On the Use of Polymer Inclusion Membranes for the Selective Separation of Pb(II), Cd(II), and Zn(II) from Seawater
Abstract
1. Introduction
2. Materials and Methods
2.1. Reactives and Equipment
2.2. PIM Preparation
2.3. Transport Experiments
2.4. PIM Optimization
2.5. Effect of the Increase in NaCl Concentration in the Feed Phase
2.6. Effect of pH in the Feed Phase
2.7. Effect of Matrix Nature
2.8. Selective Separation of Cd(II), Pb(II), and Zn(II)
2.8.1. Effect of the Initial Concentrations of the Metal Ions
2.8.2. PIM Stability and Preconcentration
3. Results and Discussion
3.1. PIM Optimization
3.2. Transport Mechanisms
3.3. Effect of NaCl in the Feed Phase
3.4. Effect of the pH in the Feed Phase
3.5. Effect of the Matrix Nature
3.6. Selective Separation of Cd(II), Pb(II), and Zn(II)
3.6.1. Effect of the Composition of the Feed Solution
3.6.2. Stability and Preconcentration Behavior
3.7. PIMs Characterization
3.8. Future Perspectives
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kroger, S.; Law, R. Sensing the sea. Trends Biotechnol. 2005, 23, 250–256. [Google Scholar] [CrossRef]
- Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT). Mares Mexicanos. México. 2020. Available online: https://www.gob.mx/semarnat/articulos/mares-mexicanos (accessed on 17 April 2023).
- Arslan, O.; Karaslan, M.A. Bioavailability of Sea Urchin to Aquatic Toxicity Tests. J. Aquat. Pollut. Toxicol. 2017, 1, 1–6. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Experientia Supplementum; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; Volume 101. [Google Scholar] [CrossRef]
- Chapman, P.M.; Allen, H.E.; Z’Graggen, M.N. Evaluation of Bioaccumulation Factors in Regulating Metals. Environ. Sci. Technol. 1996, 30, 448A–452A. [Google Scholar] [CrossRef]
- Scheifler, R.; Coeurdassier, M.; Morilhat, C.; Bernard, N.; Faivre, B.; Flicoteaux, P.; Giraudoux, P.; Noël, M.; Piotte, P.; Rieffel, D.; et al. Lead Concentrations in Feathers and Blood of Common Blackbirds (Turdusmerula) and in Earthworms Inhabiting Unpolluted and Moderately Polluted Urban Areas. Sci. Total Environ. 2006, 371, 197–205. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health. Environmental Health Concerns and Toxic Chemicals Where You Live, Work, and Play; Town, T., Ed.; U.S. National Library of Medicine: Bethesda, MD, USA, 2020. [Google Scholar]
- Jackson Davis, W. Contamination of coastal versus open ocean surface waters: A brief meta-analysis. Mar. Pollut. Bull. 1993, 26, 128–134. [Google Scholar] [CrossRef]
- Neff, J.M. Chapter 5—Cadmium in the Ocean and Chapter 9—Lead in the ocean. In Bioaccumulation in Marine Organisms; Elsevier Science: Amsterdam, The Netherlands, 2002; pp. 89–102+161–173+175–189. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. National Recommended Water Quality Criteria—Aquatic Life Criteria Table. Available online: https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table (accessed on 17 April 2023).
