Separation of n-Butanol from Aqueous Solutions via Pervaporation Using PDMS/ZIF-8 Mixed-Matrix Membranes of Different Particle Sizes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Nanoparticles
2.2.1. Synthesis of ZIF-8 Nanoparticles
2.2.2. Synthesis of Zinc Oxide (ZnO) Particles
2.3. Fabrication of Membranes
2.3.1. Neat Membrane
2.3.2. ZIF-8 and ZnO Nanoparticles Filled PDMS Mixed Matrix Membranes
2.4. Characterization of Nanoparticles and Membranes
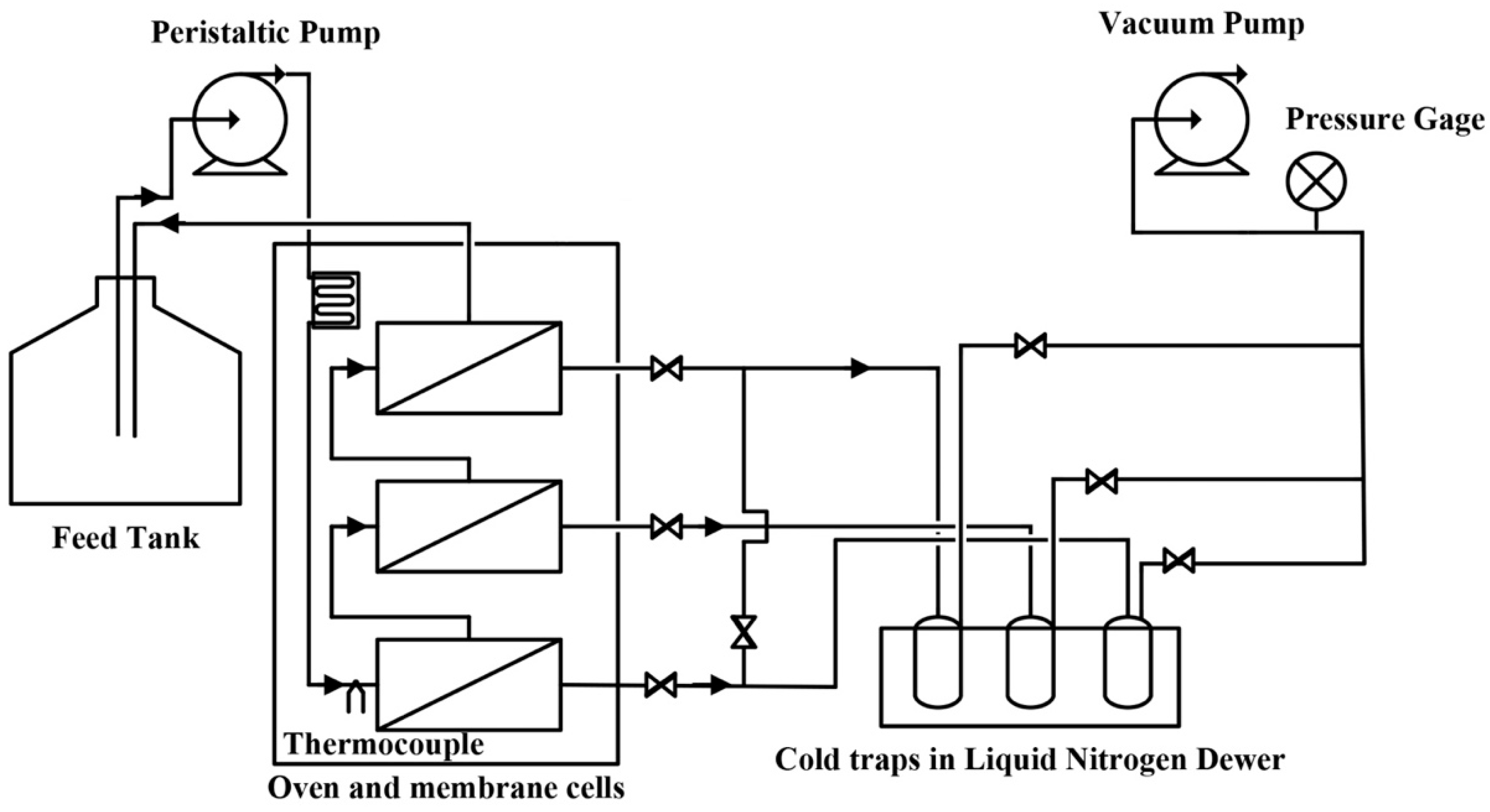
PV Experiments
3. Results and Discussion
3.1. Characterization of ZIF-8 and ZnO Nanoparticles
3.2. Characterization of ZIF-8/PDMS MMMs
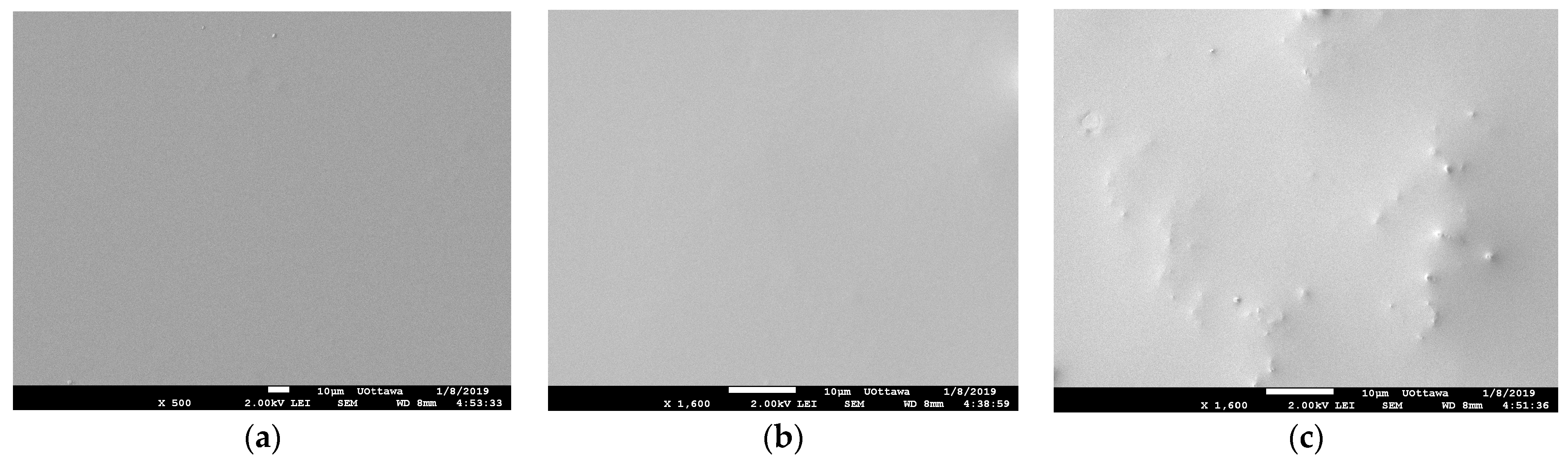
3.2.1. Scanning Electron Microscopy (SEM) Results
3.2.2. Surface Hydrophobicity
3.2.3. Effect of ZIF-8 Nanoparticle Loading on the Membrane Performance
3.2.4. Effect of the ZIF-8 Particle Size on the Membrane Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABE | Acetone: Butanol, Ethanol |
| AC | Activated Carbon |
| CNT | Carbon nanotube |
| MMM | Mixed Matrix Membrane |
| MOF | Metal organic framework |
| PAN | Polyacrylonitrile |
| PV | Pervaporation |
| PDMS | Polydimethylsiloxane |
| SCA | Static contact angle |
| SEM | Scanning Electron Microscope |
| SOD | Sodalite |
| TEM | Transmission Electron Microscopy |
| THF | Tetrahydrofuran |
| XRD | X-ray Powder Diffraction |
| ZIF | Zeolitic Imidazolate Framework |
Nomenclatures
| A | Surface area of the membrane (m2) |
| Ci−feed | Concentration of species i in the feed stream (kg·m−3) |
