Khenoussi, N.; Cheema, S.A. Oriented electrospun nanofibers on stand-alone multi-segmented cylindrical collectors. J. Text. Inst. 2021, 112, 955–964. [Google Scholar] [CrossRef]
- Munawar, M.A.; Schubert, D.W. Highly Oriented Electrospun Conductive Nanofibers of Biodegradable Polymers-Revealing the Electrical Percolation Thresholds. ACS Appl. Polym. Mater. 2021, 3, 2889–2901. [Google Scholar] [CrossRef]
- Han, Y.S.; Hong, H.J.; Park, S.M.; Kim, D.S. Metal–Electrolyte Solution Dual-Mode Electrospinning Process for In Situ Fabrication of Electrospun Bilayer Membrane. Adv. Mater. Interfaces 2020, 7, 2000571. [Google Scholar] [CrossRef]
- Maurya, A.K.; Weidenbacher, L.; Spano, F.; Fortunato, G.; Rossi, R.M.; Frenz, M.; Dommann, A.; Neels, A.; Sadeghpour, A. Structural insights into semicrystalline states of electrospun nanofibers: A multiscale analytical approach. Nanoscale 2019, 15, 7176–7187. [Google Scholar] [CrossRef]
- Shao, Z.G.; Chen, J.Y.; Ke, L.-J.; Wang, Q.F.; Wang, X.; Li, W.W.; Zheng, G.F. Directional Transportation in a Self-Pumping Dressing Based on a Melt Electrospinning Hydrophobic Mesh. ACS Biomater. Sci. Eng. 2021, 7, 5918–5926. [Google Scholar] [CrossRef]
- Sengupta, P.; Ghosh, A.; Bose, N.; Mukherjee, S.; Chowdhury, A.R.; Datta, P. A comparative assessment of poly(vinylidene fluoride)/conducting polymer electrospun nanofiber membranes for biomedical applications. J. Appl. Polym. Sci. 2020, 137, 49115. [Google Scholar] [CrossRef]
- Gwon, G.; Choi, H.J.; Bae, J.H.; Binti Zulkifli, N.A.; Jeong, W.; Yoo, S.S.; Hyun, D.C.; Lee, S.W. An All-Nanofiber-Based Substrate-Less, Extremely Conformal, and Breathable Organic Field Effect Transistor for Biomedical Applications. Adv. Funct. Mater. 2022, 32, 2204645. [Google Scholar] [CrossRef]
- Shi, S.; Si, Y.F.; Han, Y.T.; Wu, T.; Irfan Iqbal, M.; Fei, B.; Li, R.K.Y.; Hu, J.L.; Qu, J.P. Non-Toxic Crosslinking of Electrospun Gelatin Nanofibers for Tissue Engineering and Biomedicine—A Review. Adv. Mater. 2022, 34, 2107938. [Google Scholar] [CrossRef]
- Kim, H.K.; Chung, H.J.; Park, T.G. Biodegradable polymeric microspheres with “open/closed” pores for sustained release of human growth hormone. J. Control. Release 2006, 112, 167–174. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kang, J.H.; Shen, B.W.; Wang, Y.Q.; He, Y.; Lee, M.S. Open–closed switching of synthetic tubular pores. Nat. Commun. 2015, 6, 8650. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, X.P. Thermal Conductivity Analysis of High Porosity Structures with Open and Closed Pores. Int. J. Heat Mass Transf. 2022, 183A, 122089. [Google Scholar] [CrossRef]
- Yang, J.; Shi, G.X.; Bei, J.Z.; Wang, S.G.; Cao, Y.L.; Shang, Q.X.; Yang, G.H.; Wang, W.J. Fabrication and surface modification of macroporous poly(L-lactic acid) and poly(L-lactic-co-glycolic acid) (70/30) cell scaffolds for human skin fibroblast cell culture. J. Biomed. Mater. Res. 2002, 62, 438–446. [Google Scholar] [CrossRef]
- Pati, F.; Adhikari, B.; Dhara, S. Development of chitosan-tripolyphosphate non-woven fibrous scaffolds for tissue engineering application. J. Mater. Sci. Mater. Med. 2012, 23, 1085–1096. [Google Scholar] [CrossRef]
- Safari, S.; Ehsani, M.; Zandi, M. Stimuli-responsive electrospun nanofibers based on PNVCL-PVAc copolymer in biomedical applications. Prog. Biomater. 2021, 10, 245–258. [Google Scholar] [CrossRef]
- Kahdim, Q.S.; Abdelmoula, N.; Al-Karagoly, H.; Albukhaty, S.; Al-Saaidi, J. Fabrication of a Polycaprolactone/Chitosan Nano fibrous Scaffold Loaded with Nigella sativa Extract for Biomedical Applications. BioTech 2023, 12, 19. [Google Scholar] [CrossRef]
- Wang, N.; Yang, Y.J.; Al-Deyab, S.S.; El-Newehy, M.; Yu, J.Y.; Ding, B. Ultra-light 3D nanofibre-nets binary structured nylon 6–polyacrylonitrile membranes for efficient filtration of fine particulate matter. J. Mater. Chem. A 2015, 3, 23946–23954. [Google Scholar] [CrossRef]
- Chen, Y.J.; Mensah, A.; Wang, Q.Q.; Li, D.W.; Qiu, Y.Y.; Wie, Q.F. Hierarchical porous nanofibers containing thymol/beta-cyclodextrin: Physico-chemical characterization and potential biomedical applications. Mater. Sci. Eng. C 2020, 115, 111155. [Google Scholar] [CrossRef]
- Salehi, M.; Niyakan, M.; Ehterami, A.; Haghi-Daredeh, S.; Nazarnezhad, S.; Abbaszadeh-Goudarzi, G.; Vaez, A.; Hashemi, S.F.; Rezaei, N.; Mousavi, S.R. Porous electrospun poly(ε-caprolactone)/gelatin nanofibrous mat containing cinnamon for wound healing application: In vitro and in vivo study. Biomed. Eng. Lett. 2020, 10, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Ghaee, A.; Bagheri-Khoulenjani, S.; Afshar, H.A.; Bogheiri, H. Biomimetic nanocomposite scaffolds based on surface modified PCL-nanofibers containing curcumin embedded in chitosan/gelatin for skin regeneration. Comp. B Eng. 2019, 177, 107339. [Google Scholar] [CrossRef]