- Brad, M.A.; Simon, C.A.; Graeme, E.B.; Mark, D.R. Lead solubility in seawater: An experimental study. Environ. Chem. 2015, 13, 489–495. [Google Scholar] [CrossRef]
- Australian Government Initiative. Zinc in freshwater. In Australia and New Zealand Guidelines for Fresh & Marine Water Quality; Australian Government Initiative: Canberra, Australia, 2020. [Google Scholar]
- Gardner, M.; Comber, S.; Ravenscroft, J. Zinc in Estuzaries. Foundation for Water Research. R%D Note 390; National Rivers Authority: Bristol, UK, 1995. [Google Scholar]
- Saleh, T.A.; Mustaqeem, M.; Khaled, M. Water treatment technologies in removing heavy metal ions from wastewater: A review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100617. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Ge, F.; Li, M.-M.; Ye, H.; Zhao, B.-X. Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. J. Hazard. Mater. 2012, 211–212, 366–372. [Google Scholar] [CrossRef]
- Muralidhara, H.S. Chapter 2—Challenges of Membrane Technology in the XXI Century. In Membrane Technology. A Practical Guide to Membrane Technology and Applications in Food and Bioprocessing; Cui, Z.F., Muralidhara, H.S., Eds.; Butterworth-Heinemann: Amsterdam, The Netherlands, 2010; pp. 19–32. [Google Scholar] [CrossRef]
- Purkait, M.K.; Singh, R.; Mondal, P.; Haldar, D. Chapter 1—Thermal induced membrane separation processes: An introduction. In Thermal Induced Membrane Separation Processes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–16. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Porter, M.C. Handbook of Industrial Membrane Technology; Noyes Publications: Westwood, NJ, USA, 1991. [Google Scholar]
- Baker, R.W. Membrane Technology and Applications, 2nd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Staszak, K.; Wieszczycka, K.; Tylkowski, B. (Eds.) Membrane Technologies. From Academia to Industry; Walter de Gruyter: Berlin, Germany, 2022. [Google Scholar]
- Pabby, A.K.; Rizvi SS, H.; Sastre, A.M. (Eds.) Handbook of Membrane Separations: Chemical, Pharmaceutical, Food, and Biotechnological Applications; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Nghiem, L.D.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Sharaf, M.; Yoshida, W.; Kubota, F.; Kolev, S.D.; Goto, M. A polymer inclusion membrane composed of the binary carrier PC-88A and Versatic 10 for the selective separation and recovery of Sc. RSC Adv. 2018, 8, 8631. [Google Scholar] [CrossRef] [PubMed]
- Keskin, B.; Zeytuncu-Gökoglu, B.; Koyuncu, I. Polymer inclusion membrane applications for transport of metal ions: A critical review. Chemosphere 2021, 279, 130604. [Google Scholar] [CrossRef]
- Baba, Y.; Kubota, F.; Goto, M.; Cattrall, R.W.; Kolev, S.D. Separation of cobalt(II) from manganese(II) using a polymer inclusion membrane with N-[N,N-di(2-ethylhexyl)aminocarbonylmethyl] glycine (D2EHAG) as the extractant/carrier. J. Chem. Technol. Biotechnol. 2016, 91, 1320–1326. [Google Scholar] [CrossRef]
- Baczynska, M.; Regel-Rosocka, M.; Coll, M.T.; Fortuny, A.; Sastre, A.M.; WiŚniewski, M. Transport of Zn(II), Fe(II), Fe(III) across polymer inclusion membranes (PIM) and Flat sheet supported liquid membranes (SLM) containing phosphonium ionic liquids as metal ion carriers. Sep. Sci. Technol. 2016, 51, 2639–2648. [Google Scholar] [CrossRef]
- Kołodziejska, M.; Kozłowska, J.; Kozłowski, C. Separation of silver(I) and copper(II) by polymer inclusion membranes with aza[18]crown-6 derivatives as ion carriers. Desalin. Water Treat. 2017, 64, 432–436. [Google Scholar] [CrossRef]
- Meng, X.; Wang, C.; Zhou, P.; Xin, X.; Wang, L. Transport and selectivity of indium through polymer inclusion membrane in hydrochloric acid medium. Front. Environ. Sci. Eng. 2017, 11, 9. [Google Scholar] [CrossRef]
- Pospiech, B. Facilitated transport of palladium(II) across polymer inclusion membrane with ammonium ionic liquid as effective carrier. Chem. Pap. 2018, 72, 301–308. [Google Scholar] [CrossRef]