| Ci−permeate | Concentration of species i in the permeate stream (kg·m−3) |
| J | Flux (kg·m−2·s−1) |
| mi | Mass of species i in the permeate stream (kg) |
| Pi | Membrane permeability of species i (m2·s−1) |
| t | Time of permeation (h) |
| WPDMS | Weight of the PDMS polymer (g) |
| WZIF-8 | Weight of the ZIF-8 nanoparticles (g) |
| xi | Mass fraction of species i in the feed stream (gi·g−1solution) |
| yi | Mass fraction of species i in the permeate stream (gi·g−1solution) |
| σ | Membrane effective thickness (m) |
| αi | Selectivity of species i (-) |
References
- Qureshi, N.; Meagher, M.M.; Huang, J.; Hutkins, R.W. Acetone Butanol Ethanol (ABE) Recovery by Pervaporation Using Silicalite-Silicone Composite Membrane from Fed-Batch Reactor of Clostridium acetobutylicum. J. Membr. Sci. 2001, 187, 93–102. [Google Scholar] [CrossRef]
- Peng, P.; Lan, Y.; Liang, L.; Jia, K. Membranes for Bioethanol Production by Pervaporation. Biotechnol. Biofuels 2021, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, S.K.; Ahmed, A.S.; Ani, F.N. Review on Bioethanol as Alternative Fuel for Spark Ignition Engines. Renew. Sustain. Energy Rev. 2016, 56, 820–835. [Google Scholar] [CrossRef]
- Obergruber, M.; Hönig, V.; Procházka, P.; Kučerová, V.; Kotek, M.; Bouček, J.; Mařík, J. Physicochemical Properties of Biobutanol as an Advanced Biofuel. Materials 2021, 14, 914. [Google Scholar] [CrossRef]