- Esmaeili, E.; Eslami-Arshaghi, T.; Hosseinzadeh, S.; Elahirad, E.; Jamalpoor, Z.; Hatamie, S.; Soleimani, M. The biomedical potential of cellulose acetate/polyurethane nanofibrous mats containing reduced graphene oxide/silver nanocomposites and curcumin: Antimicrobial performance and cutaneous wound healing. Int. J. Biol. Macromol. 2020, 152, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, T.H.; Hua, W.K.; Li, P.Y.; Wang, X.F. 3D Porous poly(lactic acid)/regenerated cellulose composite scaffolds based on electrospun nanofibers for biomineralization. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124048. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Mansour, S.F.; Al-Wafi, R.; Abdel-Fattah, E. Nanofibers scaffolds of co-doped Bi/Sr-hydroxyapatite encapsulated into polycaprolactone for biomedical applications. J. Mater. Res. Technol. 2021, 13, 2297–2309. [Google Scholar] [CrossRef]
- Tamari, S. Optimum design of the constant-volume gas pycnometer for determining the volume of solid particles. Meas. Sci. Technol. 2004, 15, 549. [Google Scholar] [CrossRef]
- Khataei, S.; Al-Musawi, M.H.; Asadi, K.; Ramezani, S.; Abbasian, M.; Ghorbani, M. Effect of molecular weight and content of polyvinylpyrrolidone on cell proliferation, loading capacity and properties of electrospun green tea essential oil-incorporated polyamide-6/polyvinylpyrrolidone nanofibers. J. Drug Deliv. Sci. Technol. 2023, 82, 104310. [Google Scholar] [CrossRef]
- Zadeh, Z.E.; Solouk, A.; Shafieian, M.; Nazarpak, M.H. Electrospun polyurethane/carbon nanotube composites with different amounts of carbon nanotubes and almost the same fiber diameter for biomedical applications. Mater. Sci. Eng. C 2021, 118, 111403. [Google Scholar] [CrossRef]
- He, W.; Ma, Z.W.; Yong, T.; Teo, W.E.; Ramakrishna, S. Fabrication of collagen-coated biodegradable polymer nanofiber mesh and its potential for endothelial cells growth. Biomaterials 2005, 26, 7606–7615. [Google Scholar] [CrossRef]
- Hotaling, N.A.; Bharti, K.; Kriel, H.; Simon, C.G., Jr. DiameterJ: A validated open source nanofiber diameter measurement tool. Biomaterials 2015, 61, 327–338. [Google Scholar] [CrossRef]
- Bouchet, M.; Gauthier, M.; Maire, M.; Ajji, A.; Lerouge, S. Towards compliant small-diameter vascular grafts: Predictive analytical model and experiments. Mater. Sci. Eng. C 2019, 100, 715–723. [Google Scholar] [CrossRef]
- Nejad, M.R.; Yousefzadeh, M.; Solouk, A. Electrospun PET/PCL small diameter nanofibrous conduit for biomedical application. Mater. Sci. Eng. C 2020, 110, 110692. [Google Scholar] [CrossRef]
- Liu, Y.; Chaparro, F.J.; Gray, Z.; Gaumer, J.; Cybyk, D.B.; Ross, L.; Gosser, J.; Tian, Z.; Jia, Y.; Dull, T.; et al. 3D reconstruction of bias effects on porosity, alignment and mesoscale structure in electrospun tubular polycaprolactone. Polymer 2021, 232, 124120. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Chaparro, F.J.; Tian, Z.T.; Jia, Y.Z.; Gosser, J.; Gaumer, J.; Ross, L.; Tafreshi, H.; Lannutti, J.J. Visualization of porosity and pore size gradients in electrospun scaffolds using laser metrology. PLoS ONE 2023, 18, e0282903. [Google Scholar] [CrossRef]
- Agueda, J.R.S.; Madrid, J.; Mondragon, J.M.; Lim, J.; Tan, A.; Wang, I.; Duguran, N.; Bondoc, A. Synthesis and Characterization of Electrospun Polyvinylidene Fluoride-based (PVDF) Scaffolds for Renal Bioengineering. J. Phys. Conf. Ser. 2021, 2071, 012005. [Google Scholar] [CrossRef]
- Liu, W.Y.; Walker, G.; Price, S.; Yang, X.D.; Li, J.; Bunt, C. Electrospun Membranes as a Porous Barrier for Molecular Transport: Membrane Characterization and Release Assessment. Pharmaceutics 2021, 13, 916. [Google Scholar] [CrossRef]
- Tahami, S.R.; Nemati, N.H.; Keshvari, H.; Khorasani, M.T. Effect of Electrical Potential on the Morphology of Polyvinyl Alcohol/ Sodium Alginate Electrospun Nanofibers, Containing Herbal Extracts of Calendula Officinalis for Using in Biomedical Applications. J. Mod. Process. Manuf. Prod. 2020, 9, 43–46. [Google Scholar]
- Stella, S.M.; Sridhar, T.M.; Ramprasath, R.; Gimbun, J.; Vijayalakshmi, U. Physio-Chemical and Biological Characterization of Novel HPC (Hydroxypropylcellulose):HAP (Hydroxyapatite):PLA (Poly Lactic Acid) Electrospun Nanofibers as Implantable Material for Bone Regenerative Application. Polymers 2023, 15, 155. [Google Scholar] [CrossRef]
- Zhang, Q.C.; Rudolph, T.; Benitez, A.J.; Gould, O.E.C.; Behl, M.; Kratz, K.; Lendlein, A. Temperature-controlled reversible pore size change of electrospun fibrous shape-memory polymer actuator based meshes. Smart Mater. Struct. 2019, 28, 055037. [Google Scholar] [CrossRef]
- Havlícek, K.; Svobodová, L.; Bakalova, T.; Lederer, T. Influence of electrospinning methods on characteristics of polyvinyl butyral and polyurethane nanofibres essential for biological applications. Mater. Des. 2020, 194, 108898. [Google Scholar] [CrossRef]
- Krysiak, Z.J.; Szewczyk, P.K.; Berniak, K.; Sroczyk, E.A.; Boratyn, E.; Stachewicz, U. Stretchable skin hydrating PVB patches with controlled pores’ size and shape for deliberate evening primrose oil spreading, transport and release. Biomater. Adv. 2022, 136, 212786. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Jia, Z.H.; Shafiq, M.; Xie, X.R.; Xiao, X.H.; Castro, R.; Rodrigues, J.; Wu, J.L.; Zhou, G.D.; Mo, X.M. Gas foaming of electrospun poly(L-lactide-co-caprolactone)/silk fibroin nanofiber scaffolds to promote cellular infiltration and tissue regeneration. Coll. Surf. B Biointerfaces 2021, 201, 111637. [Google Scholar] [CrossRef] [PubMed]