- Fajar AT, N.; Kubota, F.; Firmansyah, M.L.; Goto, M. Separation of Palladium(II) and Rhodium(III) Using a Polymer Inclusion Membrane Containing a Phosphonium-Based Ionic Liquid Carrier. Ind. Eng. Chem. Res. 2019, 58, 22334–22342. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Pyszka, I.; Urbaniak, W. Cadmium(II) and lead(II) extraction and transport through polymer inclusion membranes with 1-alkylimidazole. Desalin. Water Treat. 2021, 214, 56–63. [Google Scholar] [CrossRef]
- Pospiech, B. Separation of Co from Ni and Li from chloride media using polymer inclusion membrane system with thiosalicylate based ionic liquid. Physicochem. Probl. Miner. Process. 2022, 58, 152997. [Google Scholar] [CrossRef]
- Kaczorowska, M.A. The Use of Polymer Inclusion Membranes for the Removal of Metal Ions from Aqueous Solutions—The Latest Achievements and Potential Industrial Applications: A Review. Membranes 2022, 12, 1135. [Google Scholar] [CrossRef] [PubMed]
- Fajar, A.T.N.; Goto, M. Enabling Metal Sustainability with Polymer Inclusion Membranes: A Critical Review. J. Chem. Eng. Japan 2023, 56, 2153547. [Google Scholar] [CrossRef]
- Pyszka, I.; Radzyminska-Lenarcik, E. New Polymer Inclusion Membrane in the Separation of Nonferrous Metal Ion from Aqueous Solutions. Membranes 2020, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Paredes, C.; Rodríguez de San Miguel, E. Selective lithium extraction and concentration from diluted alkaline aqueous media by a polymer inclusion membrane and application to seawater. Desalination 2020, 487, 114500. [Google Scholar] [CrossRef]
- Cholid Djunaidi, M.; Fauzi, H.; Hastuti, R. Desalination of Sea Water Using Polymer Inclusion Membrane (PIM) With Aliquat 336-TBP (Tributhyl Phosphate) as Carrier Compound. MATEC Web Conf. 2018, 156, 08004. [Google Scholar] [CrossRef]
- Cholid Djunaidi, M.; Tri Wahyuni, P. Influence of Different Carriers in Polymer Inclusion Membranes for Desalination of Seawater. Asian J. Chem. 2020, 32, 1869–1873. [Google Scholar] [CrossRef]
- Ait Khaldoun, I.; Mitiche, L.; Sahmoune, A.; Fontàs, C. An Efficient Polymer Inclusion Membrane-Based Device for Cd Monitoring in Seawater. Membranes 2018, 8, 61. [Google Scholar] [CrossRef]
- López-Guerrero, M.M.; Granado-Castro, M.D.; Díaz-de-Alba, M.; Lande-Durán, J.; Casanueva-Marenco, M.J. A polymer inclusion membrane for the simultaneous determination of Cu(II), Ni(II) and Cd(II) ions from natural waters. Microchem. J. 2020, 157, 104980. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M.; Pyszka, I. Application of Polymer Inclusion Membranes Doped with Alkylimidazole to Separation of Silver and Zinc Ions from Model Solutions and after Battery Leaching. Materials 2020, 13, 3103. [Google Scholar] [CrossRef]
- Ballinas MD, L.; Rodríguez de San Miguel, E.; Rodríguez MT, D.J.; Silva, O.; Muñoz, M.; De Gyves, J. Arsenic(V) Removal with Polymer Inclusion Membranes from Sulfuric Acid Media Using DBBP as Carrier. Environ. Sci. Technol. 2004, 38, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Hu, H.; Hu, F.; Tang, J.; Yang, C.; Zhou, Y.; Lin, X.; Hu, J. Simultaneous recovery of copper(II) from two different feed solutions based on a three-compartment module with selective polymer inclusion membranes. Hydrometallurgy 2019, 188, 64–72. [Google Scholar] [CrossRef]
- Han, X.; Wang, J.; Cai, W.; Xu, X.; Sun, M. The Pollution Status of Heavy Metals in the Surface Seawater and Sediments of the Tianjin Coastal Area, North China. Int. J. Environ. Res. Public Health 2021, 18, 11243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Cao, Q.; Hong, M.; Lei, Y.; Wen, D.; Zhang, D. Spatial distribution and risk assessment of heavy metals in seawater and sediments in Jieshi Bay, Shanwei, China. Front. Mar. Sci. 2022, 9, 1011564. [Google Scholar] [CrossRef]
- Panakos, Agua de Mar Profundo. Available online: https://www.panakosaguademar.com/ (accessed on 17 April 2023).