- Rakopoulos, D.C.; Rakopoulos, C.D.; Giakoumis, E.G.; Dimaratos, A.M.; Kyritsis, D.C. Effects of Butanol–Diesel Fuel Blends on the Performance and Emissions of a High-Speed DI Diesel Engine. Energy Convers. Manag. 2010, 51, 1989–1997. [Google Scholar] [CrossRef]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Butanol Fermentation Research: Upstream and Downstream Manipulations. Chem. Rec. 2004, 4, 305–314. [Google Scholar] [CrossRef]
- Chua, T.K.; Liang, D.-W.; Qi, C.; Yang, K.-L.; He, J. Characterization of a Butanol–Acetone-Producing Clostridium Strain and Identification of Its Solventogenic Genes. Bioresour. Technol. 2013, 135, 372–378. [Google Scholar] [CrossRef]
- Huang, H.; Singh, V.; Qureshi, N. Butanol Production from Food Waste: A Novel Process for Producing Sustainable Energy and Reducing Environmental Pollution. Biotechnol. Biofuels 2015, 8, 147. [Google Scholar] [CrossRef] [Green Version]
- Pugazhendhi, A.; Mathimani, T.; Varjani, S.; Rene, E.R.; Kumar, G.; Kim, S.H.; Ponnusamy, V.K.; Yoon, J.J. Biobutanol as a Promising Liquid Fuel for the Future—Recent Updates and Perspectives. Fuel 2019, 253, 637–646. [Google Scholar] [CrossRef]
- Park, C.H.; Geng, Q. Simultaneous Fermentation and Separation in the Ethanol and ABE Fermentation. Sep. Purif. Rev. 1992, 21, 127–174. [Google Scholar] [CrossRef]
- Chen, C.; Xiao, Z.; Tang, X.; Cui, H.; Zhang, J.; Li, W.; Ying, C. Acetone–Butanol–Ethanol Fermentation in a Continuous and Closed-Circulating Fermentation System with PDMS Membrane Bioreactor. Bioresour. Technol. 2013, 128, 246–251. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, G.; Jin, W. Recent Progress in Separation Membranes and Their Fermentation Coupled Processes for Biobutanol Recovery. Energy Fuels 2020, 34, 11962–11975. [Google Scholar] [CrossRef]