- McLaren, R.L.; Laycock, C.J.; Brousseau, E.; Owen, G.R. Examining slit pore widths within plasma-exfoliated graphitic material utilising Barrett–Joyner–Halenda analysis. New J. Chem. 2021, 45, 12071–12080. [Google Scholar] [CrossRef]
- Yang, H.M.; Song, X.L.; Zhang, X.C.; Ao, W.Q.; Qiu, G.H. Synthesis of vanadium-doped SnO2 nanoparticles by chemical co-precipitation method. Mater. Lett. 2003, 57, 3124–3127. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Absorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Kim, C.-Y.; Lee, J.-K.; Kim, B.-I. Synthesis and pore analysis of aerogel–glass fiber composites by ambient drying method. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313–314, 179–182. [Google Scholar] [CrossRef]
- Bazzi, M.; Shabani, I.; Mohandesi, J.A. Enhanced mechanical properties and electrical conductivity of Chitosan/Polyvinyl Alcohol electrospun nanofibers by incorporation of graphene nanoplatelets. J. Mech. Behav. Biomed. Mater. 2022, 125, 104975. [Google Scholar] [CrossRef]
- Choma, J.; Jaroniec, M.; Burakiewicz-Mortka, W.; Kloske, M. Critical appraisal of classical methods for determination of meso pore size distributions of MCM-41 materials. Appl. Surf. Sci. 2002, 196, 216–223. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Yan, D.; Zhao, L.F.; Lin, J.Y. Composite fibrous membrane comprising PLA and PCL fibers for biomedical application. Compos. Commun. 2022, 34, 101268. [Google Scholar] [CrossRef]
- Peinador, R.I.; Calvo, J.I.; Aim, R.B. Comparison of Capillary Flow Porometry (CFP) and Liquid Extrusion Porometry (LEP) Techniques for the Characterization of Porous and Face Mask Membranes. Appl. Sci. 2020, 10, 5703. [Google Scholar] [CrossRef]
- Jena, A.; Gupta, K. Pore Volume of Nanofiber Nonwovens. Int. Nonwovens J. 2005, 2. [Google Scholar] [CrossRef]
- Yunok, T.; Matsumoto, K.; Nakamura, K. Pore Size Distribution Measurements of Nonwoven Fibrous Filter by Differential Flow Method. Membrane 2004, 29, 227–235. [Google Scholar] [CrossRef]
- Fatema, N.; Bhatia, S.K. Comparisons between geotextile pore sizes obtained from capillary flow and dry sieving tests. Geotech. Test. J. 2019, 43, 853–876. [Google Scholar] [CrossRef]
- Kolb, H.E.; Schmitt, R.; Dittler, A.; Kasper, G. On the accuracy of capillary flow porometry for fibrous filter media. Sep. Purif. Technol. 2018, 199, 198–205. [Google Scholar] [CrossRef]
- He, X.; Wang, Y.-n.; Zhou, J.F.; Wang, H.B.; Ding, W.; Shi, B. Suitability of Pore Measurement Methods for Characterizing the Hierarchical Pore Structure of Leather. J. Am. Leather Chem. Assoc. 2019, 114, 41–47. [Google Scholar]
- Liu, Y.; Lannutti, J.J. Characterization of electrospun porosities: Current techniques. In Proceedings of the Nanofiber, Applications and Related Technologies NART 2021, Istanbul, Turkey, 8–10 September 2021; pp. 54–63. [Google Scholar]
- Appell, M.; Jackson, M.A. Applications of Nanoporous Materials in Agriculture. Adv. Appl. Nanotechnol. Agric. 2013, 1143, 167–176. [Google Scholar]
- Orsolini, P.; Michen, B.; Huch, A.; Tingaut, P.; Caseri, W.R.; Zimmermann, T. Characterization of Pores in Dense Nanopapers and Nanofibrillated Cellulose Membranes: A Critical Assessment of Established Methods. ACS Appl. Mater. Interfaces 2015, 7, 25884–25897. [Google Scholar] [CrossRef]
- Raja, I.S.; Fathima, N.N. Gelatin–Cerium Oxide Nanocomposite for Enhanced Excisional Wound Healing. ACS Appl. Bio Mater. 2018, 1, 487–495. [Google Scholar] [CrossRef]
- Hao, J.J.; Lu, C.X.; Zhou, P.C.; Li, D.H. Pore structure development of polyacrylonitrile nascent fibers in water stretching process. Thermochim. Acta 2013, 569, 42–47. [Google Scholar] [CrossRef]
- Kanungo, I.; Fathima, N.N.; Rao, J.R.; Nair, B.U. Influence of PCL on the material properties of collagen based biocomposites and in vitro evaluation of drug release. Mater. Sci. Eng. C 2013, 33, 4651–4659. [Google Scholar] [CrossRef]
- Landry, M.R. Thermoporometry by differential scanning calorimetry: Experimental considerations and applications. Thermochim. Acta 2005, 433, 27–50. [Google Scholar] [CrossRef]
- Ishikiriyama, K.; Todoki, M. Evaluation of water in silica pores using differential scanning calorimetry. Thermochim. Acta 1995, 256, 213–226. [Google Scholar] [CrossRef]
- Abolhasani, M.M.; Naebe, M.; Amiri, M.H.; Shirvanimoghaddam, K.; Anwar, S.; Michels, J.J.; Asadi, K. Hierarchically Structured Porous Piezoelectric Polymer Nanofibers for Energy Harvesting. Adv. Sci. 2020, 7, 2000517. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, S.; Westermann, F.; Hanrieder, T.; Jung, L.; Ruppach, H.; Mihranyan, A. Comparative Analysis of Dry and Wet Porometry Methods for Characterization of Regular and Cross-Linked Virus Removal Filter Papers. Membranes 2019, 9, 1. [Google Scholar] [CrossRef]
- Fashandi, H.; Karimi, M. Characterization of porosity of polystyrene fibers electrospun at humid atmosphere. Thermochim. Acta 2012, 547, 38–46. [Google Scholar] [CrossRef]
- Balasubramaniam, B.; Kumar, S.A.; Singh, K.A.; Bhunia, S.; Verma, K.; Tian, L.M.; Gupta, R.K.; Gaharwar, A.K. Electrically Conductive MoS2 Reinforced Polyacrylonitrile Nanofibers for Biomedical Applications. Adv. NanoBiomed Res. 2022, 2, 2100105. [Google Scholar] [CrossRef]