- Yeats, P.A. Techniques in Marine Environmental Sciences. No. 2 Trace Metals in Sea Water: Sampling and Storage Methods; International Council for the Exploration of The Sea: Copenhagen, Denmark, 1987. [Google Scholar]
- Lamb, J.D.; Nazarenko, Y.A. Selective metal ion sorption and transport using polymer inclusion membranes containing dycyclorohexano-18-crown-6. Sep. Sci. Technol. 1997, 32, 2749–2764. [Google Scholar] [CrossRef]
- García-Beleño, J.; Rodríguez de San Miguel, E. Optimization of Cr(III) transport in a polymer inclusion membrane system through experimental design strategies. Chem. Pap. 2022, 76, 2235–2247. [Google Scholar] [CrossRef]
- American National Standards Institute (ANSI). ASTM D1141—98; Standard Practice for the Preparation of Substitute Ocean Water. ANSI: Washington, DC, USA, 2021. [Google Scholar]
- Millero, F.J. Chemical Oceanography, 3rd ed.; CRC Press: New York, NY, USA, 2006. [Google Scholar]
- Briones Guerash Silva, U. Transporte de Cd (II), Fe(III), Pb(II) y Zn(II) en Membranas Poliméricas Empleando Aminas Terciarias y Sales de Amonio Cuaternarias Como Acarreadores. Master’s Thesis, Universidad Nacional Autónoma de México, Mexico City, Mexico, 2016. Available online: https://repositorio.unam.mx/contenidos/transporte-de-cdii-feiii-pbii-y-znii-en-membranas-polimericas-empleando-aminas-terciarias-y-sales-de-amonio-cuaternar-62564?c=VpebGM&d=true&q=*:*&i=2&v=1&t=search_0&as=0 (accessed on 29 March 2023).
- Salazar-Alvarez, G.; Bautista-Flores, A.N.; Rodríguez de San Miguel, E.; Muhammed, M.; de Gyves, J. Transport characterization of a PIM system used for the extraction of Pb (II) using D2EHPA as carrier. J. Membr. Sci. 2005, 250, 247–257. [Google Scholar] [CrossRef]
- Macías, M.; Rodríguez de San Miguel, E. Optimization of Ni (II) Facilitated Transport from Aqueous Solutions Using a Polymer Inclusion Membrane. Water Air Soil Pollut. 2021, 232, 62. [Google Scholar] [CrossRef]
- Zioui, D.; Arous, O.; Amara, M.; Kerdjoudj, H. Polymer inclusion membrane: Effect of the chemical nature of the polymer and plasticizer on the metallic ions transference. Macromol. Indian J. 2011, 7, 93–101. Available online: https://www.researchgate.net/publication/275097910_Polymer_inclusion_membrane_Effect_of_the_chemical_nature_of_the_polymer_and_plasticizer_on_the_metallic_ions_transference (accessed on 17 April 2023).