- Azimi, H.; Ebneyamini, A.; Tezel, F.H.; Thibault, J. Separation of Organic Compounds from ABE Model Solutions via Pervaporation Using Activated Carbon/PDMS Mixed Matrix Membranes. Membranes 2018, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Qiu, L.; Feng, X. Permeability, Solubility, and Diffusivity of Aniline in Poly(Ether-b-Amide) Membranes Pertaining to Aniline Removal from Aqueous Solutions by Pervaporation and Sorption. J. Membr. Sci. 2022, 642, 120006. [Google Scholar] [CrossRef]
- Robeson, L.M. The Upper Bound Revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Ebneyamini, A.; Azimi, H.; Tezel, F.H.; Thibault, J. Mixed Matrix Membranes Applications: Development of a Resistance-Based Model. J. Membr. Sci. 2017, 543, 351–360. [Google Scholar] [CrossRef]
- Shao, P.; Kumar, A. Separation of 1-Butanol/2,3-Butanediol Using ZSM-5 Zeolite-Filled Polydimethylsiloxane Membranes. J. Membr. Sci. 2009, 339, 143–150. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, C.; Liu, S.; Lu, C.; Liu, D.; Pan, Y.; Sakiyama, H.; Muddassir, M.; Liu, J. A New Magnetic Adsorbent of Eggshell-Zeolitic Imidazolate Framework for Highly Efficient Removal of Norfloxacin. Dalton Trans. 2021, 50, 18016–18026. [Google Scholar] [CrossRef]