- Jalalah, M.; Ahmad, A.; Saleem, A.; Bilal Qadir, M.; Khaliq, Z.; Khan, M.Q.; Nazir, A.; Faisal, M.; Alsaiari, M.; Irfan, M.; et al. Electrospun Nanofiber/Textile Supported Composite Membranes with Improved Mechanical Performance for Biomedical Applications. Membranes 2022, 12, 1158. [Google Scholar] [CrossRef]
- Chen, Y.J.; Qiu, Y.Y.; Chen, W.B.F.; Wei, Q.F. Electrospun thymol-loaded porous cellulose acetate fibers with potential biomedical applications. Mater. Sci. Eng. C 2020, 109, 110536. [Google Scholar] [CrossRef]
- Chen, S.; Shen, L.L.; Huang, D.; Du, J.; Fan, X.X.; Wie, A.L.; Chen, W.Y. Facile synthesis, microstructure, formation mechanism, in vitro biocompatibility, and drug delivery property of novel dendritic TiO2 nanofibers with ultrahigh surface area. Mater. Sci. Eng. C 2020, 115, 111100. [Google Scholar] [CrossRef]
- Cheng, H.; Li, X.N.; Li, T.H.; Qin, D.F.; Tang, T.F.; Li, Y.P.; Wang, G.X. Electrospun Nanofibers with High Specific Surface Area to Prepare Modified Electrodes for Electrochemiluminescence Detection of Azithromycin. J. Nanomater. 2021, 2021, 9961663. [Google Scholar] [CrossRef]
- Li, W.Y.; Chao, S.; Li, Y.M.; Bai, F.Q.; Teng, Y.K.; Li, X.; Li, L.J.; Wang, C. Dual-layered composite nanofiber membrane with Cu-BTC-modified electrospun nanofibers and biopolymeric nanofibers for the removal of uremic toxins and its application in hemodialysis. J. Membr. Sci. 2022, 642, 119964. [Google Scholar] [CrossRef]
- Arabpour, Z.; Baradaran-Rafii, A.; Bakhshaiesh, N.L.; Ai, J.; Ebrahimi-Barough, S.; Malekabadi, H.E.; Nazeri, N.; Vaez, A.; Salehi, M.; Sefat, F.; et al. Design and characterization of biodegradable multi layered electrospun nanofibers for corneal tissue engineering applications. J. Biomed. Mater. Res. 2019, 107, 2340–2349. [Google Scholar] [CrossRef]
- Lim, S.K.; Hwang, S.-H.; Chang, D.I.; Kim, S.H. Preparation of mesoporous In2O3 nanofibers by electrospinning and their application as a CO gas sensor. Sens. Actuators B Chem. 2010, 149, 28–33. [Google Scholar] [CrossRef]
- Prajapati, Y.N.; Verma, N. Adsorptive desulfurization of diesel oil using nickel nanoparticle-doped activated carbon beads with/without carbon nanofibers: Effects of adsorbate size and adsorbent texture. Fuel 2017, 189, 186–194. [Google Scholar] [CrossRef]
- Othman, F.E.C.; Yusof, N.; Petru, M.; Md Nordin, N.A.H.; Hamid, M.F.; Ismail, A.F.; Rushdan, A.I.; Hassan, S.A. Polyethy leneimine-impregnated activated carbon nanofiber composited graphene-derived rice husk char for efficient post-combustion CO2 capture. Nanotechnol. Rev. 2022, 11, 926–944. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Filimon, A.; Olaru, N.; Doroftei, F.; Coroaba, A.; Dunca, S. Processing of quaternized polysulfones solutions as tool in design of electrospun nanofibers: Microstructural characteristics and antimicrobial activity. J. Mol. Liq. 2021, 330, 115664. [Google Scholar] [CrossRef]
- Filimon, A.; Stoica, I.; Onofrei, M.D.; Bargan, A.; Dunca, S. Quaternized polysulfones-based blends: Surface properties and performance in life quality and environmental applications. Polym. Test. 2018, 71, 285–295. [Google Scholar] [CrossRef]
- Chaiarwut, S.; Ekabutr, P.; Chuysinuan, P.; Chanamuangkon, T.; Supaphol, P. Surface immobilization of PCL electrospun nanofibers with pexiganan for wound dressing. J. Polym. Res. 2021, 28, 344. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Maio, A.; Botta, L.; Rigogliuso, S.; Ghersi, G. Electrospun PCL/GO-g-PEG structures: Processing-morphology-properties relationships. Comp. A Appl. Sci. Manuf. 2017, 92, 97–107. [Google Scholar] [CrossRef]
- Jia, X.W.; Qin, Z.Y.; Xu, J.X.; Kong, B.H.; Liu, Q.; Wang, H. Preparation and characterization of pea protein isolate-pullulan blend electrospun nanofiber films. Int. J. Biol. Macromol. 2020, 157, 641–647. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.; Saldana, L.; McGoldrick, D.; John, J.V.; Kuss, M.; Chen, S.X.; Duan, B.; Carlson, M.A.; Xie, J.W. Large-scale synthesis of compressible and re-expandable three-dimensional nanofiber matrices. Nano Sel. 2021, 2, 1566–1579. [Google Scholar] [CrossRef]
- Dorati, R.; Chiesa, E.; Pisani, S.; Genta, I.; Modena, T.; Bruni, G.; Brambilla, C.R.M.; Benazzo, M.; Conti, B. The Effect of Process Parameters on Alignment of Tubular Electrospun Nanofibers for Tissue Regeneration Purposes. J. Drug Deliv. Sci. Technol. 2020, 58, 101781. [Google Scholar] [CrossRef]
- Murphy, R.; Turcott, A.; Banuelos, L.; Dowey, E.; Goodwin, B.; O’Halloran Cardinal, K. SIMPoly: A Matlab-Based Image Analysis Tool to Measure Electrospun Polymer Scaffold Fiber Diameter. Tissue Eng. C Methods 2020, 26, 628–636. [Google Scholar] [CrossRef]
- Götz, A.; Senz, V.; Schmidt, W.; Huling, J.; Grabow, N.; Illner, S. General image fiber tool: A concept for automated evaluation of fiber diameters in SEM images. Measurement 2021, 177, 109265. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Q.; Shen, J.; Ding, X.B.; Liu, T.; He, J.H.; Zhu, C.Y.; Zhao, D.; Zhu, J.D. Multifunctional Fibroblasts Enhanced via Thermal and Freeze-Drying Post-treatments of Aligned Electrospun Nanofiber Membranes. Adv. Fiber Mater. 2021, 3, 26–37. [Google Scholar] [CrossRef]