- Adelung, S.; Lohrengel, B.; Nghiem, L. Selective transport of Cadmium by PVC/Aliquat 336 polymer inclusion membranes (PIMs): The role of membrane composition and solution chemistry. Membr. Water Treat. 2012, 3, 123–131. [Google Scholar] [CrossRef]
- Lommelen, R.; Vander Hoogerstraete, T.; Onghena, B.; Billard, I.; Binnemans, K. Model for Metal Extraction from Chloride Media with Basic Extractants: A Coordination Chemistry Approach. Inorg. Chem. 2019, 58, 12289–12301. [Google Scholar] [CrossRef] [PubMed]
- Kebiche-Senhadji, O.; Mansouri, L.; Benamor, M. Consideration of Polymer Inclusion Membranes Containing D2EHPA for Toxic Metallic Ion (Pb2+) Extraction Recovery. In Proceedings of the 5th International Conference on Environment Science and Engineering, Istanbul, Turkey, 24–25 April 2015; Volume 83, p. 30. [Google Scholar]
- Charcosset, C. Chater. 3—Microfiltration. In Membrane Processes in Biotechnology and Pharmaceutics; Elsevier: Amsterdam, The Netherlands, 2012; pp. 101–141. [Google Scholar]
- Liu, P.C.; Chen, J.C. Effects of heavy metals on the hatching rates of brine shrimp Artemia salina cysts. J. World Aquac. Soc. 1987, 18, 78–83. [Google Scholar] [CrossRef]
- Ritcey, G.M.; Ashbrook, A.W. Solvent Extraction, 2nd ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Rodríguez de San Miguel, E.; de Gyves, J. Indium transport modelling through an SLM system with ADOGEN 364 from concentrated hydrochloric acid. In Proceedings of the ISEC 2011 International Solvent Extraction Conference, Santiago, Chile, 3–7 October 2011; Valenzuela, F.L., Moyer, B.A., Eds.; Gecamín: Santiago, Chile, 2011. Chapter 8. pp. 1–9, ISBN 978-956-8504-58-8. [Google Scholar]
- ChemEQL—A Software for the Calculation of Chemical Equilibria. Available online: https://www.eawag.ch/en/department/surf/projects/chemeql/ (accessed on 23 March 2023).
- Juang, R.S.; Kao, H.C.; Wu, W.H. Analysis of liquid membrane extraction of binary Zn(II) and Cd(II) from chloride media with Aliquat 336 based on thermodynamic equilibrium models. J. Membr. Sci. 2004, 228, 169–177. [Google Scholar] [CrossRef]
- Danesi, P.R.; Chiarizia, R.; Castagnola, A. Transfer rate and separation of Cd(II) and Zn(II) chloride species by a trilaurylammonium chloride-triethylbenzene supported liquid membrane. J. Membr. Sci. 1983, 14, 161–174. [Google Scholar] [CrossRef]
- Rojas-Challa, Y.; Rodríguez de San Miguel, E.; de Gyves, J. Response Surface Methodology Approach Applied to the Study of Arsenic (V) Migration by Facilitated Transport in Polymer Inclusion Membranes. Water Air Soil Pollut. 2020, 231, 33. [Google Scholar] [CrossRef]
- Hedwig, S.; Kraus, M.; Amrein, M.; Stiehm, J.; Constable, E.C.; Lenz, M. Recovery of scandium from acidic waste solutions by means of polymer inclusion membranes. Hydrometallurgy 2022, 213, 105916. [Google Scholar] [CrossRef]
- Motekaitis, R.J.; Martell, A.E. Speciation of metals in the oceans. I. Inorganic complexes in seawater, and influence of added chelating agents. Mar. Chem. 1987, 21, 101–116. [Google Scholar] [CrossRef]
- Kumar, R. Synthesis and characterization of Membrane Supported Metal Nanoparticles. Ph.D. Thesis, Bhabha Atomic Research Centre, Mumbai, India, 2013. Available online: http://www.hbni.ac.in/phdthesis/chem/CHEM01200604013.pdf (accessed on 3 May 2023).