- Tanh Jeazet, H.B.; Staudt, C.; Janiak, C. Metal-Organic Frameworks in Mixed-Matrix Membranes for Gas Separation. Dalton Trans. 2012, 41, 14003–14027. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal Organic Frameworks as Efficient Adsorbents for Drugs from Wastewater. Mater. Today Commun 2022, 31, 103514. [Google Scholar] [CrossRef]
- Li, M.; Yin, S.; Lin, M.; Chen, X.; Pan, Y.; Peng, Y.; Sun, J.; Kumar, A.; Liu, J. Current Status and Prospects of Metal–Organic Frameworks for Bone Therapy and Bone Repair. J. Mater. Chem. B 2022, 10, 5105–5128. [Google Scholar] [CrossRef] [PubMed]
- Azimi, H.; Tezel, F.H.; Thibault, J. Effect of Embedded Activated Carbon Nanoparticles on the Performance of Polydimethylsiloxane (PDMS) Membrane for Pervaporation Separation of Butanol. J. Chem. Technol. Biotechnol. 2017, 92, 2901–2911. [Google Scholar] [CrossRef]
- Yang, D.; Cheng, C.; Bao, M.; Chen, L.; Bao, Y.; Xue, C. The Pervaporative Membrane with Vertically Aligned Carbon Nanotube Nanochannel for Enhancing Butanol Recovery. J. Membr. Sci. 2019, 577, 51–59. [Google Scholar] [CrossRef]
- Kudo, Y.; Mikami, H.; Tanaka, M.; Isaji, T.; Odaka, K.; Yamato, M.; Kawakami, H. Mixed Matrix Membranes Comprising a Polymer of Intrinsic Microporosity Loaded with Surface-Modified Non-Porous Pearl-Necklace Nanoparticles. J. Membr. Sci. 2020, 597, 117627. [Google Scholar] [CrossRef]