- Cai, Z.J.; Xiong, P.; He, S.Q.; Zhu, C. Improved piezoelectric performances of highly orientated poly(β-hydroxybutyrate) electrospun nanofiber membrane scaffold blended with multiwalled carbon nanotubes. Mater. Lett. 2019, 240, 213–216. [Google Scholar] [CrossRef]
- Hellert, C.; Wortmann, M.; Frese, N.; Grötsch, G.; Cornelißen, C.; Ehrmann, A. Adhesion of Electrospun Poly(acrylonitrile) Nanofibers on Conductive and Isolating Foil Substrates. Coatings 2021, 11, 249. [Google Scholar] [CrossRef]
- Storck, J.L.; Grothe, T.; Mamun, A.; Sabantina, L.; Klöcker, M.; Blachowicz, T.; Ehrmann, A. Orientation of electrospun magnetic nanofibers near conductive areas. Materials 2020, 13, 47. [Google Scholar] [CrossRef]
- Bazrafshan, Z.; Stylios, G.K. Custom-built electrostatics and supplementary bonding in the design of reinforced Collagen-g-P (methyl methacrylate-co-ethyl acrylate)/nylon 66 core-shell fibers. J. Mech. Behav. Biomed. Mater. 2018, 87, 19–29. [Google Scholar] [CrossRef]
- He, H.J.; Wang, Y.M.; Farkas, B.; Nagy, Z.K.; Molnar, K. Analysis and prediction of the diameter and orientation of AC electrospun nanofibers by response surface methodology. Mater. Des. 2020, 194, 108902. [Google Scholar] [CrossRef]
- Shahverdi, F.; Barati, A.; Salehi, E.; Arjomandzadegan, M. Biaxial electrospun nanofibers based on chitosan-poly (vinyl alcohol) and poly (ε-caprolactone) modified with CeAlO3 nanoparticles as potential wound dressing materials. Int. J. Biol. Macromol. 2022, 221, 736–750. [Google Scholar] [CrossRef]
- El-Morsy, M.A.; Afifi, M.; Ahmed, M.K.; Awwad, N.S.; Ibrahium, H.A.; Alqahtani, M.S. Electrospun nanofibrous scaffolds of polycaprolactone containing binary ions of Pd/vanadate doped hydroxyapatite for biomedical applications. J. Drug Deliv. Sci. Technol. 2022, 70, 103153. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Shalaby, E.S.; Abd-Al-Aleem, A.H.; Abu-Saied, M.A.; Youssef, A.M. Synthesis of environmentally benign antimicrobial dressing nanofibers based on polycaprolactone blended with gold nanoparticles and spearmint oil nanoemulsion. J. Mater. Res. Technol. 2021, 15, 3447–3460. [Google Scholar] [CrossRef]
- Teaima, M.H.; Abdelnaby, F.A.; Fadel, M.; El-Nabarawi, M.A.; Shoueir, K.R. Synthesis of Biocompatible and Environmentally Nanofibrous Mats Loaded with Moxifloxacin as a Model Drug for Biomedical Applications. Pharmaceutics 2020, 12, 1029. [Google Scholar] [CrossRef]
- Sambaer, W.; Zatloukal, M.; Kimmer, D. 3D air filtration modeling for nanofiber based filters in the ultrafine particle size range. Chem. Eng. Sci. 2012, 82, 299–311. [Google Scholar] [CrossRef]
- Joshi, J.; Homburg, S.V.; Ehrmann, A. Atomic force microscopy (AFM) on biopolymers and hydrogels for biotechnological applications—Possibilities and limits. Polymers 2022, 14, 1267. [Google Scholar] [CrossRef]
- Beigmoradi, R.; Samimi, A.; Mohebbi-Kalhori, D. Controllability of the hydrophilic or hydrophobic behavior of the modified polysulfone electrospun nanofiber mats. Polym. Test. 2021, 93, 106970. [Google Scholar] [CrossRef]
- Sharma, D.; Dhingra, S.; Banerjee, A.; Saha, S.; Bhattacharyya, J.; Satapathy, B.K. Designing suture-proof cell-attachable copolymer-mediated and curcumin- β-cyclodextrin inclusion complex loaded aliphatic polyester-based electrospun antibacterial constructs. Int. J. Biol. Macromol. 2022, 216, 397–413. [Google Scholar] [CrossRef]
- Arumugam, M.; Murugesan, B.; Sivakumar, P.M.; Pandiyan, N.; Chinnalagu, D.K.; Rangasamy, G.; Mahalingam, S. Electrospun silk fibroin and gelatin blended nanofibers functionalized with noble metal nanoparticles for enhanced biomedical applications. Process Biochem. 2023, 124, 221–234. [Google Scholar] [CrossRef]
- Kichi, M.K.; Torkaman, R.; Mohammadi, H.; Toutounchi, A.; Kharaziha, M.; Alihosseini, F. Electrochemical and in vitro bioactivity behavior of poly (ε-caprolactone) (PCL)-gelatin-forsterite nano coating on titanium for biomedical application. Mater. Today Commun. 2020, 24, 101326. [Google Scholar] [CrossRef]
- Drobota, M.; Gradinaru, L.M.; Vlad, S.; Bargan, A.; Butnaru, M.; Angheloiu, M.; Afori, M. Preparation and Characterization of Electrospun Collagen Based Composites for Biomedical Applications. Materials 2020, 13, 3961. [Google Scholar] [CrossRef] [PubMed]
- Lasenko, I.; Sanchaniya, J.V.; Kanukuntla, S.P.; Ladani, Y.; Viluma-Gudmona, A.; Kononova, O.; Lusis, V.; Tipans, I.; Selga, T. The Mechanical Properties of Nanocomposites Reinforced with PA6 Electrospun Nanofibers. Polymers 2023, 15, 673. [Google Scholar] [CrossRef] [PubMed]
- Pakolpakcil, A.; Draczynski, Z.; Szulc, J.; Stawski, D.; Tarzynska, N.; Bednarowicz, A.; Sikorski, D.; Hernandez, C.; Sztajnowski, S.; Krucinska, I.; et al. An In Vitro Study of Antibacterial Properties of Electrospun Hypericum perforatum Oil-Loaded Poly(lactic Acid) Nonwovens for Potential Biomedical Applications. Appl. Sci. 2021, 11, 8219. [Google Scholar] [CrossRef]
- Ryu, H.I.; Koo, M.S.; Kim, S.J.; Kim, S.K.; Park, Y.-A.; Park, S.M. Uniform-thickness electrospun nanofiber mat production system based on real-time thickness measurement. Sci. Rep. 2020, 10, 20847. [Google Scholar] [CrossRef]