- O’Rourke, M.; Duffy, N.; De Marco, R.; Potter, I. Electrochemical Impedance Spectroscopy—A Simple Method for the Characterization of Polymer Inclusion Membranes Containing Aliquat 336. Membranes 2011, 1, 132–148. [Google Scholar] [CrossRef]
- Nitti, F.; Edison Selan, O.T.; Hoque, B.; Tambaru, D.; Djunaidi, M.C. Improving the Performance of Polymer Inclusion Membranes in Separation Process Using Alternative Base Polymers: A Review. Indones. J. Chem. 2022, 22, 284–302. [Google Scholar] [CrossRef]
- Mancilla-Rico, A.; de Gyves, J.; Rodríguez de San Miguel, E. Structural Characterization of the Plasticizers’ Role in Polymer Inclusion Membranes Used for Indium (III) Transport Containing IONQUEST® 801 as Carrier. Membranes 2021, 11, 401. [Google Scholar] [CrossRef]
- Rodríguez de San Miguel, E.; Monroy-Barreto, M.; Aguilar, J.C.; Ocampo, A.L.; de Gyves, J. Structural effects on metal ion migration across polymer inclusion membranes: Dependence of membrane properties and transport profiles on the weight and volume fractions of the components. J. Membr. Sci. 2011, 379, 416–425. [Google Scholar] [CrossRef]
- Henderson, C.A.; Nagul, E.A.; Cattrall, R.W.; Kolev, S.D.; Smith, T.A. Imaging chemical extraction by polymer inclusion membranes using fluorescence microscopy. Methods Appl. Fluoresc. 2014, 2, 024008. [Google Scholar] [CrossRef] [PubMed]
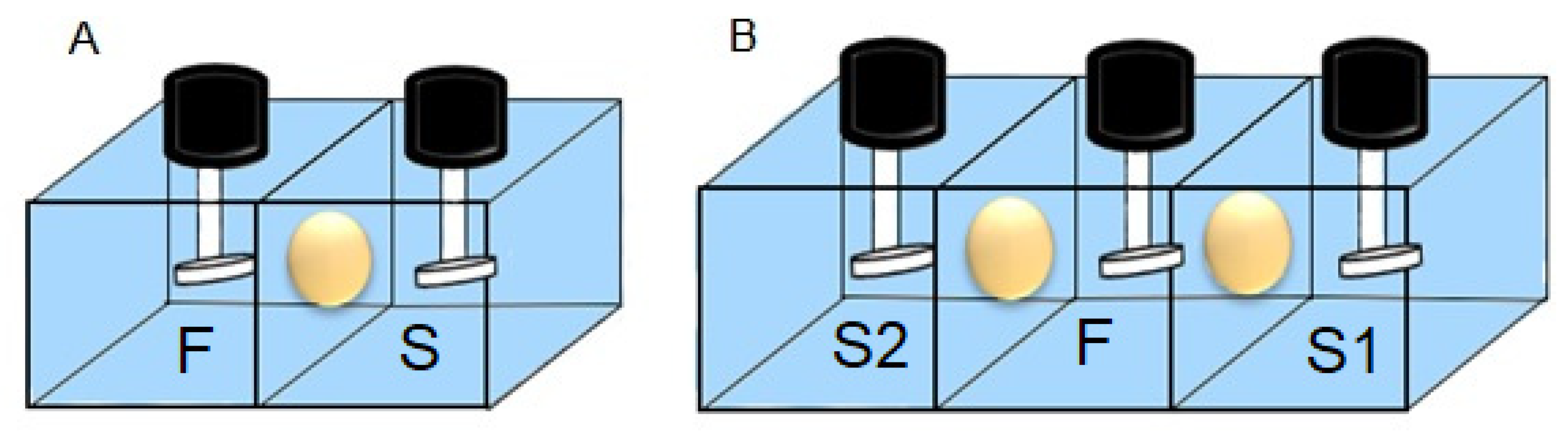







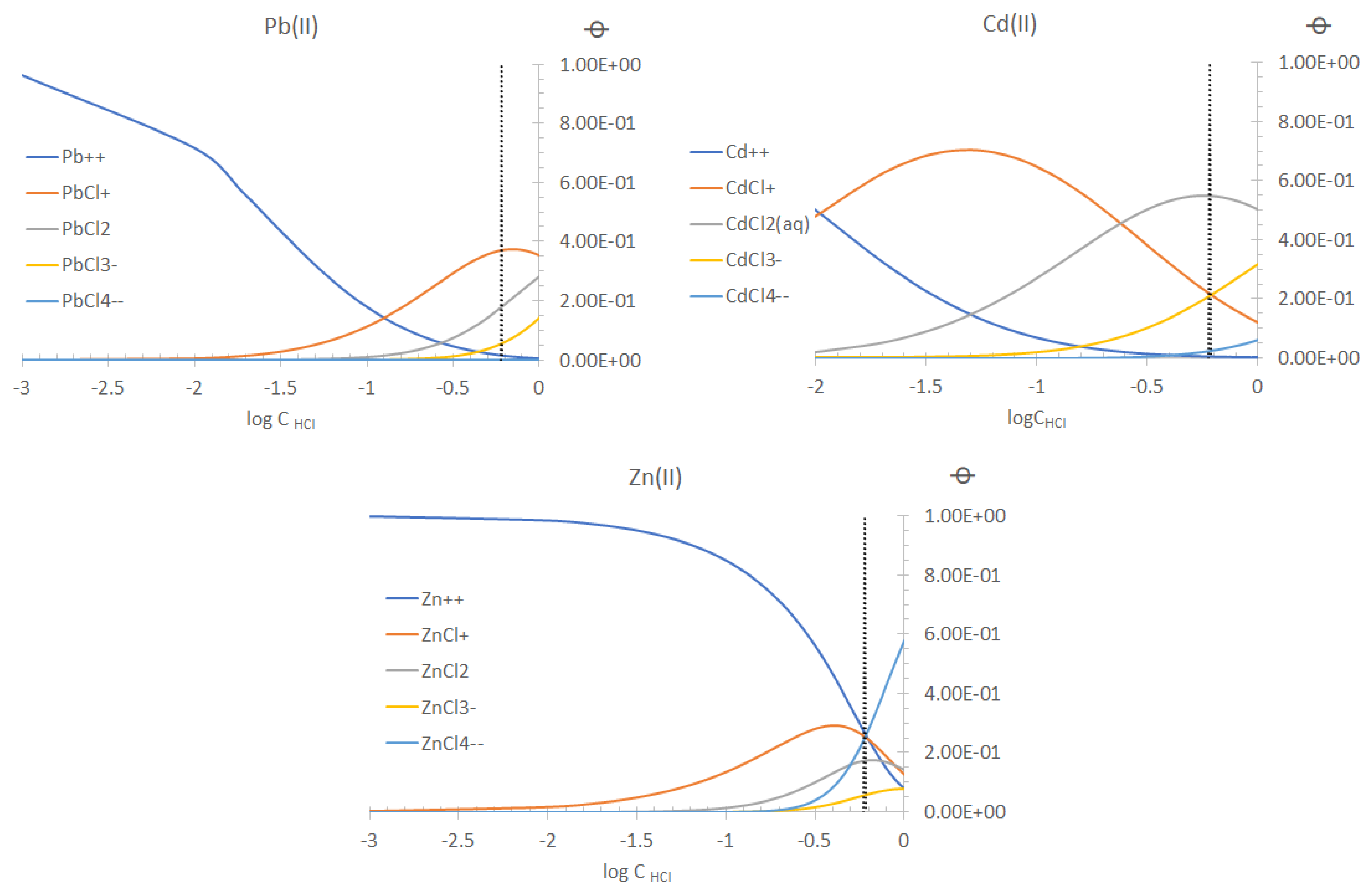






| Coded Value | CTA (g) | NPOE (g) | Aliquat 336 (g) |
|---|---|---|---|
| 1 | 0.15 | 0.0475 | 0.12 |
| 0 | 0.10 | 0.0325 | 0.08 |
| −1 | 0.05 | 0.0175 | 0.04 |