- Setiawan, W.; Chiang, K.-Y. Silica Applied as Mixed Matrix Membrane Inorganic Filler for Gas Separation: A Review. Sustain. Environ. Res. 2019, 29, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Sazanova, T.S.; Smorodin, K.A.; Zarubin, D.M.; Otvagina, K.V.; Maslov, A.A.; Markov, A.N.; Fukina, D.G.; Mochalova, A.E.; Mochalov, L.A.; Atlaskin, A.A.; et al. Morphology Effect of Zinc Oxide Nanoparticles on the Gas Separation Performance of Polyurethane Mixed Matrix Membranes for CO2 Recovery from CH4, O2, and N2. Membranes 2022, 12, 577. [Google Scholar] [CrossRef]
- Cseri, L.; Hardian, R.; Anan, S.; Vovusha, H.; Schwingenschlögl, U.; Budd, P.M.; Sada, K.; Kokado, K.; Szekely, G. Bridging the Interfacial Gap in Mixed-Matrix Membranes by Nature-Inspired Design: Precise Molecular Sieving with Polymer-Grafted Metal–Organic Frameworks. J. Mater. Chem. A 2021, 9, 23793–23801. [Google Scholar] [CrossRef]
- Pang, S.; Si, Z.; Li, G.; Wu, H.; Cui, Y.; Zhang, C.; Ren, C.; Yang, S.; Pang, S.; Qin, P. A Fluorinated, Defect-Free ZIF-8/PDMS Mixed Matrix Membrane for Enhancing Ethanol Pervaporation. J. Membr. Sci. 2022, 661, 120920. [Google Scholar] [CrossRef]
- Zhu, T.; Xu, S.; Yu, F.; Yu, X.; Wang, Y. ZIF-8@GO Composites Incorporated Polydimethylsiloxane Membrane with Prominent Separation Performance for Ethanol Recovery. J. Membr. Sci. 2020, 598, 117681. [Google Scholar] [CrossRef]
- Liu, G.; Jiang, Z.; Cao, K.; Nair, S.; Cheng, X.; Zhao, J.; Gomaa, H.; Wu, H.; Pan, F. Pervaporation Performance Comparison of Hybrid Membranes Filled with Two-Dimensional ZIF-L Nanosheets and Zero-Dimensional ZIF-8 Nanoparticles. J. Membr. Sci. 2017, 523, 185–196. [Google Scholar] [CrossRef]