- Adhikari, U.; An, X.X.; Rijal, N.; Hopkins, T.; Khanal, S.; Chavez, T.; Tatu, R.; Sankar, J.; Little, K.J.; Horn, D.B.; et al. Embedding magnesium metallic particles in polycaprolactone nanofiber mesh improves applicability for biomedical applications. Acta Biomater. 2019, 98, 215–234. [Google Scholar] [CrossRef]
- Sordini, L.; Silva, J.C.; Garrudo, F.F.F.; Rodrigues, C.A.V.; Marques, A.C.; Linhardt, R.J.; Cabral, J.M.S.; Morgado, J.; Castelo Ferreira, F. PEDOT:PSS-Coated Polybenzimidazole Electroconductive Nanofibers for Biomedical Applications. Polymers 2021, 13, 2786. [Google Scholar] [CrossRef]
- Zarei, M.; Samimi, A.; Khorram, M.; Abdi, M.M.; Golestaneh, S.I. Fabrication and characterization of conductive polypyrrole/chitosan/collagen electrospun nanofiber scaffold for tissue engineering application. Int. J. Biol. Macromol. 2021, 168, 175–186. [Google Scholar] [CrossRef]
- Conte, A.A.; Sun, K.; Hu, X.; Beachley, V.Z. Effects of Fiber Density and Strain Rate on the Mechanical Properties of Electrospun Polycaprolactone Nanofiber Mats. Front. Chem. 2020, 8, 610. [Google Scholar] [CrossRef]
- Mozaffari, A.; Gashti, M.P. Air Plasma Functionalization of Electrospun Nanofibers for Skin Tissue Engineering. Biomedicines 2022, 10, 617. [Google Scholar] [CrossRef]
- Chen, S.X.; John, J.V.; McCarthy, A.; Carlson, M.A.; Li, X.W.; Xie, J.W. Fast transformation of 2D nanofiber membranes into pre-molded 3D scaffolds with biomimetic and oriented porous structure for biomedical applications. Appl. Phys. Rev. 2020, 7, 021406. [Google Scholar] [CrossRef]
- Movahedi, M.; Salehi, A.O.M.; Hajipour, F.P.; Etemad, S. Casein release and characterization of electrospun nanofibres for cartilage tissue engineering. Bull. Mater. Sci. 2022, 45, 76. [Google Scholar] [CrossRef]
- Karim, A.M.; Kavehpour, H.P. Effect of viscous force on dynamic contact angle measurement using Wilhelmy plate method. Colloids Surf. A Physicochem. Eng. Asp. 2018, 548, 54–60. [Google Scholar] [CrossRef]
- Zefirov, V.V.; Lubimtsev, N.A.; Stakhanov, A.I.; Elmanovich, I.V.; Kondratenko, M.S.; Lokshin, B.V.; Gallyamov, M.O.; Khokhlov, A.R. Durable crosslinked omniphobic coatings on textiles via supercritical carbon dioxide deposition. J. Supercrit. Fluids 2018, 133, 30–37. [Google Scholar] [CrossRef]
- Mahltig, B.; Fischer, A. Inorganic/organic polymer coatings for textiles to realize water repellent and antimicrobial properties—A study with respect to textile comfort. J. Polym. Sci. B Polym. Phys. 2010, 48, 1562–1568. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chen, X.Q.; Xin, J.H. Hydrophobic duck feathers and their simulation on textile substrates for water repellent treatment. Bioinspiration Biomim. 2008, 3, 046007. [Google Scholar] [CrossRef]
- Gashti, M.P.; Dehdast, S.A.; Berenjian, A.; Shabani, M.; Zarinabadi, E.; Fard, G.C. PDDA/Honey Antibacterial Nanofiber Composites for Diabetic Wound-Healing: Preparation, Characterization, and In Vivo Studies. Gels 2023, 9, 173. [Google Scholar] [CrossRef]
- Merin, D.D.; Jose, R.A.; Arulananth, T.S.; Sundarraj, A.A.; Inbamalar, T.M.; Meharie, M.G. Nanoclay-Incorporated Polycaprolactone Matrix via Electrospinning Techniques-Enriched Spectroscopic Responses. J. Nanomater. 2023, 2023, 1194158. [Google Scholar]
- Serbezeanu, D.; Vlad-Bubulac, T.; Rusu, D.; Gradisteanu Pircalabioru, G.; Samoila, I.; Dinescu, S.; Aflori, M. Functional Polyimide-Based Electrospun Fibers for Biomedical Application. Materials 2019, 12, 3201. [Google Scholar] [CrossRef]
- Sazegar, M.; Bazgir, S.; Katbab, A.A. Preparation and characterization of water-absorbing gas-assisted electrospun nanofibers based on poly(vinyl alcohol)/chitosan. Mater. Today Commun. 2020, 25, 101489. [Google Scholar] [CrossRef]
- Abdolbaghian, H.; Bazgir, S. Fabrication and characterization of gas-assisted core-shell hydrogel nanofibers as a drug release system with antibacterial activity. Eur. Polym. J. 2022, 174, 111302. [Google Scholar] [CrossRef]
- Munawar, M.A.; Schubert, D.W. Revealing Electrical and Mechanical Performances of Highly Oriented Electrospun Conductive Nanofibers of Biopolymers with Tunable Diameter. Int. J. Mol. Sci. 2021, 22, 10295. [Google Scholar] [CrossRef] [PubMed]
- Pedrotty, D.M.; Koh, J.; Davis, B.H.; Taylor, D.A.; Wolf, P.; Niklason, L.E. Engineering skeletal myoblasts: Roles of threedimensional culture and electrical stimulation. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1620–H1626. [Google Scholar] [CrossRef] [PubMed]
- Schwarz-Pfeiffer, A.; Obermann, M.; Weber, M.O.; Ehrmann, A. Smarten up garments through knitting. IOP Conf. Ser. Mater. Sci. Eng. 2016, 141, 012008. [Google Scholar] [CrossRef]
- Tyurin, I.N.; Getmantseva, V.V.; Andreeva, E.G. Van der Pauw Method for Measuring the Electrical Conductivity of Smart Textiles. Fibre Chem. 2019, 51, 139–146. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, G.; Ehrmann, A. Recent Developments in Additive Manufacturing of Conductive Polymer Composites. Macromol. Mater. Eng. 2023; 2200692, early view. [Google Scholar] [CrossRef]
- Simsek, M.; von Kruechten, L.; Buchner, M.; Duerkop, A.; Baeumner, A.J.; Wongkaew, N. An efficient post-doping strategy creating electrospun conductive nanofibers with multi-functionalities for biomedical applications. J. Mater. Chem. C 2019, 7, 9316–9325. [Google Scholar] [CrossRef]