| Experiment | Zn(II) (mol/dm3) | Pb(II) (mol/dm3) | Cd(II) (mol/dm3) |
|---|---|---|---|
| A | 1 (1 × 10−4) | −1 (5 × 10−5) | −1 (5 × 10−5) |
| B | −1 (5 × 10−5) | −1 (5 × 10−5) | −1 (5 × 10−5) |
| C | 1 (1 × 10−4) | 1 (1 × 10−4) | 1 (1 × 10−4) |
| D | −1 (5 × 10−5) | 1 (1 × 10−4) | 1 (1 × 10−4) |
| E | −1 (5 × 10−5) | 1 (1 × 10−4) | −1 (5 × 10−5) |
| F | 1 (1 × 10−4) | 1 (1 × 10−4) | −1 (5 × 10−5) |
| G | −1 (5 × 10−5) | −1 (5 × 10−5) | 1 (1 × 10−4) |
| H | 1 (1 × 10−4) | −1 (5 × 10−5) | 1 (1 × 10−4) |
| Experiment | CTA | NPOE | Aliquat 336 | ΦF | ΦS |
|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0.25 | 0.63 |
| 2 | 0 | −1 | 1 | 0.41 | 0.48 |
| 3 | 0 | 0 | 0 | 0.5 | 0.36 |
| 4 | −1 | 0 | −1 | 0.42 | 0.53 |
| 5 | 1 | 0 | 1 | 0.52 | 0.37 |
| 6 | 1 | 1 | 0 | 0.24 | 0.68 |
| 7 | 0 | −1 | −1 | 0.71 | 0.22 |
| 8 | −1 | 0 | 1 | Nd * | 0.98 |
| 9 | −1 | 1 | 0 | 0.28 | 0.68 |
| 10 | 1 | −1 | 0 | 0.72 | 0.21 |
| 11 | 0 | 0 | 0 | 0.3 | 0.6 |
| 12 | 0 | 1 | 1 | 0.014 | 0.96 |
| 13 | 0 | 1 | −1 | 0.52 | 0.46 |
| 14 | 1 | 0 | −1 | 0.69 | 0.28 |
| 15 | −1 | −1 | 0 | 0.61 | 0.32 |
| Group | Wavenumber (cm−1) | PIM | CTA | Aliquat 336 | NPOE |
|---|---|---|---|---|---|
| C=O | 1750 | X | X | ||
| C-H | 2951 | X | X | X | |
| OH | 3600 | X | |||
| CH3 | 1368 | X | X | X | |
| COO | 1649 | X | |||
| C–NO2 | 1520 | X | X | ||
| COO | 1434 | X | |||
| -C-O-C- | 1217 | X | X | ||
| -C-O-C- | 1041 | X | X | ||
| C-O | 1279 | X | X | ||
| C-O | 1165 | X | |||
| N-O | 1525 | X | X | ||
| N-O | 744 | X | |||
| -C=C- | 1468 | X | |||
| -C=C- | 1608 | X | |||
| =C-H | 2923 | X | |||