- Wiebcke, M.; Cravillon, J.; Münzer, S.; Lohmeier, S.J.; Feldhoff, A.; Huber, K.; Münzer, S. Rapid Room-Temperature Synthesis and Characterization of Nanocrystals of a Prototypical Zeolitic Imidazolate Framework. Chem. Mater. 2009, 21, 1410–1412. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, C.M.; Singh, A.; Koros, W.J. Tailoring Mixed Matrix Composite Membranes for Gas Separations. J. Membr. Sci. 1997, 137, 145–154. [Google Scholar] [CrossRef]
- Bai, Y.; Dong, L.; Zhang, C.; Gu, J.; Sun, Y.; Zhang, L.; Chen, H. ZIF-8 Filled Polydimethylsiloxane Membranes for Pervaporative Separation of n-Butanol from Aqueous Solution. Sep. Sci. Technol. 2013, 48, 2531–2539. [Google Scholar] [CrossRef]
- Keser Demir, N.; Topuz, B.; Yilmaz, L.; Kalipcilar, H. Synthesis of ZIF-8 from Recycled Mother Liquors. Microporous Mesoporous Mater. 2014, 198, 291–300. [Google Scholar] [CrossRef]
- Hardian, R.; Liang, Z.; Zhang, X.; Szekely, G. Artificial Intelligence: The Silver Bullet for Sustainable Materials Development. Green Chem. 2020, 22, 7521–7528. [Google Scholar] [CrossRef]
- Wu, C.; Qiao, X.; Chen, J.; Wang, H.; Tan, F.; Li, S. A Novel Chemical Route to Prepare ZnO Nanoparticles. Mater. Lett. 2006, 60, 1828–1832. [Google Scholar] [CrossRef]
- Fang, M.; Wu, C.; Yang, Z.; Wang, T.; Xia, Y.; Li, J. ZIF-8/PDMS Mixed Matrix Membranes for Propane/Nitrogen Mixture Separation: Experimental Result and Permeation Model Validation. J. Membr. Sci. 2015, 474, 103–113. [Google Scholar] [CrossRef]