- Archana, D.; Dutta, J.; Dutta, P.K. Evaluation of chitosan nano dressing for wound healing: Characterization, in vitro and in vivo studies. Int. J. Biol. Macromol. 2013, 57, 193–203. [Google Scholar] [CrossRef]
- Mi, F.-L.; Shyu, S.-S.; Wu, Y.-B.; Lee, S.-T.; Shyong, J.-Y.; Huang, R.-N. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials 2001, 22, 165–173. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Wang, S.; Zhang, X.; Yu, J.; Wang, C. Mussel-inspired polydopamine-assisted bromelain immobilization onto electrospun fibrous membrane for potential application as wound dressing. Mater. Sci. Eng. C. Mater. Biol. Appl. 2020, 110, 110624. [Google Scholar] [CrossRef]
- Zhong, G.F.; Qiu, M.Y.; Zhang, J.B.; Jiang, F.C.; Yue, X.; Huang, C.; Zhao, S.Y.; Zeng, R.; Zhang, C.; Qu, Y. Fabrication and characterization of PVA@PLA electrospinning nanofibers embedded with Bletilla striata polysaccharide and Rosmarinic acid to promote wound healing. Int. J. Biol. Macromol. 2023, 234, 123693. [Google Scholar] [CrossRef]
- Gu, S.-Y.; Wang, Z.-M.; Ren, J.; Zhang, C.-Y. Electrospinning of gelatin and gelatin/poly(l-lactide) blend and its characteristics for wound dressing. Mater. Sci. Eng. C 2009, 29, 1822–1828. [Google Scholar] [CrossRef]
- Naseri-Nosar, M.; Farzamfar, S.; Sahrapeyma, H.; Ghorbani, S.; Bastami, F.; Vaez, A.; Salehi, M. Cerium oxide nanoparticle-containing poly (ε-caprolactone)/gelatin electrospun film as a potential wound dressing material: In vitro and in vivo evaluation. Mater. Sci. Eng. C 2017, 81, 366–372. [Google Scholar] [CrossRef]
- Samadian, H.; Zamiri, S.; Ehterami, A.; Farzamfar, S.; Vaez, A.; Khastar, H.; Alam, M.; Ai, A.; Derakhshankhah, H.; Allahyari, Z.; et al. Electrospun cellulose acetate/gelatin nanofibrous wound dressing containing berberine for diabetic foot ulcer healing: In vitro and in vivo studies. Sci. Rep. 2020, 10, 8312. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, Y.W.; Shi, X.Q.; Xie, Z.G.; Hu, J.L.; Liu, Y.C. Effects of preparation parameters on the properties of the crosslinked pectin nanofiber mats. Carbohydr. Polym. 2021, 269, 118314. [Google Scholar] [CrossRef]
- Salemi, M.S.; Bahrami, G.; Arkan, E.; Izadi, Z.; Miraghaee, S.; Samadian, H. Co-electrospun nanofibrous mats loaded with bitter gourd (Momordica charantia) extract as the wound dressing materials: In vitro and in vivo study. BMC Complement. Med. Ther. 2021, 21, 111. [Google Scholar] [CrossRef]
- Xia, Y.; He, L.F.; Feng, J.D.; Xu, S.J.; Yao, L.R.; Pan, G.W. Waterproof and Moisture-Permeable Polyurethane Nanofiber Membrane with High Strength, Launderability, and Durable Antimicrobial Properties. Nanomaterials 2022, 12, 1813. [Google Scholar] [CrossRef]
- Mustapha, R.; Zoughaib, A.; Ghaddar, N.; Ghali, K. Modified upright cup method for testing water vapor permeability in porous membranes. Energy 2020, 195, 117057. [Google Scholar] [CrossRef]
- Beristain-Bauza, S.C.; Mani-López, E.; Palou, E.; López-Malo, A. Antimicrobial activity and physical properties of protein films added with cell-free supernatant of Lactobacillus rhamnosus. Food Control 2016, 62, 44–51. [Google Scholar] [CrossRef]
- Shekarabi, A.S.; Oromiehie, A.R.; Vaziri, A.; Ardjmand, M.; Safekordi, A.A. Investigation of the effect of nanoclay on the properties of quince seed mucilage edible films. Food Sci. Nutr. 2014, 2, 821–827. [Google Scholar] [CrossRef]
- Sabantina, L.; Hes, L.; Mirasol, J.R.; Cordero, T.; Ehrmann, A. Water Vapor Permeability through PAN Nanofiber Mat with Varying Membrane-Like Areas. Fibres Text. East. Eur. 2019, 27, 12–15. [Google Scholar] [CrossRef]
- Huang, J.H.; Qian, X.M. Comparison of Test Methods for Measuring Water Vapor Permeability of Fabrics. Text. Res. J. 2008, 78, 342–352. [Google Scholar] [CrossRef]
- Sun, N.; Wang, G.-G.; Zhao, H.-X.; Cai, Y.-W.; Li, J.-Z.; Li, G.-Z.; Zhang, X.-N.; Wang, B.-L.; Han, J.-C.; Wang, Y.H.; et al. Waterproof, breathable and washable triboelectric nanogenerator based on electrospun nanofiber films for wearable electronics. Nano Energy 2021, 90, 106639. [Google Scholar] [CrossRef]
- Yardimci, A.I.; Durmus, A.; Kayhan, M.; Tarhan, O. Antibacterial Activity of AgNO3 Incorporated Polyacrylonitrile/Polyvinylidene Fluoride (PAN/PVDF) Electrospun Nanofibrous Membranes and Their Air Permeability Properties. J. Macromol. Sci. B 2022, 61, 749–762. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.H. Reinforced tensile strength and wettability of nanofibrous electrospun cellulose acetate by coating with waterborne polyurethane and graphene oxide. J. Eng. Fibers Fabr. 2022, 17, 15589250221127353. [Google Scholar] [CrossRef]
- Sarwar, M.N.; Ali, H.G.; Ullah, S.; Yamashita, K.; Shahbaz, A.; Nisar, U.; Hashmi, M.; Kim, I.-S. Electrospun PVA/CuONPs/Bitter Gourd Nanofibers with Improved Cytocompatibility and Antibacterial Properties: Application as Antibacterial Wound Dressing. Polymers 2022, 14, 1361. [Google Scholar] [CrossRef]
- Kawabata, S. Method and Apparatus for Measuring Air Permeability of Fiber Material Such as Cloth or Nonwoven Fabric of Every Kind. Patent No. JPH056133B2, 14 December 1987. [Google Scholar]