| N-C | 3373 | X | X | ||
| CH2 | 1466 | X | |||
| N-C | 2923 | X | X |
| Group | Wavenumber (cm−1) | PIM | D2EHPA | TEHP | CTA |
|---|---|---|---|---|---|
| CH | 2959, 881 | X | X | X | X |
| CH2 | 1465 | X | X | X | X |
| CH3 | 1379 | X | X | X | X |
| P=O | 1282 | X | X | ||
| P-O-C | 1022 | X | X | X | |
| P-O-H | 2282 | X | |||
| Hydrogen bond | 1680 | X | |||
| P=O | 1221 | X | X | X | |
| C=O | 1750 | X | X | ||
| OH | 3600 | X | |||
| COO | 1649 | X | |||
| COO | 1520 | X | |||
| COO | 1434 | X | |||
| -C-O-C- | 1217 | X | |||
| -C-O-C- | 1041 | X | |||
| CH2 | 727 | X | X | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macías, M.; Rodríguez de San Miguel, E. On the Use of Polymer Inclusion Membranes for the Selective Separation of Pb(II), Cd(II), and Zn(II) from Seawater. Membranes 2023, 13, 512. https://doi.org/10.3390/membranes13050512
Macías M, Rodríguez de San Miguel E. On the Use of Polymer Inclusion Membranes for the Selective Separation of Pb(II), Cd(II), and Zn(II) from Seawater. Membranes. 2023; 13(5):512. https://doi.org/10.3390/membranes13050512
Chicago/Turabian StyleMacías, Mariana, and Eduardo Rodríguez de San Miguel. 2023. "On the Use of Polymer Inclusion Membranes for the Selective Separation of Pb(II), Cd(II), and Zn(II) from Seawater" Membranes 13, no. 5: 512. https://doi.org/10.3390/membranes13050512
APA StyleMacías, M., & Rodríguez de San Miguel, E. (2023). On the Use of Polymer Inclusion Membranes for the Selective Separation of Pb(II), Cd(II), and Zn(II) from Seawater. Membranes, 13(5), 512. https://doi.org/10.3390/membranes13050512