- Yin, H.; Khosravi, A.; O’Connor, L.; Tagaban, A.Q.; Wilson, L.; Houck, B.; Liu, Q.; Lind, M.L. Effect of ZIF-71 Particle Size on Free-Standing ZIF-71/PDMS Composite Membrane Performances for Ethanol and 1-Butanol Removal from Water through Pervaporation. Ind. Eng. Chem. Res. 2017, 56, 9167–9176. [Google Scholar] [CrossRef]
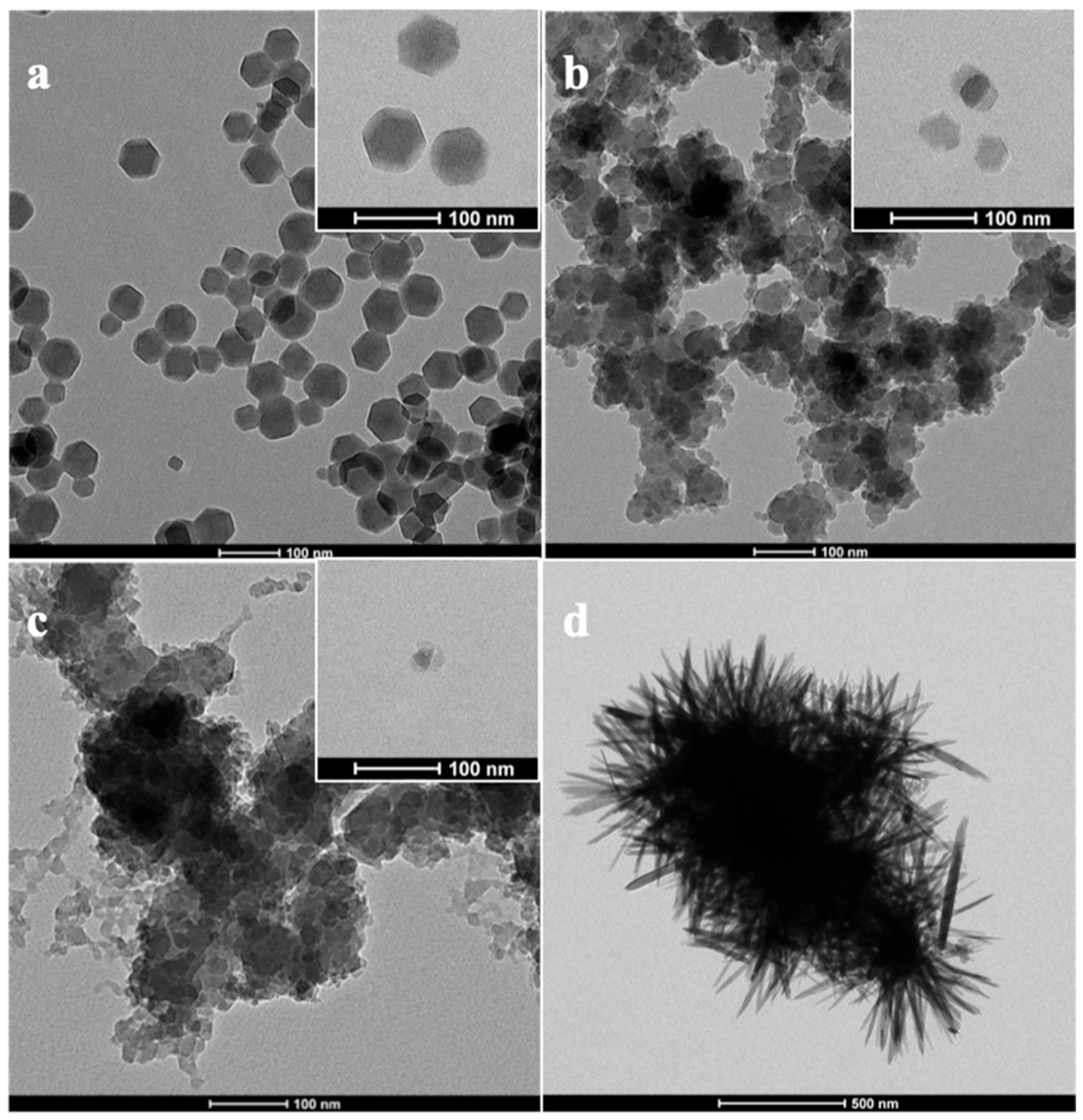
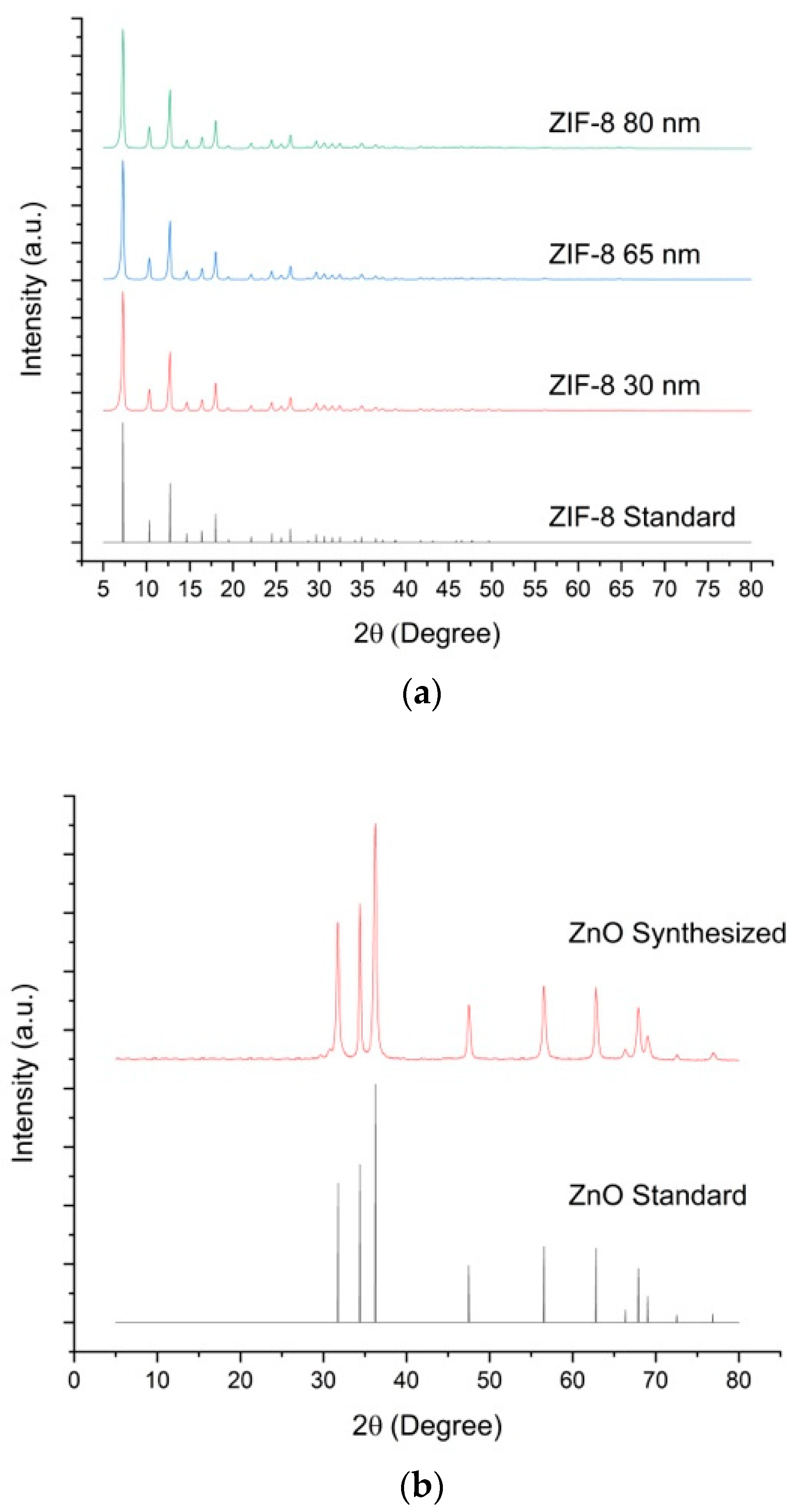
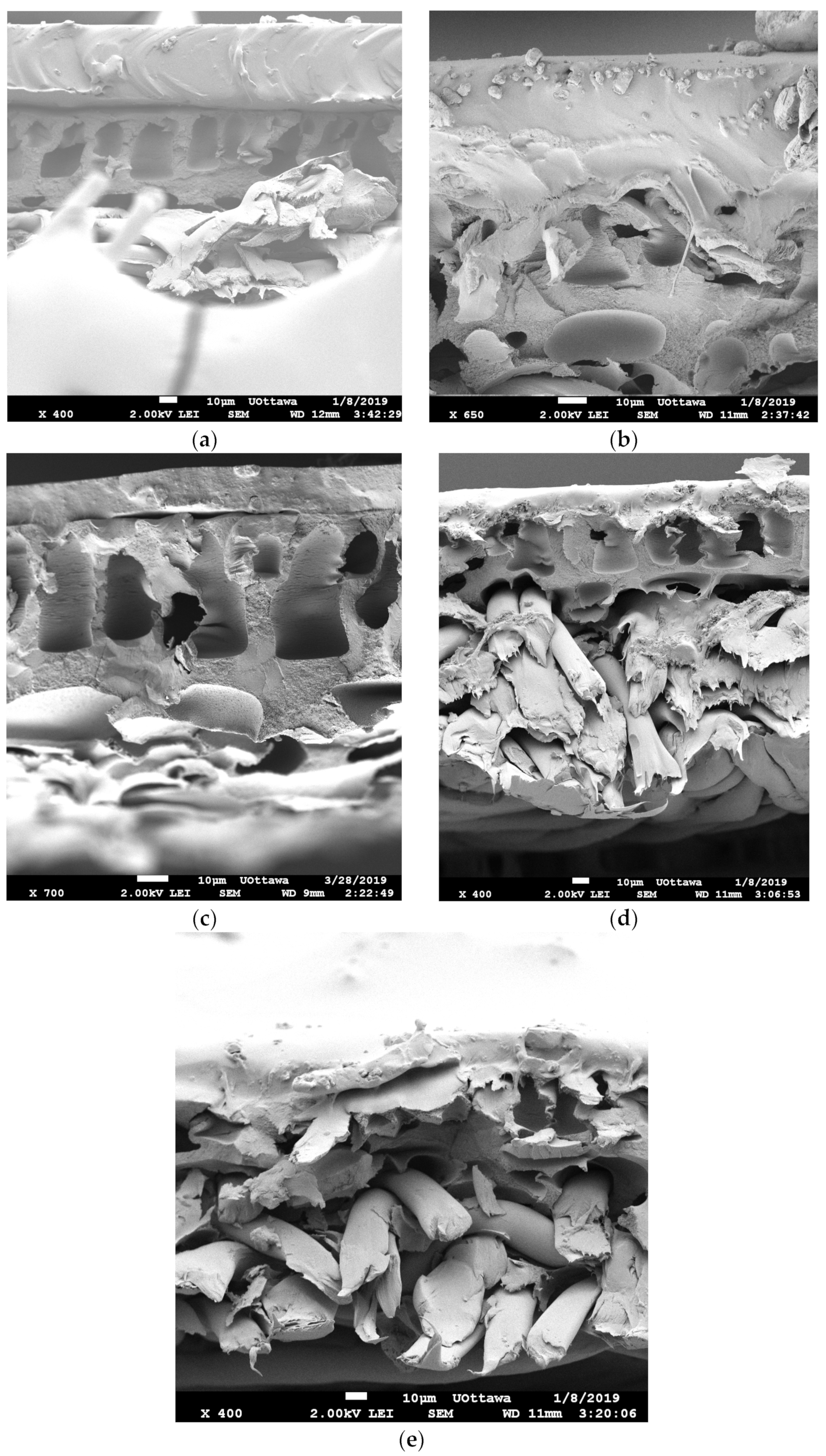
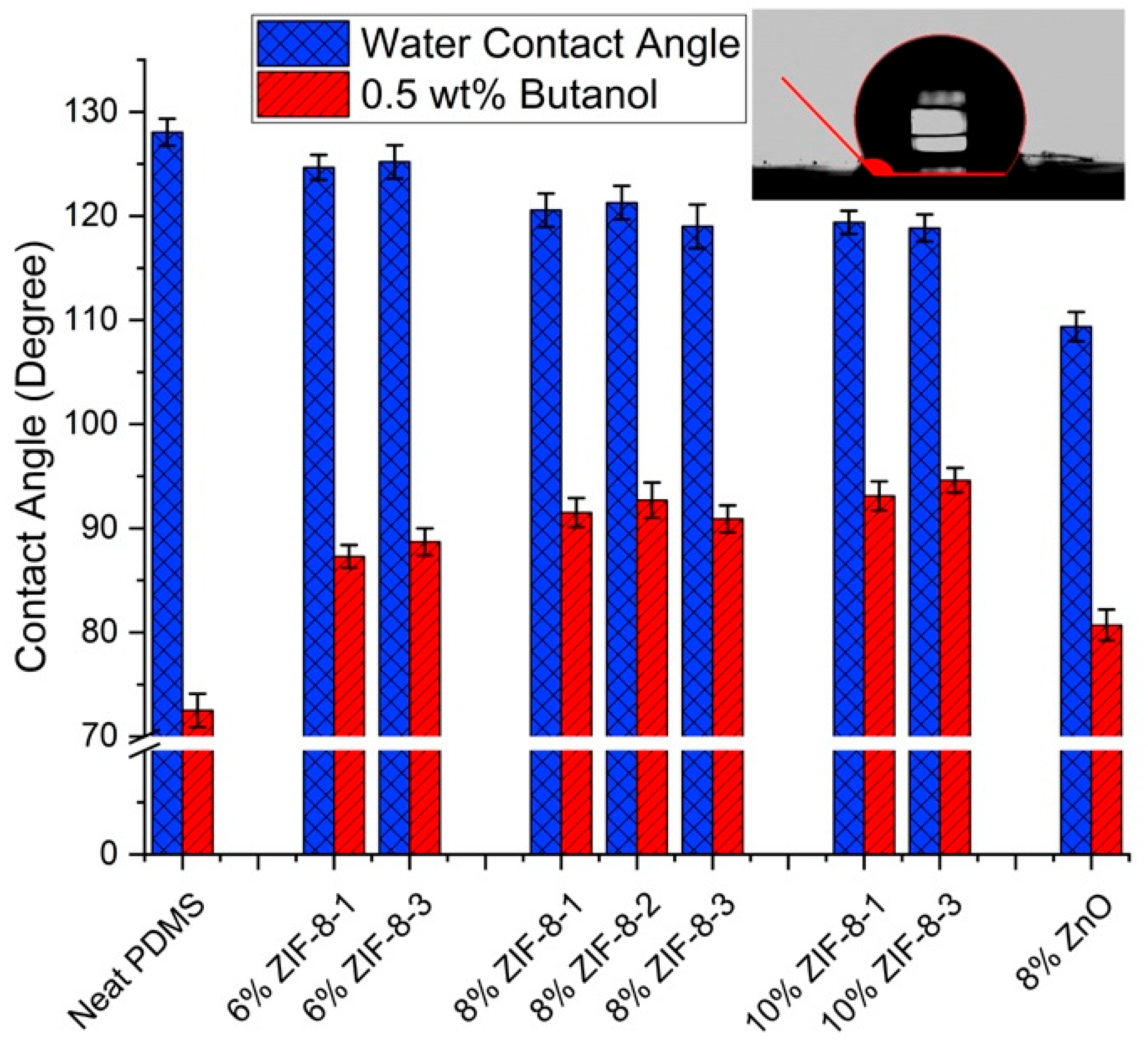
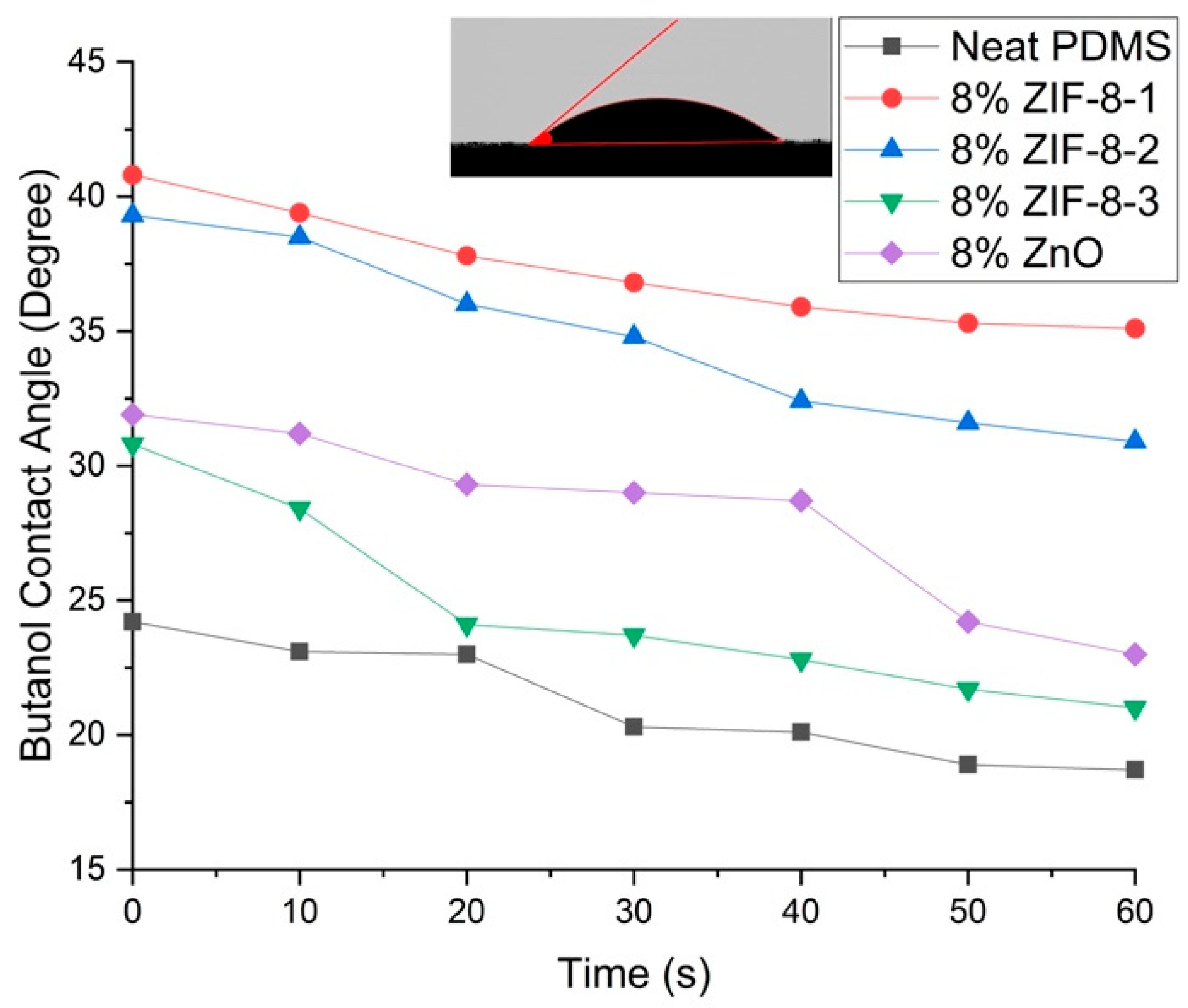
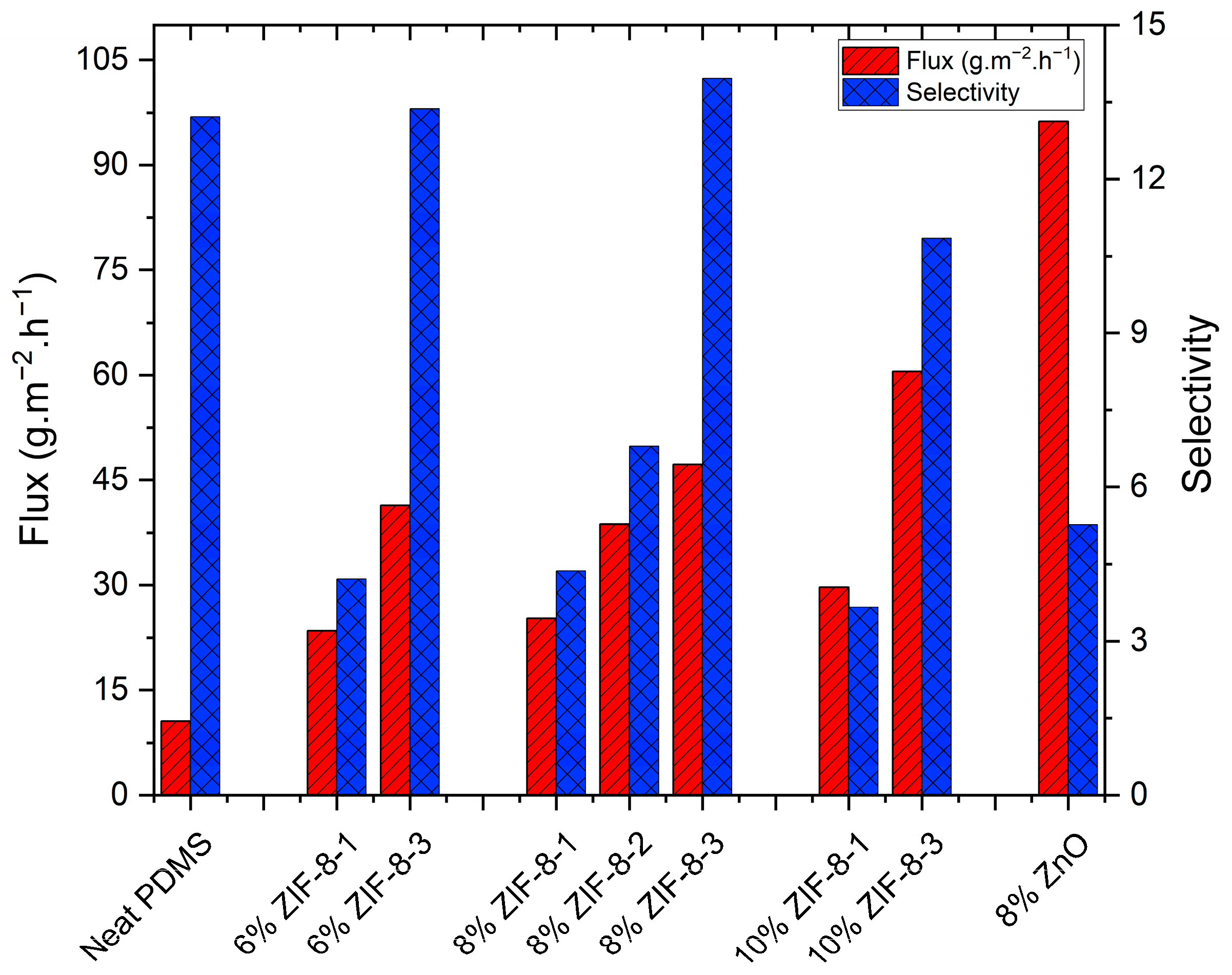

| Particle Name | Particle Size (nm) |
|---|---|
| ZIF-8-1 | 80 ± 20 |
| ZIF-8-2 | 65 ± 08 |
| ZIF-8-3 | 30 ± 15 |
| ZnO | 500 ± 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamani, A.; Thibault, J.; Tezel, F.H. Separation of n-Butanol from Aqueous Solutions via Pervaporation Using PDMS/ZIF-8 Mixed-Matrix Membranes of Different Particle Sizes. Membranes 2023, 13, 632. https://doi.org/10.3390/membranes13070632
Zamani A, Thibault J, Tezel FH. Separation of n-Butanol from Aqueous Solutions via Pervaporation Using PDMS/ZIF-8 Mixed-Matrix Membranes of Different Particle Sizes. Membranes. 2023; 13(7):632. https://doi.org/10.3390/membranes13070632
Chicago/Turabian StyleZamani, Ali, Jules Thibault, and Fatma Handan Tezel. 2023. "Separation of n-Butanol from Aqueous Solutions via Pervaporation Using PDMS/ZIF-8 Mixed-Matrix Membranes of Different Particle Sizes" Membranes 13, no. 7: 632. https://doi.org/10.3390/membranes13070632