- Wang, Y.W.; Pan, J.Q.; Liu, H.Y.; Liu, K.; Huang, F.H.; Niu, S.X.; Cheng, G.Y.; Wang, D.D. Test Equipment Used for Air Circulation Performance of Diesel Soot Particulate Filter. Patent CN108019263A, 11 May 2018. [Google Scholar]
- Wagner, C.G.; Cain, D.E. Method and Apparatus for Determining Permeability and Thickness of Refractory Coatings on Foundry Molds and Cores. U.S. Patent US4366703A, 4 January 1983. [Google Scholar]
- Lyu, L.X.; Daichi, K.; Yang, Y.; Xu, T. Gas Permeability Detecting Device and Determination Method for Tissue Engineering Porous Scaffold. Patent CN106596374A, 26 April 2017. [Google Scholar]
- Sabantina, L.; Ehrmann, A. New testing device for air permeability. Commun. Dev. Assem. Text. Prod. 2023; in print. [Google Scholar]
- Lv, X.H.; Tang, Y.; Tian, Q.F.; Wang, Y.P.; Ding, T. Ultra-stretchable membrane with high electrical and thermal conductivity via electrospinning and in-situ nanosilver deposition. Compos. Sci. Technol. 2020, 200, 108414. [Google Scholar] [CrossRef]
- Zhang, X.S.; Wang, B.; Wu, N.; Han, C.; Wu, C.Z.; Wang, Y.D. Flexible and thermal-stable SiZrOC nanofiber membranes with low thermal conductivity at high-temperature. J. Europ. Ceram. Soc. 2020, 40, 1877–1885. [Google Scholar] [CrossRef]
- Li, Z.J.; Cheng, B.; Ju, J.G.; Kang, W.M.; Liu, Y. Development of a novel multi-scale structured superhydrophobic nanofiber membrane with enhanced thermal efficiency and high flux for membrane distillation. Desalination 2021, 501, 114834. [Google Scholar] [CrossRef]
- Han, Z.Y.; Cheng, Z.Q.; Chen, Y.; Liang, Z.W.; Li, H.F.; Ma, Y.J.; Feng, X. Fabrication of highly pressure-sensitive, hydrophobic, and flexible 3D carbon nanofiber networks by electrospinning for human physiological signal monitoring. Nanoscale 2019, 11, 5942–5950. [Google Scholar] [CrossRef]
- Datsyuk, V.; Trotsenko, S.; Trakakis, G.; Boden, A.; Vyzas-Asimakopoulos, K.; Parthenios, J.; Galiotis, C.; Reich, S.; Papagelis, K. Thermal properties enhancement of epoxy resins by incorporating polybenzimidazole nanofibers filled with graphene and carbon nanotubes as reinforcing material. Polym. Test. 2020, 82, 106317. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, X.D.; Shang, Y.; Xu, P.H.; Pan, D.; Su, F.M.; Ji, Y.X.; Feng, Y.Z.; Liu, Y.Z.; Liu, C.T. Highly thermally conductive polyvinyl alcohol/boron nitride nanocomposites with interconnection oriented boron nitride nanoplatelets. Compos. Sci. Technol. 2021, 201, 108521. [Google Scholar] [CrossRef]
- Yin, C.-G.; Ma, Y.; Liu, Z.-J.; Fan, J.-C.; Shi, P.-H.; Xu, Q.-J.; Min, Y.-L. Multifunctional boron nitride nanosheet/polymer composite nanofiber membranes. Polymer 2019, 162, 100–107. [Google Scholar] [CrossRef]










| Physical Property | Test Procedures | Dimensions, Standards | References |
|---|---|---|---|
| Porosity | Fluid uptake | [39,40,41,42,43,44,45] | |
| Gas pycnometer | ASTM D2000 | [47] | |
| Apparent density | [57] | ||
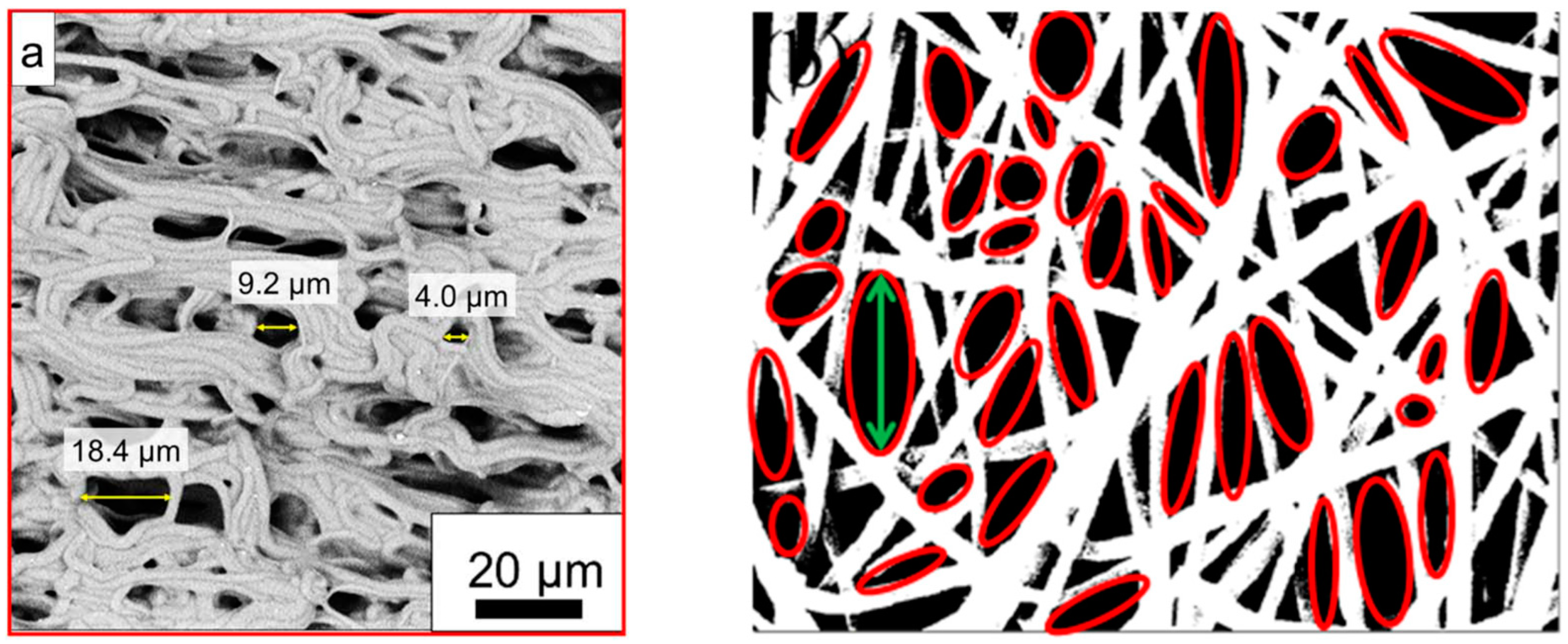
| Pore size distribution | SEM images, ImageJ | [52,61] | |
| Thermoporometry | [85,86,87] | ||
| Specific surface area | BET isotherms | [88,89,90] | |
| Nanofiber diameter | SEM images, DiameterJ | [51,60] | |
| Nanofiber orientation | SEM images, ImageJ | [54] | |
| Surface roughness | CLSM | [61] | |
| SEM and Fiji software | [114] | ||
| SEM and Gwyddion software | [115,116] | ||
| Atomic force microscopy | [119,120,121] | ||
| Nanofiber mat thickness | Textile thickness tester | [52] | |
| Laser profilometer | [54] | ||
| Micrometer caliper | [57] | ||
| Hydrophobicity | Sessile drop | [43,44,50,53,88] | |
| Water uptake | Mass difference dry/wet | [140,141,142,143] | |
| Mechanical properties | Tensile tests | 10 mm × 10 mm | [131] |
| 15 mm × 20 mm | [45] | ||
| Length 100 mm, ASTM D882 | [46] | ||
| 10 mm × 30 mm, ASTM D882 | [48] | ||
| 10″ × 3″, EN ISO 13934:1:1999 | [89] | ||
| Electrical conductivity | Impedance measurement | [88] | |
| Conductivity meter | [114] | ||
| Water vapor permeability | Bottle permeation test | 1.18 cm2 | [42] |
| 1.77 cm2 | [44] | ||
| Air permeability | Air permeability tester | 20 cm2 | [126] |
| 50 cm2 | [166] | ||
| 38.3 cm3 | [167] | ||
| Thermal conductivity | Hot plate | [175,176,177] | |
| Light flash | [178,179,180] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langwald, S.V.; Ehrmann, A.; Sabantina, L. Measuring Physical Properties of Electrospun Nanofiber Mats for Different Biomedical Applications. Membranes 2023, 13, 488. https://doi.org/10.3390/membranes13050488
Langwald SV, Ehrmann A, Sabantina L. Measuring Physical Properties of Electrospun Nanofiber Mats for Different Biomedical Applications. Membranes. 2023; 13(5):488. https://doi.org/10.3390/membranes13050488
Chicago/Turabian StyleLangwald, Sarah Vanessa, Andrea Ehrmann, and Lilia Sabantina. 2023. "Measuring Physical Properties of Electrospun Nanofiber Mats for Different Biomedical Applications" Membranes 13, no. 5: 488. https://doi.org/10.3390/membranes13050488
APA StyleLangwald, S. V., Ehrmann, A., & Sabantina, L. (2023). Measuring Physical Properties of Electrospun Nanofiber Mats for Different Biomedical Applications. Membranes, 13(5), 488. https://doi.org/10.3390/membranes13050488








